Fibularia coffea, Tanaka & Wakabayashi & Fujita, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4543.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:2A1736C7-FB28-4C8E-9954-B17BA60B6E57 |
|
DOI |
https://doi.org/10.5281/zenodo.4562871 |
|
persistent identifier |
https://treatment.plazi.org/id/6B335535-DD20-0D28-FF4B-BD97C7280FBB |
|
treatment provided by |
Plazi |
|
scientific name |
Fibularia coffea |
| status |
sp. nov. |
Fibularia coffea sp. nov.
[New Japanese name: Kohi-mame-uni]
Figs. 3–8 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 ; Tables 1 View TABLE 1 , 2 View TABLE 2 ; Electronic Supplementary Table S1 View TABLE 1 .
Non Fibularia japonica Shigei 1982: 11 –16, figs. 1–48 (part).
Non Fibularia plateia Schultz 2005: 321 , fig. 603.
Fibularia n. sp. “bean” Gomes & Mooi 2015: poster presentation.
Material examined. Holotype: NSMT E-10371, whole, with spines, Ayamaru Cape , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°28′26″N, 129°43′09″E), depth 1 m, snorkeling, coll. H. Tanaka, 23 Mar. 2015 GoogleMaps . Paratypes: 1 specimen, NSMT E-10372, whole, denuded, SEM stub, Tsuchi-Hama , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°24′36″N, 129°40′47″E), depth 2 m, snorkeling, coll. H. Tanaka and L. Sakamoto, 20 Mar. 2015 GoogleMaps ; 2 specimens, NSMT E-10373, whole, denuded, SEM stub, Kunigami , Nishinoomote city, Tanegashima Island, Kagoshima Prefecture, Japan (30°48′19″N, 131°01′24″E), depth 4–5 m, scuba diving, coll. H. Yamasaki and R. Yoshida, 1 Mar. 2014 GoogleMaps ; 1 specimen, NSMT E-10374, whole, denuded, SEM stub, Tateyama Bay , Tateyama city, Chiba Prefecture, Japan (34°58′56″N, 139°48′43″E), depth 6.9–8.1 m, dredging, coll. Y. Yoshida, 14 Jan. 2015 GoogleMaps ; 1 specimen, NSMT E-10375, whole, denuded, SEM stub, Tateyama Bay , Tateyama city, Chiba Prefecture, Japan (34°59′05″N, 139°48′57″E), depth 9–12 m, dredging, coll. Y. Yoshida, 12 May. 2014 GoogleMaps ; 1 specimen, NSMT E-10376, whole, denuded, SEM stub, Tateyama Bay , Tateyama city, Chiba Prefecture, Japan (34°58′40″N, 139°46′05″E), depth 5 m, scuba diving, coll. K. Kosoba, 30 Aug. 2015 GoogleMaps ; 29 specimens, NSMT E-10377, dead tests, Tomioka , Amakusa group, Kumamoto Prefecture, Japan, dredging, coll. H. Tanaka, 21 Mar. 2015 ; 1 specimen, NSMT