Caenis pycnacantha, Jia, Yan-Yan, Qin, Jia-Zhang, Ju, Min & Zhou, Chang-Fa, 2010
|
publication ID |
https://doi.org/10.5281/zenodo.196612 |
|
DOI |
https://doi.org/10.5281/zenodo.6209883 |
|
persistent identifier |
https://treatment.plazi.org/id/6F7F87D0-FF93-D770-02EE-7F4BFBA8FA05 |
|
treatment provided by |
Plazi |
|
scientific name |
Caenis pycnacantha |
| status |
sp. nov. |
Caenis pycnacantha View in CoL sp. nov.
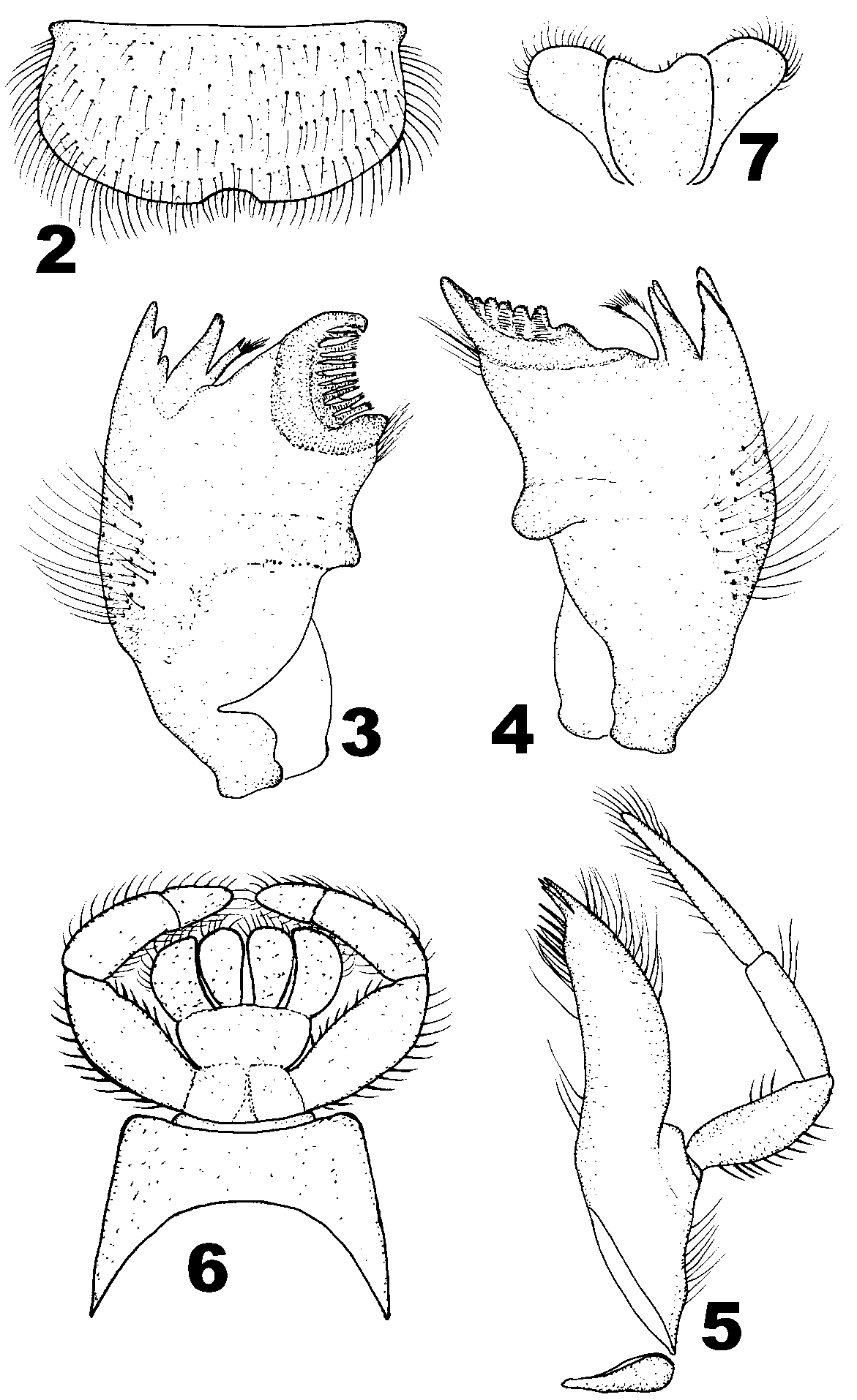
Mature larvae (in alcohol): Body length 3.6–5.4 mm, caudal filaments 3.7–4.9 mm. Body generally pale to slightly pale brown or brown. Vertex dark brown with pale ecdysis suture. Ocelli pale, compound eyes dark black ( Figs. 1, 12–13). Mouthparts: labrum slightly concave medially, and with dense short hair and spines on anterior margin and dorsal surface ( Fig. 2 View FIGURES 2 – 7 ). Mandibles: with long marginal setae on surface and seven to eight spines on inner margin. Left mandible ( Fig. 3 View FIGURES 2 – 7 ): outer incisor with three denticles while inner incisor with two; prostheca clavate, with long fine setae and two sharp bristles at apex. Right mandible ( Fig. 4 View FIGURES 2 – 7 ): both of the incisors with two denticles, the apex of prostheca represented by a tuft of branched setae. Maxillae ( Fig. 5 View FIGURES 2 – 7 ) with 3 obvious bristles at apex and scattered long, fine setae and spines along inner and outer margin. Maxillary palpi segment 2 is the shortest in the three segments (length ratio of them: 1: 0.7: 1.1); segment 1 and 3 with sparse long hair and short spines along both inner and outer margins while segment 2 with three long setae on outer margin subapically only. Segment 1 and 2 of labial palpi subequal in length, segment 3 shorter than any of them; segment 2 and 3 decorated with dense long setae. Glossae and paraglossae with dense tiny setae at apex ( Fig. 6 View FIGURES 2 – 7 ). Hypopharynx shown in figure 7, superlinguae with a row of long hair along free margin ( Fig. 7 View FIGURES 2 – 7 ).
