Varanus obor
|
publication ID |
https://doi.org/ 10.5281/zenodo.194776 |
|
DOI |
https://doi.org/10.5281/zenodo.6210845 |
|
persistent identifier |
https://treatment.plazi.org/id/6C7F8780-DF16-1F47-D8FE-FB6EFBE7F829 |
|
treatment provided by |
Plazi |
|
scientific name |
Varanus obor |
| status |
|
Varanus obor species novum
( Figs. 1–7 View FIGURE 1 , 9 View FIGURE 9 )
Holotype. RMNH. RENA 7225. Soela-Bési [now known as Pulau Sanana, Maluku Utara province], Indonesia. Leg. et don. D.S. Hoedt. Collection date was apparently not recorded, but probably between 1863– 1866 as Dirk Samuel Hoedt (1815–1893) was Secretary of the Moluccas from 1853–1867, and travelled extensively while collecting for the Natural History Museum, Leiden, between 1863–1866 (Steenis- Kruseman, 1950).
Diagnosis. The new species is a member of the Varanus indicus species group of the subgenus Euprepiosaurus . It is distinguished from other Euprepiosaurus by a combination of the following features. (1) Dorsum dark brown, charcoal gray or black, often with dispersed single tan or pale yellowish brown scales, most evident on nape and sacral regions. From a distance the dorsum appears uniformly glossy black. (2) Anterior facial region with variable orange or orange-red markings in life, always including the supra-and infralabial regions anterior to the eye, extending to include the entire snout and anterior interorbital region in some individuals. (3) Tail strongly compressed distally, dark brown or black with 17–20 indistinct, narrow pale bands, most prominent in the middle third. (4) Venter dark gray with diffuse paler areas arrayed in irregular incomplete transverse bands. (5) Throat gray-brown, with variable white markings that are usually arranged in 4–8 transverse patches and sometimes extend to the lateral neck or even the temporal-facial region. The mental region is usually white, grading into orange laterally. (6) Tongue pink with dark pigmentation on the distal half of the tines. (7) Middorsal scales comparatively large and highly polished, in 136–148 transverse rows from occiput to cloaca, and 119–134 rows at midbody.
Description of holotype. A subadult female, total length 885 mm (snout-vent length 355mm, tail 530mm). The keratin layer has been lost on the dorsum, venter and partially on limbs, tail and throat during storage. Shotgun wounds are visible as a number of holes in the skin in the lateral chest region. Body, head and tail are dark brown where the keratin layer remains, otherwise a pale gray. The tail is round at its base, becoming laterally compressed and triangular at 1/7 of its length. At its midpoint the tail is nearly twice as high as it is wide. One to four middorsal caudal scale rows form a sharp double ridge extending from the transition to a triangular cross-section nearly to the tip. The tail is muscular with two distinct longitudinal grooves separating muscle bundles. It is marked by 17 discernible paler crossbands that are 3–5 scale rows wide.
The venter is lighter than the dorsum with about 9 regular but ill-defined crossbands, which are most prominent laterally and fade out towards the center. Nostrils are large and slightly oval in outline, positioned nearer to the snout than to the anterior margin of eye. The cartilaginous nasal capsules are slightly expanded, forming a shallow saggital groove on the rostrum. The tongue is pale, with dark pigment on the distal half of each tine. The limbs are robust, feet are large, and the claws are strongly recurved. One claw is missing from the third digit of the left front foot.
Nuchal and dorsal scales are large, round to oval, and flattened, each with 4–6 small scale glands (sensu Andres et al. 1999). The oval middorsal scales are about 2.5 times longer than the circular lateral and ventrolateral scales, with scale size declining in a smooth gradient. Granular scales completely surround each dorsal scale from nape to sacral region, but they are confined to the anterior and posterior edges on the hind limbs and base of the tail, and receding to the posterior edges of scales at 1/5th of the tail length and absent past ½ the tail length. From around mid-body posteriorly the dorsal scales have a poorly defined keel.
Dorsal head scales are irregularly polygonal and flattened with the intraocular scales in 3–4 enlarged rows. There are 5, 6 (r, l) enlarged supraocular scales that are bordered by 1–2 arcuate rows of smaller scales medially and 2–3 supraciliary rows laterally. The rostral scale is paired. Supralabials 27, 27, with 8–12 scale gland ducts/scale anterior to the orbit, increasing to 60–80 gland ducts/scale posteriorly. Infralabials 29, 29, with 6–14 gland ducts/scale anterior to the orbit, increasing to 20–60 gland ducts/scale posteriorly. Temporal scales are rounded oval and slightly domed, with the largest over the postorbital arch and bordering the supralabials, having from 4–20 gland ducts/scale. Three rows of scales separate the nostril from the supralabials, and two rows lie between the supralabials and the ventral margin of the orbit.
Gular scales are small and squared, mostly surrounded by a single row of granules, with 5–15 gland ducts/ scale between the mandibles, and 3–7 gland ducts/scale near the gular fold. The gular scales are enlarged laterally where 2–6 rows border the infralabials, each scale containing up to 60 gland ducts. Mental and submental scales are pentagonal, but the latter are smaller. Ventral scales on the chest are flat and mostly hexagonal, becoming longitudinally rectangular on the sternum and trunk, grading into ovoid at the pelvis and increasing in size near the cloaca. Ventral scales near the cloaca are surrounded by granules and have 5–30 centrally positioned gland ducts. These structures are probably not femoral pores (sensu Andres et al. 1999).
Scales on the dorsal surface of the forelimbs above the elbow are slightly domed, small and oval, becoming enlarged on the anterodorsal aspect of the forearm. Scales on the anterior aspect of the hind limbs are large and flat, hexagonal or oval, becoming smaller and keeled on the posterior surface. Ventral scales of the thighs are equipped with single gland ducts at their posterior ends. Ventral scales of the upper forelimbs each have 3–7 ducts. The anterior ventral margin of the proximal phalanx of the fourth toe has a row of 11 distinctly enlarged hemispherical scales that have flattened anterior margins forming a prominent knobbed ridge. There are 31 rows of lamellae beneath the fourth toe. Palms and soles are light brown, with a few dark pigmented scales in the center; the plantar scales are small, rounded and domed and surrounded by granules. Scales on the ventral surface of the tail are rectangular and strongly keeled. Each ventral scale corresponds to two rows of lateral and dorsal caudal scales. Lateral caudal scales are small and rectangular, each with a domed median ridge, most having a single duct pit near the posterior edge.
The oviducts of the holotype are translucent and thin, and there are no enlarged ovarian follicles. The hemiclitori are inverted, and the condition of the specimen is such that dissection or an attempt to evert them for study was deemed unwarranted in lieu of providing a description from fresh material.
Scale counts. The holotype has 120 midbody scale rows (S). There are 122 dorsal scale rows (XY), and 136 dorsal scale rows counted from the last occipital scale to a point dorsal to the cloaca (DOR). There are 90 transverse rows of ventral scales from the gular fold to the anterior edge of the hind legs (T), and 113 ventral scale rows counted from the gular fold to the cloaca (VEN). There are 35 transverse rows of dorsal scales from the hind margin of the tympanum to the gular fold (X) and 85 scales around the neck at the anterior edge of the gular fold (m). There are 47 scales from rictus to rictus (P) and 80 scales around the base of the tail (Q). Measurements. Snout to vent length (SVL) is 355mm; tail length (F) is 530mm; total length (TL) is 885mm; body length from gular fold to cloaca (E) is 230mm; head-neck length from tip of snout to gular fold (D) is 125mm; head length from the snout to anterior border of tympanum (A) is 57mm; maximum head width behind the eyes (B) is 28mm; head depth measured over the eyes (C) is 22mm; distance from anterior border of eye to middle of nostril (G) is 16mm; from middle of nostril to tip of snout (H) is 13mm; from anterior border of tympanum to anterior border of eye (I) is 35mm.
Variation and color in life. Most of the dorsum, venter and head of the type specimen has lost its keratin layer and original pigmentation during preservation and storage. The pale grey dorsal regions of the holotype are brown or black in living animals. The nearly white venter and throat of the holotype are grey-brown in life, and the brown facial regions of the type are faded from the bright orange-red coloration of living V. obor sp. n.
Thirty eight free-living specimens showed little morphological variation from the holotype and permit a full description of coloration in life ( Figs. 3–6 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 9 View FIGURE 9 ). The most variable features are the intensity of dorsal and facial pigmentation, and the extent and configuration of white areas on the throat, lateral neck and face. Dorsal coloration varies from very dark brown or black to medium brown ( Figs. 3–5 View FIGURE 3 View FIGURE 4 View FIGURE 5 ). The density of isolated yellow scales on the dorsum, nuchal region and top of head varies among individuals, such that the neck occasionally may appear almost brown because a majority of nape scales have some yellow markings. Yellow or yellowish brown scales may align into a few transverse bars 2–4 scales wide on the posterior trunk ( Fig. 5 View FIGURE 5 b). The redorange pigmentation of the face varies in saturation from grey-orange to tomato red, and the extent of coverage also varies ( Fig. 6 View FIGURE 6 ). This coloration may be most intense in males; the single juvenile observed had a dull orange-gray facial region, and the facial region of adult females is strongly suffused with orange. One individual had an irregular band of white extending along the supralabials from anterior to the orbit to the ear ( Fig. 6 View FIGURE 6 b). Throat pattern is highly variable; among 12 wild individuals and the holotype the amount of white varies from ca. 0–80% coverage. The mental region is always white, blending into orange on the medial surfaces of the mandibles. The mental patch varies in posterior extent from the anterior border of the orbit nearly to the level of the ear. Behind this point the throat may be entirely dark gray (two animals), or show a highly irregular series of about 4–8 transversely elongated white patches that often run together on the ventral midline. The white areas diminish in area and lateral extent posteriorly, and may contact the gular fold.
The tongue is pale pink in life, with indistinct and irregular gray mottling on the lateral surfaces of the tines. The iris is medium brown, usually with a paler middorsal quadrant.
Varanus obor sp. n. differs from all other species in the V. indicus group in having a predominantly dark tail with 17–20 narrow, regularly-spaced pale bands ( Fig. 4 View FIGURE 4 ). These bands are 3–5 scale rows wide laterally (though usually only two adjoining rows form a continuous ring), and are separated by from 13–17 rows of dark scales near the tail base to 19–22 scale rows distally. All other species in the V. indicus group (except V. zugorum Böhme & Ziegler ) have a flecked or mottled tail base, with the light markings organizing into progressively more distinct rings that transition into bands distally, with the pale components of the bands becoming as wide as or wider than the dark components. As far as is known, V. zugorum has a nearly patternless tail with faint dark crossbands only near the end ( Böhme & Ziegler 2005).
Scale counts are available for six free-living animals in addition to the type, and are presented in Table 1.
Table 1. Measurements (in mm) and counts of Varanus obor sp. n., comparing the holotype with values for six animals examined (and released) in the field. Abbreviations (letters) are defined in the text; 4th toe refers to the number of enlarged scales along the leading edge of the proximal phalanx, plus the number of enlarged scales extending onto the base of digit 3.
means 125.4 123.4 142 92.4 113.4 18.4 The hemipenis of a 42 cm SVL wild male was everted and examined ( Fig. 7 View FIGURE 7 ). The hemipenis was about 4 cm in length, with strongly asymmetrical lobes. The sulcus spermaticus was oblique basally, deflecting onto the lateral lobe at the base of the apical platform, and terminating at the base of the lateral hemibaculum. The apical platform was asymmetrically extended toward the sulcal surface of the lateral lobe. Hemibacula were weakly mineralized and covered with a thin layer of tissue. The lateral hemibaculum was developed as a decurved cone with a single point in the individual examined. The medial hemibaculum was flattened, decurved and quadrangular, bearing 8 small denticles on its distal margin. Paraphasmata were present only on the lateral lobe, in about 11 rows; the individual elements were white and strongly mineralized, with free and slightly spinose proximal margins. The truncus and pedicel of the hemipenis were smooth, without basal pigmentation.
Comparisons with other species. Varanus obor sp. n. is unlikely to be confused with any other species in the Varanus indicus species group. The black dorsum, dark throat with irregular white patches, grey venter, orange-red labial region, and dark tail with widely-spaced narrow pale bands are unique characters. Varanus obor sp. n. can additionally be distinguished from the melanistic members of the V. prasinus species group by the laterally compressed, non-prehensile tail and much larger adult size.
The species most likely to be confused with V. obor sp. n. are the melanistic populations of the V. s a l v a t o r complex (subgenus Soterosaurus) of Thailand, Sulawesi and the Sula Islands, and V. togianus (Peters) of the Togian Islands northeast of Sulawesi. These differ from V. obor sp. n. by having a dark grey-blue tongue (vs. pink), lacking orange labial pigmentation, having 10–11 enlarged supraocular scales (vs. 3–4), and more anteriorly placed nares. Additionally, Soterosaurus species have pigmented hemipenes with the sulcus bifurcating to the bases of both hemibacula, and strongly enlarged, imbricate rows of paraphasmata (Zeigler & Boehme 1997). The dark-colored V. m a b i t a n g Gaulke & Curio of Panay Island in the Philippines can be distinguished from V. obor sp. n. by lacking the orange-red facial markings, having smaller scales ( V. mabitang dorsals [XY] 124–175, mean 139; ventrals [T] 111–143, mean 127 [ Gaulke et al. 2005], vs. XY 119–127, mean 123, T 88–94, mean 92 in V. obor sp. n), having strongly keeled ventral scales (smooth in V. obor sp. n.) and having a reddish instead of brown iris.
Within the V. i n d i c u s group V. obor sp. n. aligns with the derived clade recognized by Böhme and Ziegler (1997) and Ziegler and Böhme (1999). These species are characterized by the following features (vs. the primitive state, in parentheses): dark blue pigment on the tongue (tongue pale); large dorsal scales reflected in midbody scale counts <150 (counts usually>160); blue pigment on the tail or limbs (blue lacking); and lack of paraphasmata on the medial lobe of the hemipenis (vestigial paraphasmata present). Mitochondrial DNA sequences ( Ast 2001, Ziegler et al. 2007b) support this polarity, although there is poor resolution at critical parts of both trees. As a general practice the reconstruction of species trees from single gene trees (especially with uniparentally inherited loci) is of questionable value ( Avise 2000) because stochastic processes can introduce misleading variation ( Irwin 2002, Kuo & Avise 2005). Further, the locality data for specimens acquired from animal dealers must be regarded as uncertain.
Scale counts and body proportions ally V. obor sp. n. with V. melinus of nearby Mangole and Taliabu islands, V. cerambonensis Philipp, Böhme & Ziegler of Buru, Seram and Ambon, and V. i n d i c u s (islands of Sahul Shelf and eastward). Comparisons of 7 scale counts for V. obor sp. n. to values provided for 11 other members of the V. indicus group in Table 5 of Ziegler et al. (2007b), and values for V. lirungensis Koch, Arida, Schmitz, Böhme & Ziegler (Koch et al. 2009) show overlaps in 7 ( V. cerambonensis ), 6 ( V. indicus , V. melinus ), 4 ( V. lirungensis ), 3 ( V. caerulivirens Ziegler, Böhme & Philipp, V. f i n s c h i Böhme, Horn & Ziegler, V. juxtindicus Böhme, Philipp & Ziegler , V. rainerguentheri Ziegler, Böhme & Schmitz ), 2 ( V. doreanus (Meyer) , V. jobiensis Ahl , V. zugorum ) or 1 character states ( V. yuwonoi Harvey & Barker ).
The three species having 6 or 7 range overlaps in scalation differ strikingly from V. obor sp. n. in coloration and pattern development. Varanus cerambonensis and V. indicus (as redefined by Philipp et al. 1999) have white or yellow scales distributed across the dorsal and lateral surfaces, unmarked pale venters, and partly or entirely blue tongues. Adult Varanus melinus are pale yellow with highly variable dark markings on the trunk and tail, and display complex pattern ontogeny as they grow. Hatchling V. melinus are dark brown dorsally with prominent transverse rows of yellow spots on the trunk; the head is yellowish brown grading to black on the neck, and the tail is black with 22–24 cream bands, with the light and dark bands being of equal width on the distal third of the tail. As they mature, young V. melinus rapidly lose the dark dorsal coloration, initially leaving dark borders around each of the yellow spots. These borders then break down to leave a highly variable pattern of irregular dark markings on a dull yellow ground color, and the dark markings continue to fade with age. This follows an anterior-posterior gradient such that dark pigmentation is least on the head and nape (although a dark postorbital bar often persists), and persists longest on the pelvic region. The dark tail bands become subdivided by light scales proximally, and are visible only on the dorsal aspect of the tail in adults. None of these features is seen in V. obor sp. n. where individuals do not seem to undergo any radical ontogenetic change in patterning. A juvenile specimen observed in the field was of similar coloration as the adults, with the exception of more subdued facial markings.
Etymology. The specific epithet obor is from Bahasa Indonesia. Obor means torch, in reference to the striking facial coloration of this species which is unique in the subgenus Euprepiosaurus . The term is employed as a noun in apposition. We suggest the common name Sago Monitor in recognition of the association of this species with Metroxylon palm swamps. The local name for V. obor sp. n. is “soa-soa hitam” (black monitor).
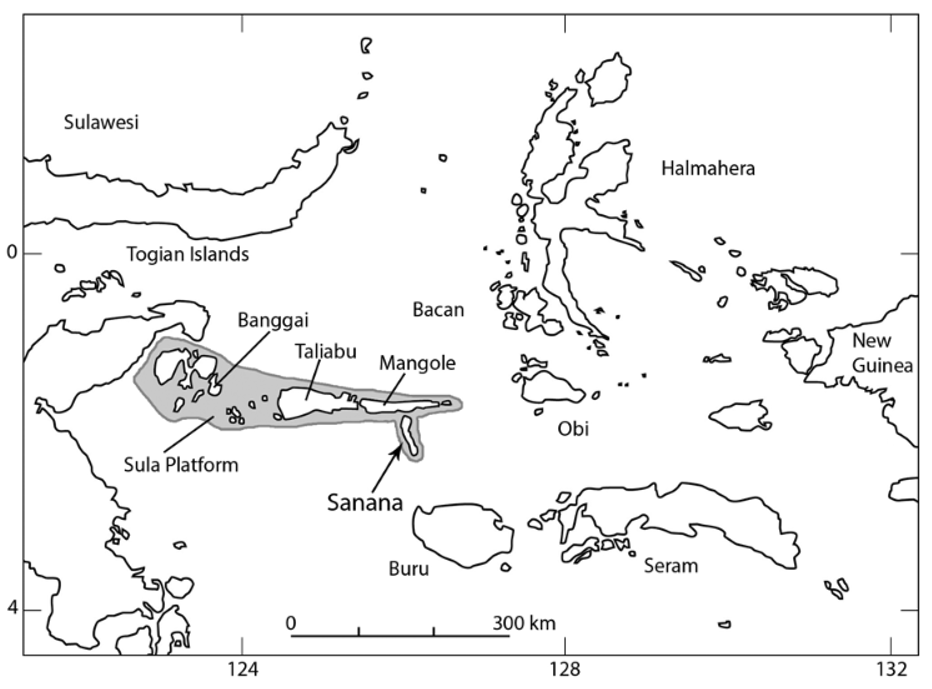
Distribution. Varanus obor sp. n. is endemic to the relatively small (568 km 2) island of Sanana ( Fig. 8 View FIGURE 8 ) in Maluku Utara province (the north Moluccas). The locality data with the holotype were recorded as “Soela Bési”, an old synonym for Sanana. No precise locality accompanies the holotype, but field investigations show that the animal is widely distributed on the coastal margins of Sanana. Fieldwork and enquiries with animal traders and rural residents of nearby Mangole and Taliabu islands have established that V. obor sp. n. is restricted to Sanana.
Ecological observations. Varanus obor sp. n. occurs widely on the NE quarter of Sanana covered by survey work, and is reported from other areas by local informants. Habitat features common to all sites where it was observed include partial to full canopy closure, trees with stem diameters> 30 cm, and dense vegetation at ground level. Field observations indicate that the Sanana monitor reaches the highest densities in coastal sago palm ( Metroxylon sagu Rottboell ) swamps ( Fig. 10 View FIGURE 10 ). In this habitat individuals were seen digging through decomposing palm trunks in search of beetle grubs ( Rynchophorus sp.). The sago palm is extensively utilized as a source of starch by the human population on Sanana, a use that quite possibly has favored monitors. Opened and decomposing trunks and stumps accessible for beetle and insect infestation are much more frequent in places where the palm starch is consumed by people and there is a constant supply of degrading wood. On several occasions monitors were first observed while investigating these felled palms stumps or even immersed in the fermenting pulp ( Figs. 3 View FIGURE 3 , 9 View FIGURE 9 b).
In addition to sago swamps, V. obor sp. n. also frequents forested inland areas, especially riparian habitats around streams and streambeds ( Fig. 11 View FIGURE 11 ), as well as coastal forest and coconut plantations at the margins of mangrove stands. Out of 38 field observations 20 were made in sago swamps, and 14 along freshwater streams up to 180 m elevation. Two individuals were observed in coconut plantations with mature undergrowth, one at a beach near a small patch of sago palms, and one in primary hill forest at over 400 m elevation ( Fig. 12 View FIGURE 12 ).
As far as is known, V. obor sp. n. forages on or near the ground. In addition to observations in sago swamps, one individual was observed digging into exposed roots of a large fallen tree in mature forest near the central divide of Sanana. Animals were observed scavenging on a dead piglet in a sago swamp and a juvenile cuscus along a streambed, confirming that like its relatives this species also feeds on carrion at opportunity. A freshly-captured animal defecated two lizard egg shells (ca. 10 mm in length), two 12 mm brown beetle elytra and portions of the exoskeleton of a large mole cricket.
Varanus obor sp. n. is active from about 0900–1700 hrs on clear or partly cloudy days, but seeks shelter during even brief rainfall events and appears to be inactive on overcast or rainy days. They are adept climbers that seek refuge in trees as well as utilizing the upper trunks and canopy for basking.
Nothing has been published on the ecology of Moluccan varanids previously, but field observations by Weijola on several islands suggest that V. obor sp. n. differs from the other species of this region. Whereas many of the V. indicus group monitors on single-species islands (Kei, Tanimbar, Seram, Buru, etc.) utilize a wide variety of habitats from coastal mangroves and sago to hill forests and riparian habitats, none appeared to have a strong preference for sago swamps. On some of the islands with more complex communities, such as Halmahera and Bacan, none of the resident species were observed in this habitat. It should still be emphasized that on both Seram and Kei Besar, monitors frequently hunt for Rynchophorus larvae in sago, and that this probably is a resource widely utilized by many of the Pacific monitor species.
Varanus melinus , the species morphologically and by scutellation most similar (and possibly a sister taxon) to V. obor sp. n., differs in ecology by preferring more open habitats away from the immediate mangrove zone (Weijola unpub.). The coastal sago swamps and swamp and mangrove forests of Mangole and Taliabu are instead inhabited by a representative of the V. salvator complex. Varanus salvator was also recorded for the first time on Sanana in November 2009, where it occupies coastal habitats, riparian and upland forests in microsympatry with V. obor sp. n. (Weijola & Sweet, in prep.).
| RMNH |
National Museum of Natural History, Naturalis |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.