E-10378, dead test, Ayamaru Cape , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°28′30″N, 129°43′05″E), beachcombing, coll. H. Tanaka, 23 Mar. 2015 GoogleMaps ; 7 specimens, NSMT E-10379, dead tests, Ayamaru Cape , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°28′30″N, 129°43′05″E), beachcombing, coll. H. Arima, 11 Dec. 2013 – 27 Mar. 2014 GoogleMaps ; 7 specimens, NSMT E-10380, dead tests, Kosyuku , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°24′10″N, 129°28′04″E), beachcombing, coll. H. Arima, 13 Dec. 2013 – 22 Mar. 2015 GoogleMaps ; 2 specimens, NSMT E-10381, dead tests, Kosyuku , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°24′10″N, 129°28′04″E), beachcombing, coll. H. Tanaka, 22 Mar. 2015 GoogleMaps ; 5 specimens, NSMT E-10382, dead tests, Tsuchi-Hama , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°24′39″N, 129°40′37″E), beachcombing, coll. H. Tanaka, 15 Jul. 2014 GoogleMaps ; 3 specimens, NSMT E-10383, dead tests, Tsuyazaki , Fukutsu city, Fukuoka Prefecture, Japan (33°45′59″N, 130°23′03″E), beachcombing, coll. K. Wakabayashi, 17 Jun. 2005 GoogleMaps ; 10 specimens, NSMT E-10384, dead test, Wada Beach , Oi county, Fukui Prefecture, Japan (35°29′41″N, 135°34′32″E), beachcombing, coll. H. Tanaka, 3 Jan. 2014 GoogleMaps ; 4 specimens, NSMT E-10385, dead tests, Yoan Beach , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°24′12″N, 129°38′35″E), beachcombing, coll. H. Tanaka and L. Sakamoto, 31 Jan. 2016 GoogleMaps ; 48 specimens, NSMT E-10386, dead tests, Zushi Beach , Zushi city, Kanagawa Prefecture, Japan (35°17′18″N, 139°34′25″E), beachcombing, coll. H. Tanaka, 21 Jul. 2012 – 2 Feb. 2014 GoogleMaps ; 2 specimens, NSMT E-10387, whole, denuded, Tsuchi-Hama , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°24′36″N, 129°40′47″E), intertidal, snorkeling, coll. H. Tanaka and L. Sakamoto, 24 Mar. 2015 GoogleMaps ; 1 specimen, NSMT E-10388, whole, denuded, Tsuchi-Hama , Amami-Oshima Island, Kagoshima Prefecture, Japan (28°24′36″N, 129°40′47″E), intertidal, snorkeling, coll. H. Tanaka and L. Sakamoto, 22 Oct. 2016 GoogleMaps .
One specimen, UMUTZ-Ecn-SG10-17T No. 7 (as a paratype of Fibularia japonica ), dead test, off Misaki Marine Biological Station, Sagami Bay, sublittoral zone, coll. K. Aoki, J. Deguchi, T. Sekimoto, H. Suzuki, and M. Shigei, 1926–1978.
GenBank accession number. LC388935 View Materials (holotype: NSMT E-10371, Amami-Oshima Island , Kagoshima Prefecture, Japan) , LC388934 View Materials (paratype: NSMT E-10374, Tateyama Bay , Tateyama city, Chiba Prefecture, Japan) .
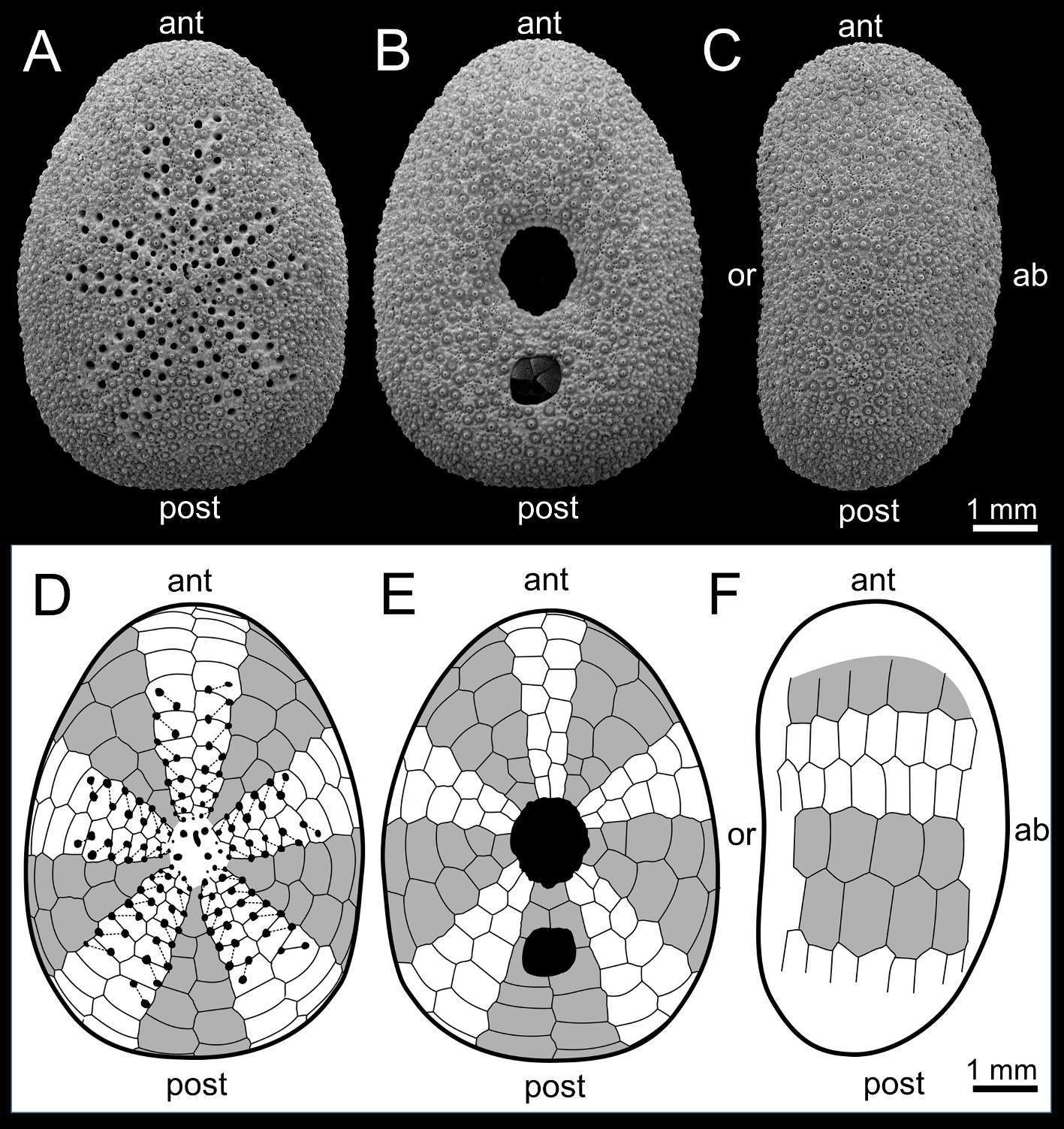
Diagnosis. Test outline elliptical when viewed from above; height low; oral surface slightly depressed toward the peristome. Periproct outline round square shaped. Petaloid region large; number of pores of petal III continues to increase with the test growth, reaching over 20 at TL> 5 mm. Diameter of genital pores equal to or smaller than that of petaloid pores in mature individuals. Two hydropores opening in an irregularly-shaped groove. Black pigments on each aboral perradius, forming symmetric pentaradial in living animals.
Description. The test is very small (TL = 1.53–9.73 mm) ( Fig. 3 View FIGURE 3 ), flattened (TH/TL = 0.38–0.55) ( Fig. 3C View FIGURE 3 ), elliptical when viewed from above (TW/TL = 0.67–0.86), and truncated posteriorly ( Figs. 3A, B View FIGURE 3 ). The test proportion hardly changes with the test growth (slope value is 1.0 between TW and TL, and 1.1 between TH and TL in the allometry regression, Table 1 View TABLE 1 ). The oral surface is slightly depressed around the peristome. The aboral surface is slightly arched convex. There are no internal buttresses. Food grooves are absent ( Fig. 3B View FIGURE 3 ). The ambulacra are almost the same width as the interambulacra ( Figs. 3 View FIGURE 3 D–F). The height of both ambulacral and interambulacral plates are lower than the width at the ambitus ( Fig. 3F View FIGURE 3 ). The petaloid region is large (PL/TL = 0.46–0.75, PW/TL = 0.33–0.61). The ratio of petaloid region size to test size becomes larger with test growth (slope value is 1.3 between PL and TL, as well as between PW and TL in the allometry regression). Each petal is composed of almost parallel series of pore pairs lying oblique, and crossing the ambulacral plates ( Fig. 3D View FIGURE 3 ). The number of pores of petal III, IV, and V is continuously increasing up to 40, 28 and 36, respectively, with the test growth ( Fig. 4 View FIGURE 4 ). The pores become larger towards the distal tip of the petals.
The peristome, situated at the center of the oral side, is small (SL/TL = 0.17–0.34, SW/TW = 0.14–0.30) and slightly elongated antero-posteriorly ( Figs. 3B, E View FIGURE 3 ). The ratio of peristome size to test size becomes smaller with the test grows (slope value is 0.8 between SL and TL, as well as between SW and TL in the allometry regression). The peristomial membrane lacks spine and pedicellariae. Two buccal pores are situated in each ambulacrum at the edge of the peristome. Single sphaeridium is fully enclosed within the test, and in the sphaeridial chamber in each ambulacrum near the peristome.
The rounded square shaped periproct is located halfway between the peristome and posterior margin of the test and is smaller than the peristome (AL/TL = 0.08–0.15, AW/TW = 0.08–0.16) ( Figs. 3B, E View FIGURE 3 ) and covered by 4–6 (mainly 5) naked radiating periproctal plates. The ratio of periproct size to test size hardly changes with the test grows (slope value is 1.0 between AL and TL, and 0.9 between AW and TL in the allometry regression).
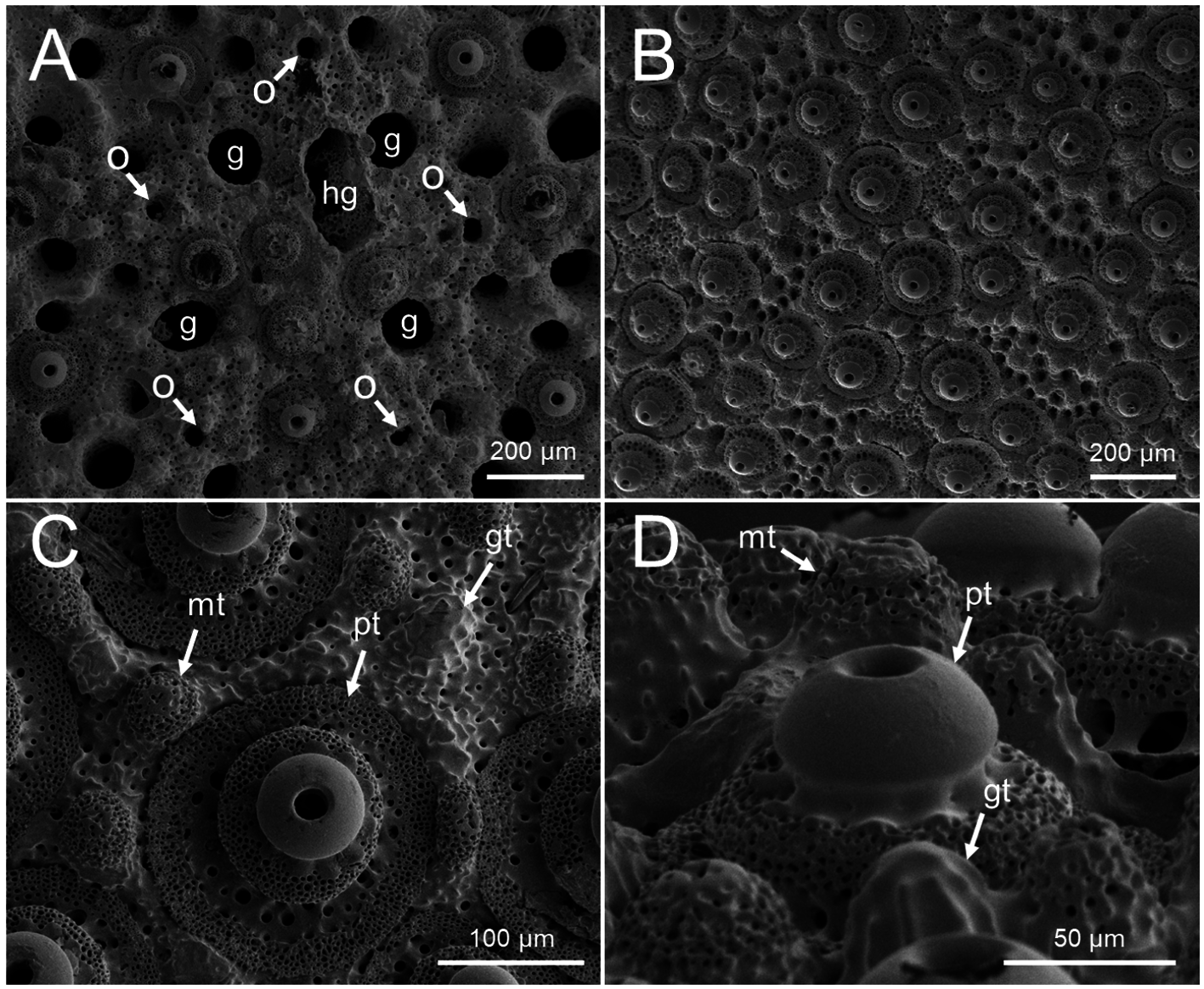
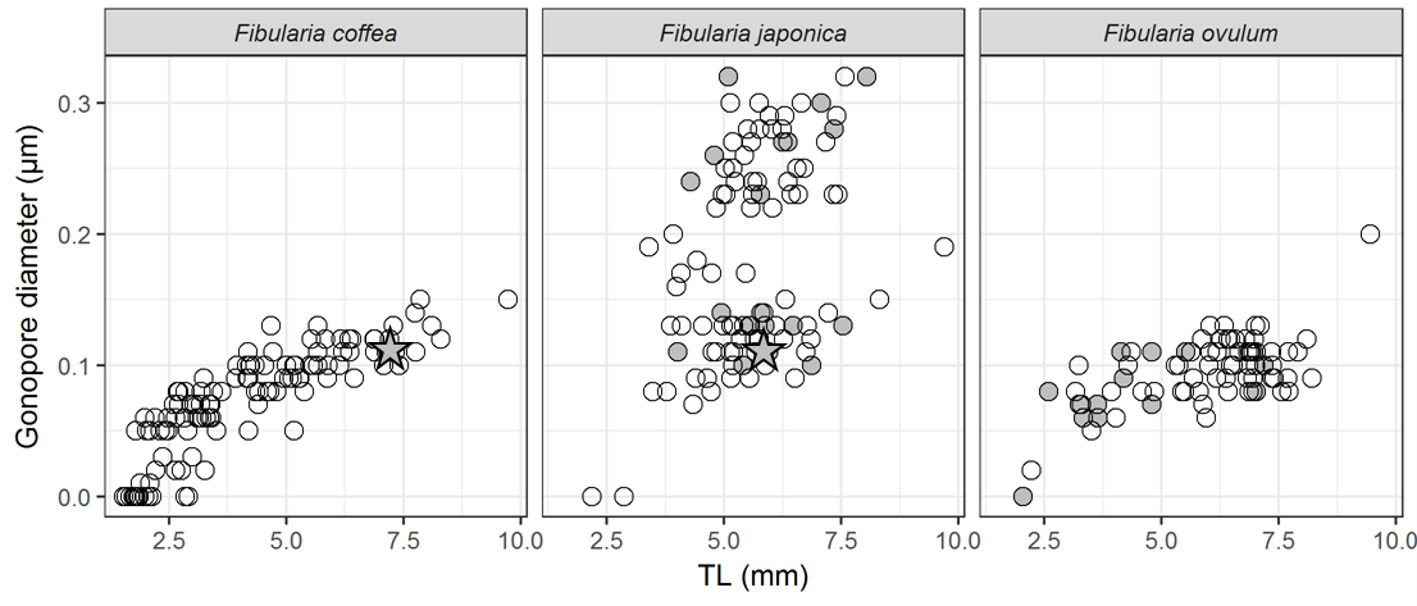
The apical system is situated slightly anterior on the aboral surface ( Figs. 3A, D View FIGURE 3 ). It consists of four genital pores, five ocular pores in small ocular plates, and two hydropores in a deep, irregularly-shaped groove ( Fig. 5A View FIGURE 5 ). The diameter of the genital pores (<150 µm) is equal to or smaller than that of the largest petaloid pore (<ca. 150 µm). There is no obvious dimorphism in gonopore size ( Fig. 6 View FIGURE 6 ). The gonopores open in specimens as small as TL = 1.79 mm. The diameter of the ocular pores (ca. 40 µm) is much larger than that of the accessory pores (ca. 25 µm). Accessory pores are situated in oblique patches in the centers of ambulacral plates ( Fig. 5B View FIGURE 5 ).
The primary tubercles are hemispherical, crenulate, and perforate ( Figs. 5C, D View FIGURE 5 ). The diameters of oral and aboral primary tubercles are almost equal, ca. 150–200 µm. Their mamelons are constricted at the base ( Fig. 5D View FIGURE 5 ). The miliary tubercles are hemispherical, poorly to non-crenulate, and indistinctly to non-perforate ( Figs. 5C, D View FIGURE 5 ). They scattered around the primary tubercles. The diameters of oral and aboral miliary tubercles are almost equal, ca. 50–60 µm. The glassy tubercles occur between primary and miliary tubercles ( Fig. 5C, D View FIGURE 5 ).
The primary spines are ca. 350–450 µm in length ( Fig. 7 View FIGURE 7 Ai). The number of wedges in a primary spine is 8–10, and each wedge has many small granules and a series of distinct denticles ( Figs. 7 View FIGURE 7 Aii, Aiii). The tip of each primary spine is somewhat truncated ( Fig. 7 View FIGURE 7 Aii). The distal end of primary spines around the peristome is slightly curved towards the peristome, and their shafts are somewhat broadened and flattened ( Fig. 7B View FIGURE 7 ). The miliary spines are ca. 250–350 µm in length ( Fig. 7 View FIGURE 7 Ci). Each miliary spine bears a distal crown ( Fig. 7 View FIGURE 7 Cii), and the number of wedges in a miliary spine is six for all examined spines. Each wedge has many small granules along its outer surface ( Fig. 7 View FIGURE 7 Ciii).
Two types of pedicellariae, ophicephalous and tridentate, are present ( Figs. 7D, E View FIGURE 7 ). These two types pedicellariae occur on small tubercles similar to those of miliary spines. The ophicephalous pedicellariae ( Fig. 7D View FIGURE 7 ) are numerous and occur over the entire test surface. The head is ca. 80–100 µm in length ( Fig. 7 View FIGURE 7 Dii), and consists of three valves that differ from each other in size and shape. The largest valve has a large, bilaterally symmetric handle. The medium-sized valve has a left-right asymmetric handle. The smallest valve has a small, bilaterally symmetric handle. The left and right ends of each valve have a finger-like structure near the hinge ( Fig. 7 View FIGURE 7 Dvi), making an “intertwined loop” ( Mooi 1990). The valves are connected to each other by these intertwined loops ( Figs. 7 View FIGURE 7 Dii, Dv–Dvii). Each valve has 20–25 teeth, and each tooth has 1–2 denticles ( Fig. 7 View FIGURE 7 Div). The proximal end of the handle on the largest valve is inserted into a depression at the distal end of the pedicellarial stalk ( Figs. 7 View FIGURE 7 Di– Diii).
The tridentate pedicellariae ( Fig. 7E View FIGURE 7 ) occur only around the peristome and periproct. These pedicellariae consist of a head with three slender valves ( Fig. 7 View FIGURE 7 Ei), short neck, and stem. The valves possess ca. 11–15 teeth on the edge and without denticles ( Figs. 7E View FIGURE 7 ). Some valves have ca. 1–4 teeth in the inner area ( Figs. 7 View FIGURE 7 Ei, Eii, Eiv).
The accessory tube feet lack a calcareous disk or spicules.
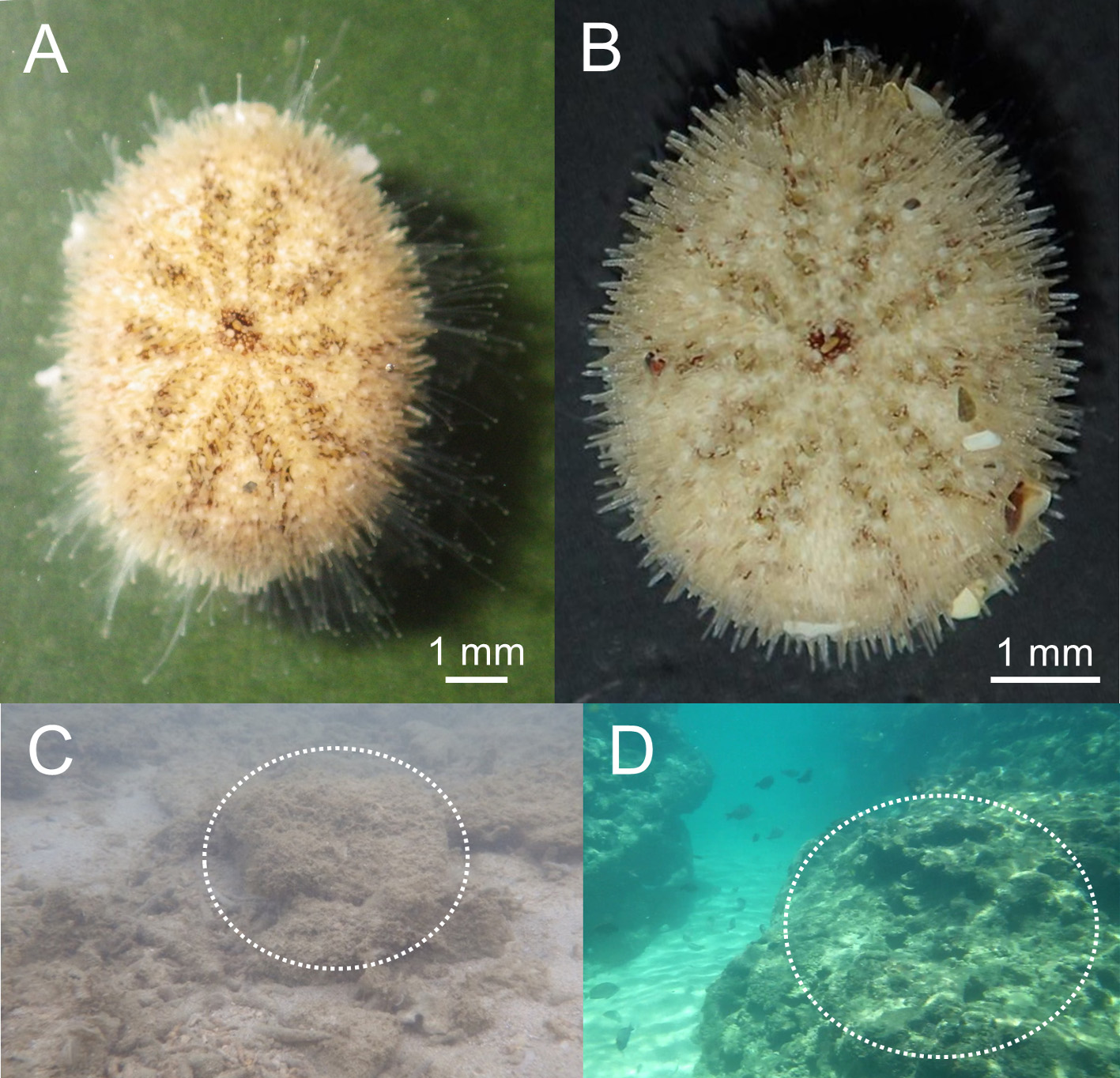
Color. The color is yellow to brown in life ( Figs. 8A, B View FIGURE 8 ) but changes to green when preserved in ethanol. Black pigments are apparent in each ambulacrum along the pore pair columns, forming a pentaradially symmetric pattern on the aboral surface ( Figs. 8A, B View FIGURE 8 ). The black pigments remain even after preservation in ethanol. The denuded test is whitish.
Distribution. This species is recorded in Japanese waters from Sagami Bay to the Amami-Oshima Islands; 1– 12 m in depth (present study). Schultz (2005) recorded F. plateia from Queensland, Australia but the figured specimen seems to be F. coffea from the flattened test and the large number of pores of petals (the number of pores of petal III, IV and V are 32, 22 and 32, respectively). In addition, Gomez & Mooi (2015) recorded an unknown species Fibularia n. sp. “bean” from the Philippines and Micronesia that seems to be the same as F. coffea from the flattened test and the large number of pores of petals (the number of pores of petal III, IV and V are 34, 26 and 33, respectively; counted from the sketch image). Therefore, this species is considered to be widely distributed across the Indo-Pacific.
Habitat. The micro-habitat of this new species is presumed to thin sand deposits directly on a hard substrate like rock-reefs. No living individual of this species was found in the sandy or muddy substrate around rock-reef although such substrates are inhabited by many species of clypeasteroids ( Mooi 1990). At Amami-Oshima Island, six live sea urchins (NSMT E-10371, E-10372, E-10373, and E-10387) were collected from the thinly deposited sand of 1–2 cm thick on a rock-reef ( Figs. 8C, D View FIGURE 8 ). In addition, one live specimen (NSMT E-10388) was found in the thin accumulation of sand of ca. 1 cm in thickness trapped by branched calcareous algae on a vertical surface of a rock-reef. In Tateyama, one live F. coffea (NSMT E-10376) was also collected from a 1–2 cm thin sand layer on a rock-reef. Two sea urchins (NSMT E-10374 and NSMT E-10375) were collected by dredging from sediments containing rubble and stones where rock-reef and sand bottom are interfingering.
Etymology. The species name is derived from the Latin “ coffea ”, meaning “coffee”, because the elliptical outline of test and the brownish color of living specimens resemble coffee beans.
Remarks. F. coffea can be easily distinguished from all other extant species of Fibularia except F. ovulum by the mode of increase of the number of pores in the petal. The number of pores in the petal III of F. coffea is greater than in F. japonica , F. plateia , F. cribellum , and F. nutriens , reaching 20 in specimens of TL> 5.0 mm and 30 in specimens of TL> 7.5 mm ( Fig. 4 View FIGURE 4 ). On the other hand, the number of pores of petal III is up to 14 for F. japonica even in the specimens of TL> 7.5 mm ( Fig. 4 View FIGURE 4 ), 7 for the holotype of F. plateia (TL = 6.25 mm) ( H.L. Clark 1928), 8 or less for F. cribellum (TL = 6.0– 6.1 mm) (de Meijere 1903; Schultz 2009). Moreover, the number of pores of petal III of F. coffea continues to increase even after TL = 5.0 mm ( Fig. 4 View FIGURE 4 ). In contrast, in F. japonica , F. plateia , F. cribellum and F. nutriens , increase in pore pair number significantly slows down at TL = ca. 3.0 mm and almost stops after TL = ca. 4.0 mm ( Fig. 4 View FIGURE 4 ) ( Gomez & Mooi 2015).
F. ovulum is the most similar species to F. coffea . The number of pores of petal III of F. ovulum increases like in F. coffea below TL = 5.0 mm. However, in F. ovulum , that increase slows down above 5.0 mm TL, and only a maximum of 30 pores is reached in the largest specimens studied (9.45 mm TL). In F. coffea , that increase continues, up to 40 ( Fig. 4 View FIGURE 4 ). F. coffea can be more clearly distinguished from F. ovulum by its lower (TH/TL = 0.38–0.55 in F. coffea vs. 0.59–0.84 in F. ovulum ) and less wide test (TW/TL = 0.67–0.86 in F. coffea vs. 0.77–0.92 in F. ovulum ). The difference in these proportions of the test is consistent throughout their growth (slope value is 1.0 and 1.0 between TW and TL, and 1.1 and 1.1 between TH and TL in the allometry regression for F. coffea and F. ovulum ), so it is more useful for identification between two species. Moreover, F. coffea can be distinguished by the larger peristome (SL/TL = 0.17–0.34 in F. coffea vs. 0.12–0.24 in F. ovulum ; SW/TL = 0.14–0.30 in F. coffea vs. 0.11–0.23 in F. ovulum ). In addition, the area around the peristome is depressed in F. coffea but inflated in F. ovulum . In life, F. coffea can be distinguished from F. ovulum by its coloration: the former has black pigment on the aboral surface ( Fig. 7A, B View FIGURE 7 ), and the latter lacks this pigment and usually has purplish accessory tube feet ( Mortensen 1948).
| NSMT |
National Science Museum (Natural History) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Fibularia coffea
| Tanaka, Hayate, Wakabayashi, Kaori & Fujita, Toshihiko 2019 |
Fibularia plateia
| Schultz, H. 2005: 321 |
Fibularia japonica Shigei 1982 : 11
| Shigei, M. 1982: 11 |