Thorax: Pronotum expanded outwards slightly, with three irregular pale spots dorsally, a narrow and shallow subanterior ridge located near anterior margin; anterolateral angles of pronotum expanded forwards slightly, lateral margin with sparse hairs. Mesonotum with small expanded anterolateral lobes, a pair of coneshaped pale dots near anterior margin; medial line prominent, forming a longitudinal ridge sometimes. Legs pale brown, with dense setae and spines; femora with blackish brown band apically; forefemur with a row of transverse spine-like setae near apex ( Figs. 1, 12); Length ratio of femora: tibiae: tarsi of foreleg = 1.5: 1.0: 1.0; claw single, with 6 denticles at base. Tibiae and tarsi pale brown. Mid- and hindlegs with similar color pattern but without transverse setae on femora; mid claw with 8–10 denticles at base, hind claw with many denticles from base to subapex, basal 3 ones slightly larger.
Abdomen: terga 1, 2, 10 darker than others; terga 7–9 with three longitudinal pale stripes, one median, two near middle line ( Figs. 1, 12); terga 2–9 with sharp posterolateral projections; a row of strong and acute denticle on posterior margin of each sternum. Apex of sternum 9 with blunt margin. Operculate gill 2 subquadrate; dorsal surface pale-brown, color pattern similar to abdominal terga; Y-shape ranges distinct, with dense setae along free margins and few longer setae on lateral edge; ventral surface of gills 2 with tiny palmate outgrowths near outer and posterior margins ( Figs. 14–15 View FIGURES 14 – 15 ). Caudal filaments pale, with sparse setae between segments.
Male imago (in alcohol): Body length 2.8 mm, caudal filaments 8.0 mm. Head and thorax dark brown; abdomen pale, with diffused brown marks ( Fig. 16 View FIGURE 16 ). Antennae pale, pedicel 1.5X as long as scape, flagellum expanded at base and tapered to apex ( Fig. 8 View FIGURES 8 – 11 ). Ocelli pale, compound eyes dark brown. Forefemur washed with brownish black markings; mid and hind femora pale; forefemur: tibiae: tarsi=1.0: 2.9: 1.9; femora of all legs with slightly pigmented apex; legs with paired claws, one hooked, one blunt. Wing pale, transparent, with brown veins, subcosta robust, yellow-brown ( Figs. 9 View FIGURES 8 – 11 , 16 View FIGURE 16 ). Genitalia: forceps covered with dense, tiny setae on surface; four spines located on the apex. Penis lobes fused, posterior margin with slight median emargination. Styliger plate pale yellow, with brown markings on the middle of the base ( Figs. 10–11 View FIGURES 8 – 11 , 17–18 View FIGURES 17 – 18 ). Caudal filaments pale.
Female imago (in alcohol): Body length 2.6 mm, caudal filaments 1.3 mm. Similar to male.
Etymology. The epithet pycnacantha is from the Latin pycnacanthus (dense), in reference to the surface of the forceps which are covered with dense, tiny setae.
Diagnosis and discussion. To date only six Caenis species have been reported from Chinese Mainland ( Eaton 1884; Klapálek 1905; Ulmer 1936; Zhou et al. 1997, 2000; Gui et al. 1999; Zhou & Zheng 2004). C. pycnacantha sp. nov. is similar to C. nigropunctata (see Ulmer 1940) because both have three longitudinal pale stripes on larval abdominal terga 7–9, and the forceps have dense setae on the surface and spines on the apex. However, they can be separated by the following characters: 1. The larval legs of C. pycnacantha sp. nov. have obvious dark brown markings, which are absent in C. nigropunctata ; 2. The larval abdomen of C. nigropunctata is slightly paler than in C. pycnacantha sp. nov., especially that of terga 10; 3. The apical segment of maxillary palpi of C. pycnacantha sp. nov. is slightly longer than second one, but those of C. nigropunctata are subequal; 4.The adult antennae of the new species are pale and the flagellum is slightly expanded at the base; in C. nigropunctata the antennal base is broader. Moreover, in the male imago of C. pycnacantha sp. nov., the forceps have four large spines at apex compared against C. nigropunctata with a tuft of spines on the same position.
Kang & Yang (1994, 1996) and Tong & Dudgeon (2002) described totally 10 Caenis species from Chinese Taiwan and Hong Kong area, but all of them are reported from larval stages only and are distinguished by color pattern and/or mandibular seta number or length. It is believed here that those characters can vary in certain level. Without imaginal materials, it is very difficult to compare the new species here to these species.
Malzacher (1984) divided the European Caenis species into two lineages based on forceps characteristics: horaria –lineage (with straight, strongly sclerotised tips of forceps) and macrura -lineage (bent forceps with some bristles or a tuft of long spines on tips). The macrura -lineage is divided further into five species groups. C. pycnacantha sp. nov. can be placed into the macrura -lineage and macrura -group because of the apical spines on the forceps; it is distinguished from other members of the group by the dense setae on the surface of the forceps.
Biology. Most nymphs of C. pycnacantha sp. nov. were collected from small pools in streams with sediments of thick leaves and soil. Nymphs were often found under surface of stones in water. This species can be found and collected in most seasons except cold winter (usually from end of November to February). The adults are easily reared from mature nymphal stage, those having dark wing pads can become adults in 2– 3 days in the rearing tank. However, we did not find living adults during our daytime field collections or at light in the evening although some dead imagos were seen on the water surface during the day. Zhou et al. (2004) reported that in dawn the collect light can attract many caenid adults and subimagos. It is suspected the new species emerges in the early morning too.
The width of streams during collection in Zijin Hill was about 1 m and depth of water was less than 40 cm (some places only 10 cm). The streams are often represented by small lentic pools and water flows under the stream bed in some sections. Except after rain, the streams flow slowly, less than 20 cm /s. There is a heavy tree canopy shading most of the stream ( Fig. 19 View FIGURE 19 ).
Material examined. Holotype: 1 male, Ying-Tuo village, May 24, 2004, Peng-LI; Paratypes: 24 Nymphs, 6 males 20 females (reared from larvae in lab), same data as for holotype; 12 Nymphs, Ling-Gu-Si Temple, May 24, 2006, Peng-LI; 3 Nymphs, Water-side Pavilion, May 29, 2007, Dong LIU, Hui XIE and Shi-Lei WANG; 10 Nymphs, Ying-Tuo village, May 11, 2008, Shi-Lei WANG, Hui XIE, Yan-Yan JIA and Ping CHEN; 2 Nymphs, Ying-Tuo village, Sep. 22, 2008, Shi-Lei WANG, Yan-Yan JIA and Ping CHEN. All types and specimens are collected from Zijin Hill, Nanjing City, Jiangsu Province, China and deposited in the Mayfly Collection, College of Life Sciences, Nanjing Normal University, China.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |