Chalceus, CUVIER, 1817
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2004.00090.x |
|
publication LSID |
lsid:zoobank.org:pub:F3937F3D-4F72-4D5A-A8A6-52592942C933 |
|
persistent identifier |
https://treatment.plazi.org/id/623087C7-FF9F-FFD8-FC48-7AF1FB48FD87 |
|
treatment provided by |
Carolina |
|
scientific name |
Chalceus |
| status |
|
CHALCEUS CUVIER, 1817 View in CoL View at ENA
Chalceus Cuvier, 1817: 454 View in CoL (type species Chalceus macrolepidotus Cuvier, 1817: 454 View in CoL , by monotypy).
Plethodectes Cope, 1870: 563 (type species Plethodectes erythrurus Cope, 1870: 563 View in CoL , by monotypy).
Pellegrinina Fowler, 1906: 442 (type species Pellegrinina heterolepis Fowler, 1906: 442 View in CoL , by original designation and monotypy).
Diagnosis: Chalceus is phylogenetically diagnosed on the basis of the following synapomorphies (but see comments in previous section):
(1) Presence of supramaxilla;
(2) Three series of teeth on premaxilla;
(3) Internal series of dentary teeth formed by a large symphyseal conical tooth followed by a gap and a series of smaller conical teeth;
(4) Reduced anterolateral process of mesethmoid;
(5) Scales situated dorsal to lateral line much larger than those ventral to it;
(6) Relative size of scales along lateral line other than on caudal peduncle alternatively large and small.
In addition, the combination of the following characters is useful to distinguish the species of Chalceus among characiforms: bright silvery body, red fins and short anal fin.
KEY TO THE SPECIES OF CHALCEUS View in CoL
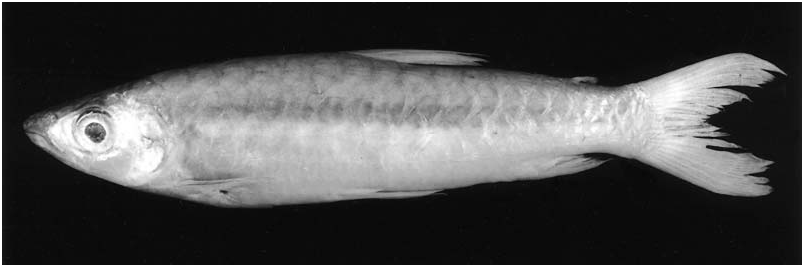
(1) Absence of distinct spots or conspicuous stripes of dark pigmentation on body (except for wide and inconspicuous longitudinal band formed by chromatophores located superficially in the skin in some specimens during reproductive period) (See ‘Comments on the colour pattern of Chalceus View in CoL species’) ( Figs 4 View Figure 4 , 5 View Figure 5 ) ........................................... C. macrolepidotus View in CoL
(1¢) Presence of dark pigmentation forming humeral spots or stripes................................................................................2
(2) Humeral spot absent or poorly defined, round to vertically elongate and located deep under scales; presence of narrow longitudinal dark stripe from posterodorsal margin of opercle to caudal peduncle; snout relatively acute; median fontanel between frontals and parietals absent................................................................................................4
(2¢) Presence of conspicuous humeral spot, round in shape and located superficially beneath scales; lateral surface of body usually with reticulate pattern; snout rounded; median fontanel present between frontals and parietals....................................................................3
(3) Humeral spot with notch on its posterodorsal margin; reticulate pattern of body coloration more evident along series of scales posterior to humeral blotch; pelvic and anal fins dark; caudal-fin lobes robust and rounded ( Fig. 10 View Figure 10 ) ................................................. C. erythrurus
(3¢) Humeral spot without notch on its posterodorsal margin; reticulate pattern of body coloration uniformly distributed over lateral and dorsal portions of body; pelvic and anal fins hyaline; caudal-fin lobes elongate and slender ( Figs 15 View Figure 15 , 16 View Figure 16 ) ................................. C. spilogyros
(4) Number of branched pelvic-fin rays 7 ( Figs 22 View Figure 22 , 23 View Figure 23 ).............................................................................. C. guaporensis
(4¢) Number of branched pelvic-fin rays 8 ( Figs 18 View Figure 18 , 19 View Figure 19 )...................................................................................... C. epakros
CHALCEUS MACROLEPIDOTUS CUVIER, 1817 View in CoL
( FIGS 4–9 View Figure 4 View Figure 5 View Figure 6 View Figure 7 View Figure 8 View Figure 9 ; TABLE 1)
Chalceus macrolepidotus Cuvier, 1817: 454 View in CoL , pl. I, fig. 1 [original description, type locality: Brazil]. Schomburgk, 1841: 216, pl XIV [description, Guyana: Essequibo River, Camuti mountain]. Cuvier & Valenciennes, 1850: 240 [description based on same specimen of Cuvier, 1817]. Günther, 1864: [in part: British Guiana (= Guyana), Essequibo River; not the specimen cited for Brazil: Rio Cupai (= Cupari); Chalceus ararapeera Cuvier & Valenciennes View in CoL placed as synonym]. Cope, 1872: 262 [species listed, Brazil: Rio Solimões]. Eigenmann & Eigenmann, 1891: 55 [literature compilation, in part: British Guiana (= Guyana), not including the citation of species for the Rio Cupai (= Cupari) ( Brazil) and (Río) Ambyiacu (= Ampiyacu) ( Peru)]. Regan, 1905: 190 [Rio Negro, based on drawings made by A. R. Wallace]. Eigenmann, 1910: 439 [in part: not including the synonymy of Pellegrinina heterolepis View in CoL ]. Eigenmann: 1912: 372 [ British Guiana (= Guyana)]. Regan, 1912: 388 [in part: British Guiana (= Guyana: Essequibo River; Surinam; not including the synonymy of Pellegrinina heterolepis Fowler View in CoL ]. Cockerel, 1914: 107, pl. 27, fig. 5 [scale morphology]. Eigenmann & Allen, 1942: 277 [literature compilation in part, not including the citation of species for The Río Ambyiacu (= Ampiyacu) ( Peru)]; not including the listed specimens; not including the synonymy of Chalceus macrolepidotus iquitensis View in CoL and Pellegrinina heterolepis View in CoL ]. Bertin, 1948: 9 [type specimen at MNHN, Paris]. Puyo, 1949: 129 [French Guiana]. Fowler, 1950: 364 [literature compilation, in part, not including the synonymy of Pellegrinina heterolepis View in CoL and Chalceus macrolepidotus iquitensis Nakashima View in CoL ; common name: Saragui, São Pedro]. Boeseman, 1952: 189 [ Surinam (specimen not examined, identification inferred by geographical distribution), local name: morokò]. Mago Leccia, 1971: 10 [Rio Casiquiare (specimen not examined, identification on geographical distribution)]. Heyer, 1975: 343 [aquarium; photograph of live specimens]. Cala, 1977: 7 [ Colombia, Rio Orinoco basin, Río Inirida and Río Vichada; (specimens not examined); common name Arari, Rabirrojo]. Géry, 1977: 342 [diagnosis]. Azuma, 1979: 58–62 [aquarium, spawning and photographs of live specimen and fry]. Lauder, 1981: 162 [functional morphology: feeding mechanism]. Géry & Planquette, 1982: 73 [French Guiana (specimen not examined, identification inferred by geographical distribution)]. Géry, Planquette & Le Bail, 1991: 43 [French Guiana: Fleuve Oyapock and Fleuve Approuague (specimen not examined, identification inferred by geographical distribution)]. Planquette, Keith & Le Bail, 1996: 230 [French Guiana: Fleuve Maroni, local common names, photograph of live specimen]. Taphorn et al., 1997: 70 [list of species of Venezuela].
Brycon macrolepidotus Müller & Troschel, 1845 [diagnosis; incorrect placement in the genus Brycon View in CoL ].
Chalceus ararapeera Cuvier & Valenciennes, 1850: 244 View in CoL [original description, type locality Essequibo River, Guyana; common name: Arara-pira, Parshama (= poisson perroquet)]. Bertin, 1948: 9 [syntypes at MNHN, Paris].
Creagrutus pellegrini Puyo, 1943: 143 View in CoL , fig. 2 [original description, type locality French Guiana: upper Fleuve Itany, upper Fleuve Maroni system]. Puyo, 1949: 128, fig. 66 [description; based on Puyo, 1943]. Myers, 1960: 211 [placement of species in genus Chalceus View in CoL ]. Géry, 1977: 654 [referred species to Chalceus macrolepidotus View in CoL ]. Vari & Harold, 2001: 2, 43 [discussion of taxonomic status, placement of species in genus Chalceus View in CoL ].
Diagnosis
Chalceus macrolepidotus can be readily distinguished from all other Chalceus species by the absence of any distinct spots of pigmentation or stripes ( Figs 4–6 View Figure 4 View Figure 5 View Figure 6 ) (except for some sexually mature specimens, see next section: ‘Comments on the colour pattern of Chalceus species’, henceforth abbreviated to ‘Comments...’). It differs from C. erythrurus and C. spilogyros by the lack of a humeral spot and from C. epakros and C. guaporensis by the lack of a longitudinal band along the body sides located deep under the skin. In life C. macrolepidotus can be distinguished from C. epakros by the lack of red pigmentation on the central portions of scales on the longitudinal series above the lateral line and from C. erythrurus by having the pelvic and anal fins tinged with red or hyaline (vs. yellow; compare photographs in Géry, 1977: 329 and 332). Chalceus macrolepidotus may be further distinguished from C. erythrurus , C. spilogyros and C. guaporensis in having the first small inner dentary row tooth originating very close to the symphyseal tooth and forming a nearly continuous series (vs. having first small tooth of inner dentary row located well behind the fourth or fifth tooth of the outer row with a distinct gap between the symphyseal tooth and first small conical tooth). Chalceus macrolepidotus can be further distinguished from C. epakros and C. guaporensis by the presence of a fontanel between the contralateral frontal and parietal bones in all but the larger specimens (vs. fontanel absent in all specimens of C. epakros and C. guaporensis ).
Description
Morphometric data presented in Table 1. Maximum size 228.0 mm SL. Body robust, relatively elongate, greatest body depth slightly anterior to dorsal-fin origin. Dorsal profile of head distinctly convex anteriorly along snout region, nearly straight to posterodorsally inclined from anterior end of snout to tip of supraoccipital spine. Dorsal profile of head in large specimens (over 200 mm SL) slightly convex and continuous with dorsal body profile. Anterior margin of snout somewhat acute in dorsal view. Interorbital distance wide, proportionally wider relative to SL in large specimens. Dorsal body profile convex from tip of supraoccipital spine to dorsal-fin origin, posteroventrally inclined along dorsal-fin base, straight to relatively convex to adipose fin and concave along dorsal profile of caudal peduncle to origin of procurrent caudal-fin rays. Overall dorsal profile of head and body of juveniles up to 50 mm SL nearly straight to slightly convex. Ventral profile of head distinctly convex in region of lower jaw, resembling a chin. Ventral body profile gently convex from the posterior limit of isthmus to anal-fin origin. Body profile along anal-fin base posterodorsally inclined, slightly concave along ventral margin of caudal peduncle. Head robust in large specimens (over 180 mm SL). Smaller specimens with relatively longer heads and more acute snout. Dorsal surface of head with distinct medial fontanel restricted to small region anterior to epiphyseal bar between contralateral frontals and completely separating parietals. Fontanel wide in small specimens and progressively narrower in larger individuals. Fontanel completely closed in 200 mm SL specimen. Mouth terminal, large, upper jaw slightly longer than lower jaw, tip of premaxillary teeth extending below margin of upper lip giving saw-like appearance to margin of premaxilla even in closed mouth. Maxilla extending approximately to vertical through anterior margin of orbit. Supramaxilla present.
Dorsal-fin rays ii,10 (ii,10, n = 92). Dorsal-fin origin posterior to vertical through insertion of innermost pelvic-fin rays. First basal dorsal-fin pterygiophore inserting behind neural spine of 14th vertebra (n = 1). Distal margin of dorsal fin nearly straight to convex. Adipose fin present. Anal-fin rays iii,9 (iii,9; iii, 8 in one specimen, n = 87). First basal anal-fin pterygiophore inserting behind haemal spine of 26th vertebra (n = 1). Distal margin of anal fin straight to emarginate with anterior branched rays approximately 3 times length of ultimate ray. Pectoral-fin rays i,15 (range 14–18, mean 16, n = 88), pointed distally, with unbranched- and first branched rays longest. Tip of pectoral fin not reaching pelvic-fin insertion. Pelvic-fin rays i,8 (i,8; i, 7 in 3 specimens, n = 92); fin pointed distally. Caudal fin forked, with lobes slender, especially in specimens up to 120 mm SL, lower fin lobe slightly more developed than upper lobe.
Premaxillary teeth in three rows. Outer row 9 (range 7–10; 11 in one specimen, mean 8.5, n = 92) tricuspid or pentacuspid in large specimens; smaller specimens with tricuspid or conical teeth, with medial cusp larger. Tooth close to premaxillary symphysis slightly larger than others in series. Remaining teeth of similar size except for slightly smaller one or two lateralmost teeth. Cusps slightly curved with concave portion facing mouth cavity. Inner row 6 (range 6–8; 5 in one specimen, 9 in two, mean 6.4, n = 92) with largest, symphyseal tooth usually asymmetric with one cusp on the medial side and two on the lateral side of tooth. Remaining teeth penta- or heptacuspid in large specimens, all teeth tricuspid in small specimens, with second tooth from symphysis larger and teeth gradually diminishing in size laterally. Cusps slightly curved, with concave portion opposite of mouth cavity. Intermediate row 2 (2; 1 in three specimens, n = 91) pentacuspid (rarely tricuspid) teeth more spaced than teeth of other rows and of intermediate size. Cusps straight.
Maxillary teeth 12 (range 8–13, with single specimens each having 7, 14, 16 and 17 teeth, mean 10.9, n = 90); smaller specimens usually with higher tooth counts. First teeth pentacuspid followed by tricuspid and conical teeth distally. Maxillary dentition not extending along entire margin of ossification in large specimens. Small specimens with maxillary teeth conical and extending along entire margin of ossification. Dentary teeth in two rows. Outer row 8 (range 8–13, 14 in one specimen, 16 in 2, mean 10.9, n = 90). Teeth large and pentacuspid anteriorly, sometimes heptacuspid in large specimens or tricuspid in small specimens, gradually diminishing in size and number of cusps posteriorly. Posteriormost teeth conical. Cusps slightly curved with concave portion facing mouth cavity. Inner row consists of large conical symphyseal tooth (tricuspid in few larger specimens) followed by series of approximately 30 minute conical teeth. First tooth origi- nates very close to symphyseal tooth, forming an almost continuous series.
Scales cycloid, large overall, approximately twice as large above lateral line as below it. Circuli on exposed portion of scales not concentric with those of anterior portion; circuli on exposed portion of scales straight and extending to posterior margin of scale in small specimens; restricted to upper and lower portion of scale in specimens around 140 mm SL; disorganized and with labyrinthic pattern in specimens around 170 mm SL. Radii originating on centre of scale and radiating anteriorly and posteriorly on scale surface.
Lateral line low on body sides, complete, with alternating large and small perforated scales from posterior margin of opercle to vertical through base of last anal-fin ray; scales smaller and of similar size from that point to end of caudal peduncle. Canals in large specimens with 3–9 elevated branches, forming ridges on scale surface; ridges more evident on region of caudal peduncle. Number of branches decreases toward caudal peduncle with posterior scales unbranched. Small specimens (less than 140 mm) with branching pattern less developed. Lateral-line scales 38 (range 36–40, mean 38, n = 90). Scale rows between dorsalfin origin and lateral line 3; between lateral line and pelvic-fin insertion 2. Scales around caudal peduncle 12. Vertebrae 39 (n = 1).
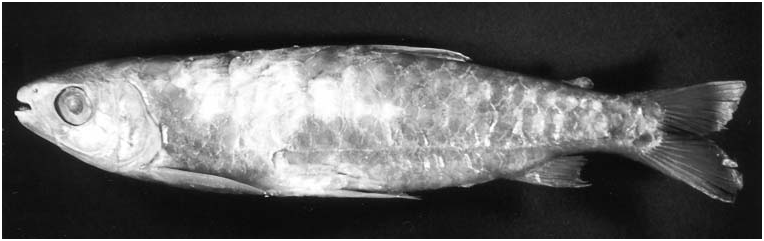
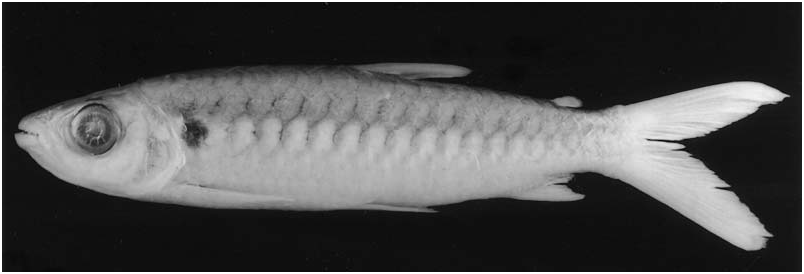
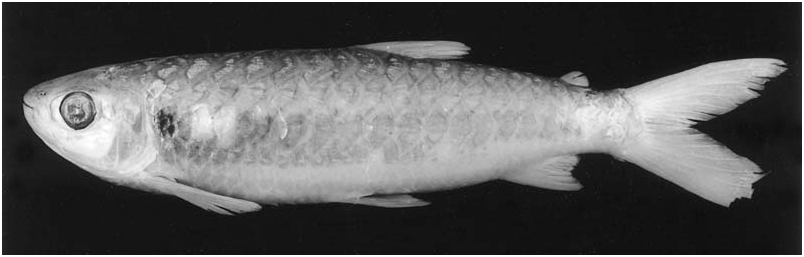
Colour in life. (Description based on photographs in Géry, 1977: 329 and Planquette et al., 1996: 231). Overall coloration of head and body bright silver. No conspicuous humeral spot. Dorsal portion of eye yellow. One specimen ( Géry, 1977: 329) with dorsal profiles of head and body darker. Somewhat indistinct longitudinal stripe extending from rear of orbit through opercle to vertical through adipose fin and patch of dark pigmentation present on middle portion of opercle. Caudal fin bright red, adipose yellowish, all other fins hyaline. Other specimen ( Planquette et al., 1996: 231) with dorsal portions of head and body darker. Margins of scales on dorsal portions of body with light concentration of chromatophores, forming fine reticulate pattern. All fins (except pectoral) bright red, more so on their proximal portions.
Colour in alcohol. All available specimens lacking guanine on body except for few that retain silvery pigmentation on infraorbital and opercular regions. Ground coloration of head and body in specimens ≥ 60 mm SL yellowish to tan ( Figs 4 View Figure 4 , 5 View Figure 5 ), becoming darker dorsal of horizontal line through dorsal margin of orbit. No longitudinal body stripe or humeral blotch. Dark chromatophores scattered over infraorbitals and opercular region. Scales on body with chromatophores slightly more concentrated along posterior margin, forming fine reticulate pattern more evident in recently collected specimens (although less evident in large specimens). All fins hyaline. Some specimens with dark tips on dorsal- and caudal-fin rays.
Ground coloration of specimens £ 60 mm SL ( Fig. 6 View Figure 6 ) pale yellowish, somewhat darker dorsally, but difference between dorsal and ventral regions of body less pronounced than in larger specimens. Reticulate pattern of scales more conspicuous than in larger specimens. Pectoral and pelvic fins hyaline. Dorsal and adipose fins dusky. Lower and ventralmost rays of the upper caudal–fin lobes and anal fin dark.
Some specimens (MZUSP 58962 (1 of 2); MZUSP 43291; and ANSP 161220) with a dark and wide longitudinal band along the body sides (see ‘Comments...’).
Distribution
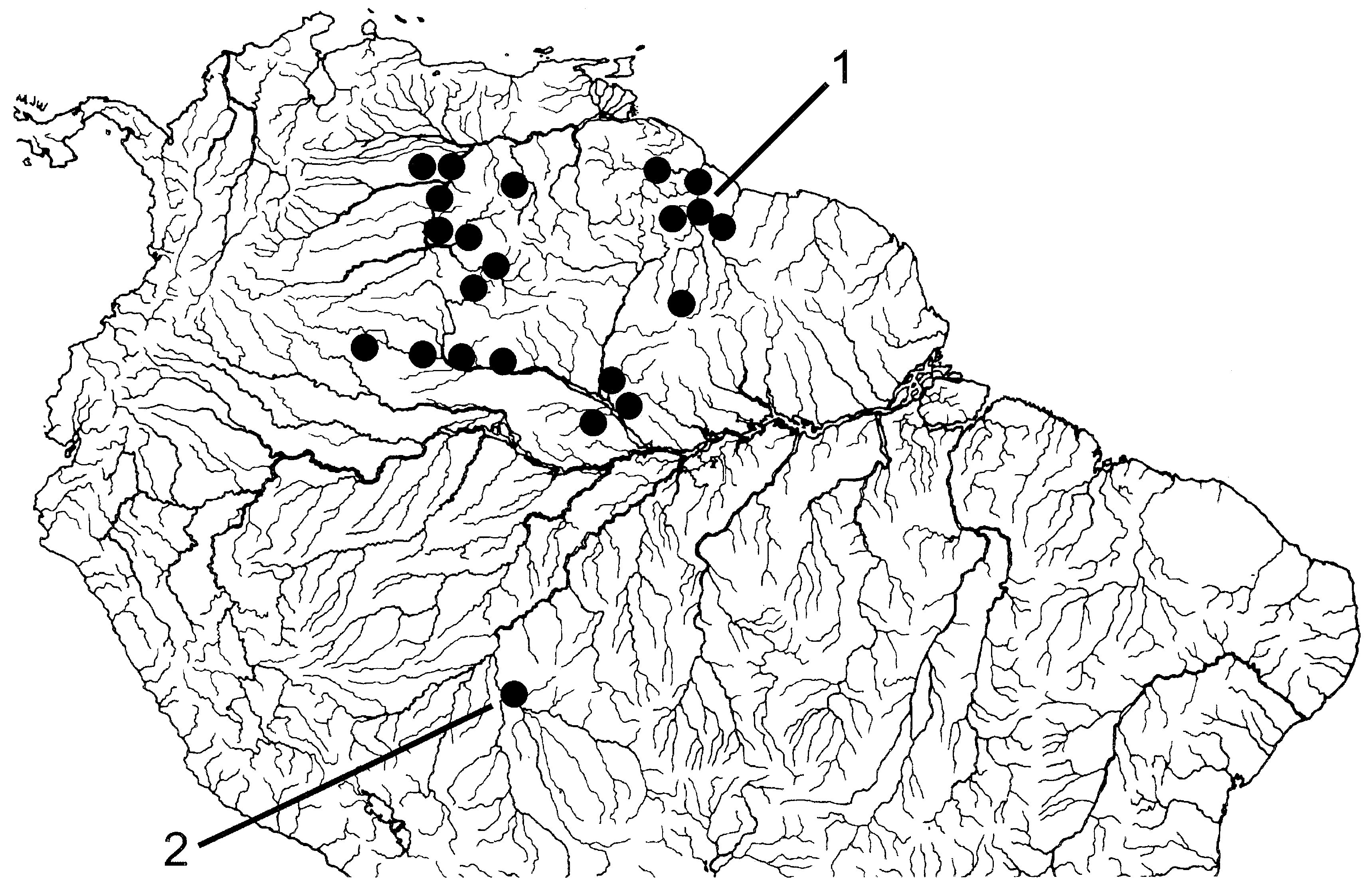
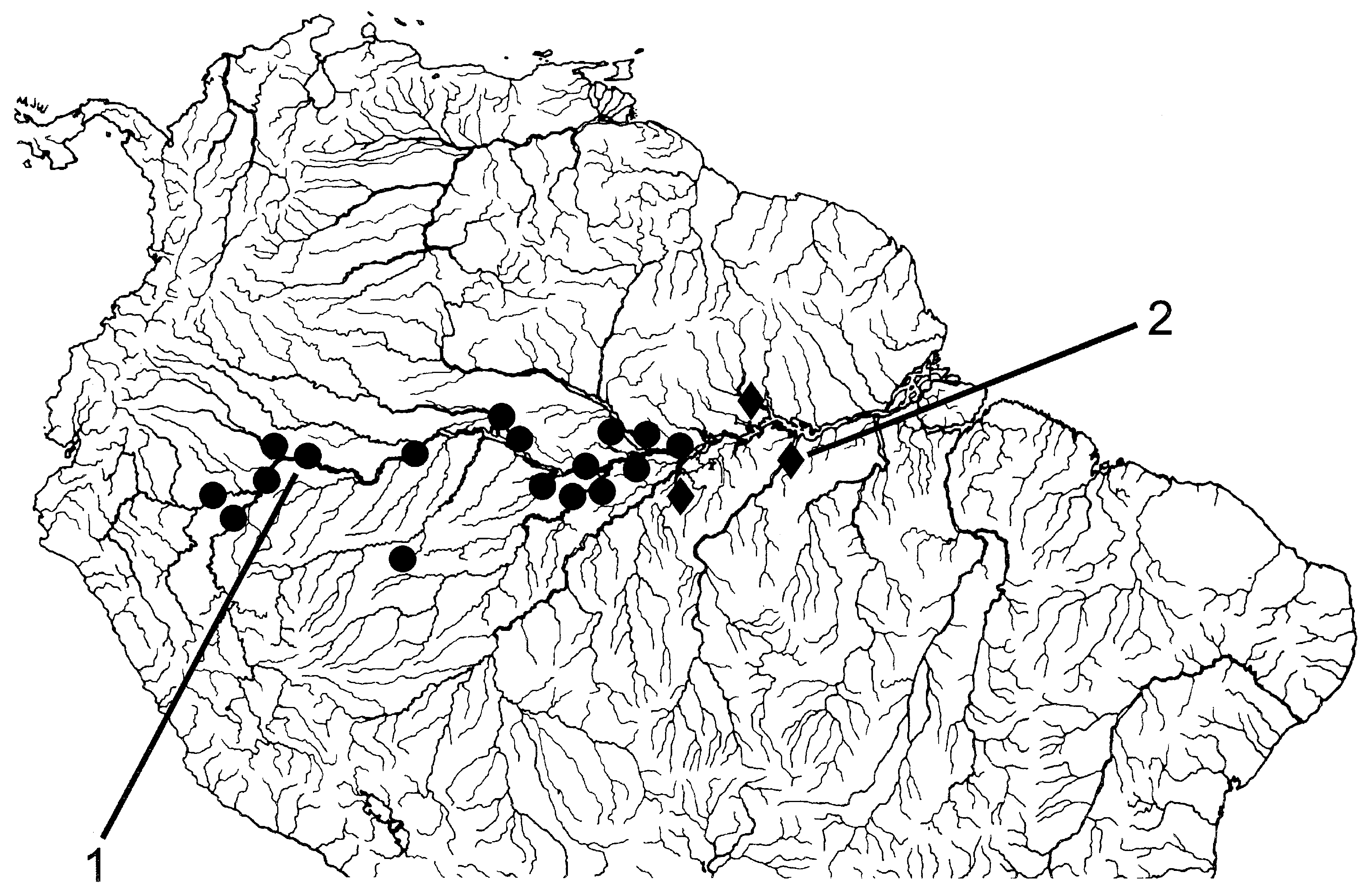
Rio Negro and its tributaries in Amazon basin, middle and upper Río Orinoco basin, Essequibo River drainage in Guyana, Corantijn River drainage in Suriname and perhaps Río Baures, a tributary of Rio Guaporé, along Brazilian-Bolivian border (12∞32¢S; 64∞19¢W) (for latter locality see ‘Remarks’) ( Fig. 9 View Figure 9 ).
Ecology
Puyo (1943: 130) and Planquette et al. (1996: 230) report Chalceus macrolepidotus from well oxygenated waters in regions of rapids; the latter authors mention that the species is uncommon in the lower portions of river drainages.
Remarks
All samples of C. macrolepidotus examined in the present study, with one exception, originated in the Rio Negro, Río Orinoco and in the Atlantic drainages of Guyana and Suriname. A single sample (UMMZ 204688) is from a southern locality, in the Río Baures, a tributary of the Rio Guaporé, along the Bolivian- Brazilian border (indicated by 2 in Fig. 9 View Figure 9 ). The series of 15 specimens from this locality have a cranial fontanel, lack any conspicuous body pigmentation such as a longitudinal stripe and/or a humeral blotch and cannot be distinguished from the population samples of C. macrolepidotus from the Rio Negro, Río Orinoco and the Guianas. Therefore, they were tentatively identified as C. macrolepidotus ; this population sample occurs sympatrically with C. guaporensis , the only other Chalceus species that occurs in the Rio Guaporé drainage. This record represents a major range extension to the south for that species. A similar disjunct distribution pattern was reported by Vari & Harold (2001) for Creagrutus maxillaris (Myers) .
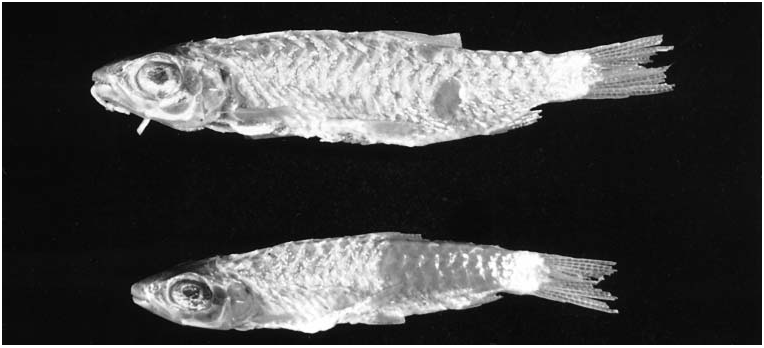
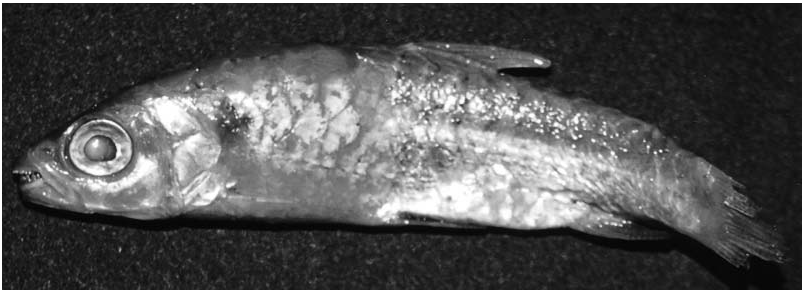
The description of Chalceus ararapeera Cuvier & Valenciennes, 1850 was based on two specimens collected by Robert Schomburgk in the Essequibo River in Guyana. These two syntypes (MNHN A9830) ( Fig. 8 View Figure 8 ) were examined in the present study and although in poor condition have a cranial fontanel and can be identified as C. macrolepidotus ( Fig. 7 View Figure 7 ). The only other species that occurs in Guyana is C. epakros , which lacks a cranial fontanel. Chalceus ararapeera is herein considered a junior synonym of C. macrolepidotus as first proposed by Günther (1864: 333). The type locality of C. macrolepidotus is indicated as originating in Brazil. The specimen on which Cuvier (1817) based the original description of the species was originally deposited in the ‘Cabinet de Lisbonne’ (Lisbon, Portugal). At the beginning of the 19th century, this and additional specimens from that collection were transferred to MNHN, Paris. They were originally collected by the Brazilian naturalist Alexandre Rodrigues Ferreira in the Brazilian Amazon ( Vanzolini, 1996: 196–197).
The generic placement of Creagrutus pellegrini Puyo (1943: 143) was discussed by Vari & Harold (2001: 43) who suggested that it should be assigned to Chalceus and considered it to be a doubtful species, C. pellegrini , following Myers (1960: 211). We follow these authors in assigning the species to Chalceus . The question of the taxonomic status of Chalceus pellegrini (Puyo) is somewhat difficult to address in view of the apparent loss of type material of various species described by Puyo ( Géry, 1959: 345) and the lack of information in Puyo’s (1943: 143) description which would permit an unambiguous identification of the species. In addition, and as was also pointed out by Myers (1960: 211), Puyo (1949: 128–130) provided a description for C. macrolepidotus Cuvier following that of Creagrutus pellegrini , but surprisingly failed to recognize any relationship between the two nominal forms.
Puyo (1943: 143) described C. pellegrini on the basis of two specimens, one from the Fleuve Maroni and one from Fleuve Itany in French Guiana. Based solely on Puyo’s description it is impossible to readily determine whether the specimens he examined represent C. macrolepidotus or C. epakros , the two Chalceus species known to occur in the Guianas. The currently known distribution of C. macrolepidotus and C. epakros in the Guianas helps to resolve this problem. Of the two species, only C. macrolepidotus has been reported in the Fleuve Maroni system ( Planquette et al., 1996: 230). In addition, C. macrolepidotus also occurs in the Corantijn River in Suriname and in the Essequibo River in Guyana. Chalceus epakros has a more restricted distribution in the Guianas, being recorded only from the middle Essequibo River. Therefore, we consider Creagrutus pellegrini to be a junior synonym of Chalceus macrolepidotus (see Géry, 1977: 654).
Material examined
Type material. MNHN 2634 View Materials , 1, 244.6 mm SL, Brazil, holotype of Chalceus macrolepidotus Cuvier, 1817 ; MNHN A9830 View Materials , 2 View Materials , 66.9–80.2 mm SL; Guyana, Essequibo River , syntypes of Chalceus ararapeera Cuvier & Valenciennes, 1850 .
Non-type material. BRAZIL: AMAZONAS: MZUSP 44570, 2, 100.9–141.0 mm SL; Rio Negro, São João, near Tapurucuara. MZUSP 20201, 3, 104.0– 123.0 mm SL; Rio Negro, Rio Jauaperi, from mouth to 100 km upstream. MZUSP 43291(*), 7, 183.2– 227.8 mm SL; Rio Negro, Cantagalo. – MZUSP 59046, 1, 197.4 mm SL; Rio Negro, lake in São João, near Tapurucuara. – MZUSP 58962(*), 2, 206.9– 210.5 mm SL; Rio Negro, Cantagalo, floodplain lake. – MZUSP 62223, 2, 71.9–72.1 mm SL; lagoon in island of Rio Negro, Paricatuba. – MZUSP 63649, 3, 84.5–129.0 mm SL; Rio Uaupés. – MZUSP 66482, 2, 91.6–96.2 mm SL; Rio Tiquié, c. 1 h downriver from the Indian community of Cunuri, below Cachoeira do Tucano. – CAS 156171, 1, 49.9 mm SL; Rio Negro or Río Orinoco. – CAS 156172, 1, 86.1 mm SL; Rio Negro, Caranguejo, above São Gabriel. – CAS 156170, 1, 95.7 mm SL; CAS 156830, 3, 149.0– 168.0 mm SL; Rio Negro, Bucuri. – INPA 16950, 6, 175.0–221.0 mm SL; Rio Jaú, Lago Ibama.
GUYANA: ANSP 176678, 1, 200.3 mm SL; Siparuni River, Tumbledown Rapids. FMNH 59467, 2, 166.7– 198.0 mm SL; FMNH 7473, 1, 161.6 mm SL; AMNH 7082, 1, 178.0 mm SL; USNM 66156, 1, 161.0 mm SL; FMNH 53479, 5, 67.4–200.2 mm SL; FMNH 69807, 2, 75.3–188.3 mm SL; lower Potaro River, Tumatumari. – CAS 69079, 1, 65.1 mm SL; Essequibo River drainage, above the falls at Tumatumari Cataract. – CAS 121888, 1, 164.0 mm SL; Potaro River at Tumatumari Cataract. – AMNH 14331, 15, 82.4–105.4 mm SL; AMNH 214975, 6, 70.5–84.7 mm SL; AMNH 13399, 2, 35.5–57.5 mm SL; AMNH 14322, 1, 104.3 mm SL; Essequibo River, Rockstone. – AMNH 214938, 6, 35.8–43.4 mm SL; junction Mazaruni-Cuyuni and Essequibo Rivers, Bartica. – AMNH 43352, 9, 31.1– 60.7 mm SL; BMNH 1936.4.4.5–6, 2, 41.4–44.9 mm SL; Essequibo, Bartica. – CAS 69081, 3, 160.0– 190.0 mm SL; BMNH 64.1.21.46, 1, 134.2 mm SL; Essequibo River drainage. – CAS 69084, 2, 76.6– 90.1 mm SL; Essequibo River drainage, Konawaruk pool, near the mouth of the Konawaruk River. – FMNH 53477, 3, 67.6–104.5 mm SL; Konawaruk. – FMNH 53478, 3, 74.2–83.4 mm SL; Gluck Island. – CAS 69086, 2, 83.3–84.7 mm SL; Essequibo River channels around Gluck Island, near Rockstone. – AMNH 216222, 1, 171.0 mm SL; headwaters between Mandi and Kuyuwini rivers, Essequibo River drainage. – AMNH 73020, 1, 54.2 mm SL; sandbar on north bank Cuyuni River, just upstream of Caowry Creek, Essequibo. – AMNH 220375, 2, 56.9–76.5 mm SL; USNM 94124, 1, 135.2 mm SL; Essequibo River drainage, Kartabo. – AMNH 221068, 2, 22.0– 27.5 mm SL; Akima Island. – BMNH 1972–10.17: 1438, 1, 68.1 mm SL; Amatuk Creek, Potaro River. – BMNH 1972– 10.17: 1440, 2, 66.5–68.5 mm SL; Kanaima Creek, Potaro River. – AMNH 16792, 3, 77.6–88.1 mm SL; AMNH 14313, 4, 88.5–101.2 mm SL; unspecified localities in Guyana.
SURINAME: AMNH 16421, 1, 168.0 mm SL; BMNH 70.3.10.53, 1, 55.3 mm SL; unspecified locality in Suriname. – AMNH 54870, 5, 89.1–95.3 mm SL; Toeboeroe Creek. – AMNH 54810, 2, 67.2–90.5 mm SL; Dalbana Creek, 150 m upstream from junction with Kabelebo River. – USNM 226115, 46, 58.0– 127.9 mm SL; Corantijn River at km 180, side channel of main river along Surinamese shore. – USNM 226114, 1, 198.0 mm SL; Corantijn River, Matapi Creek. – USNM 225373, 1, 76.4 mm SL; Corantijn River, about 2 km N of Matapi.
VENEZUELA: AMAZONAS: ANSP 161220(*), 1, 164.4 mm SL; Río Iguapo c. 1 h. above its mouth, Río Orinoco drainage. – ANSP 161221, 2, 131.7–138.0 mm SL; Rio Ventuari c. 12 km from its confluence with Río Orinoco. – ANSP 161223, 2, 89.1–125.5 mm SL; Río Pamoni, lagoon c. 0.5 km from confluence of Río Casiquiare. FMNH 103882, 1, 84.8 mm SL; backwater and beach of Río Atabapo c. 40 ft above mouth. – FMNH 103883, 2, 79.1–98.9 mm SL; Río Atabapo (pools) c. 1.2 h. above San Fernando de Atabapo. – FMNH 103885, 1, 76.9 mm SL; Río Autana at Playa Cucurito. – FMNH 103884, 1, 164.9 mm SL; Caño Guasuripana at Guasuripana, tributary of Río Atabapo, c. 7 min from San Fernando de Atabapo. – FMNH 103886, 3, 77.3–91.8 mm SL; pool of Río Ventuari above mouth in Río Orinoco Laguna Pavon. – FMNH 103881, 2, 80.9–89.9 mm SL; Caño Tuparero c. 2.5 h above San Fernando de Atabapo in Río Orinoco. – MZUSP 62454, 1, 125.8 mm SL; lagoon on Río Pamoni, c. 0.5 km from mouth in Río Casiquiare. – CAS 154592, 1, 92.2 mm SL; Río Casiquiare. – MZUSP 62455, 2, 78.8–92.5 mm SL; Laguna Pavon in pond behind beach of Río Ventuari, c. 30 min. from mouth. APURE: ANSP 165684, 4, 74.2–170.4 mm SL; Caño Potrerito, 24 km S of Rio Cinaruco on San Fernando de Apure, Puerto Paez Highway. – FMNH 69909, 1, 104.1 mm SL; FMNH 69910, 13; Río Cinaruco. – USNM 270135, Balneario Pozo Azul, c. 1 km E of Puerto Ayacucho to Solano road. BOLIVAR: MZUSP 62456, 2, 83.6–87.5 mm SL; Departamento Cedeño, mouth of caño tributary to Río Nichare. – FMNH 85686, 6, 122.6– 125.8 mm SL (1 C & S); Río Orinoco drainage, 50 km toward Puerto Ayacucho from Puerto Nuevo.
The following sample is tentatively identified as Chalceus macrolepidotus (see ‘Remarks’, above):
BOLIVIA: UMMZ 204688 View Materials , 15 View Materials , 70.7 View Materials –91.0 mm SL; El Beni: Río Baures at mouth on right bank, 6 km SW of Costa Marques, Brazil.
CHALCEUS ERYTHRURUS ( COPE, 1870) View in CoL
( FIGS 10–14 View Figure 10 View Figure 11 View Figure 12 View Figure 13 View Figure 14 , TABLE 2)
Plethodectes erythrurus Cope, 1870: 563 View in CoL [original description, type locality Pebas, Peru]. Fowler, 1906: 441 [description, based on type specimen]. Eigenmann, 1910: 439 [ Peru]. Eigenmann & Allen, 1942: 278 [Pebas, Peruvian Amazon; not Rio Cupai (= Rio Cupari)]. Fowler, 1950: 365 [literature compilation].
Plethodectes erythrinus: Eigenmann et al., 1891: 51 [incorrect spelling of P. erythrurus Cope, 1870 View in CoL ].
Chalceus erythrurus Cope, 1872: 262 View in CoL [ Ecuador (now Peru) Río Ambyiacu (= Ampiyacu)]. Eigenmann & Eigenmann, 1891: 55 [literature compilation]. Regan, 1912: 388 [in part: Upper Amazon (not including the reported specimen from Rio Cupaí (= Cupari)]. Géry, 1977: 332 [photograph of live specimen]. Ortega & Vari, 1986: 7 [list of species: Peru].
Chalceus macrolepidotus View in CoL (not of Cuvier, 1817). Misidentification: Eigenmann & Allen, 1942) [listed specimens]. La Monte, 1935: 7 [Rio Juruá, Brazil].
Pellegrinina heterolepis Fowler, 1906: 442 View in CoL , fig. 39 [original description; type locality erroneously cited as from West Africa].
Chalceus macrolepidotus iquitensis Nakashima, 1941 View in CoL : fig. on p. 76 [original description, type locality, Peru, surroundings of Iquitos , common name, San Pedro].
Diagnosis
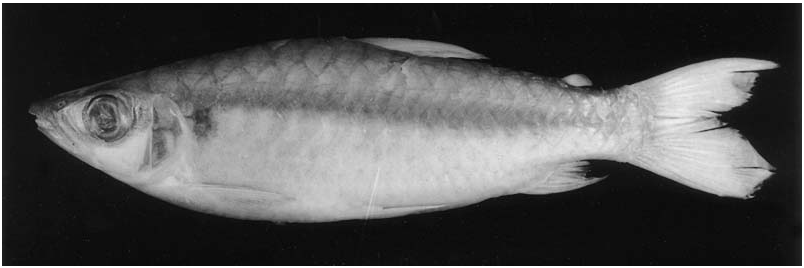
The presence of a conspicuous rounded humeral spot with a notch on its posterodorsal margin, the dark pelvic and anal fins and the presence of dark chromatophores on the posterior margin of the longitudinal series of scales posterior to the humeral spot distinguishes C. erythrurus ( Fig. 10 View Figure 10 ) from its congeners (in some sexually mature specimens, the humeral spot may be obliterated by a wide dark longitudinal band, see ‘Comments...’, below) ( Fig. 11 View Figure 11 ). Although C. spilogyros also has a rounded humeral spot, that spot is not notched and is relatively smaller than the spot in C. erythrurus . A humeral spot is present in C. guaporensis and sometimes in C. epakros , however, in these species it is usually rounded to vertically elongate and located deeper in the skin being consequently less conspicuous than the humeral spots of C. erythrurus and C. spilogyros in which the spot is located superficially on the skin (see ‘Comments...’, below). In addition, the caudal-fin lobes in C. erythrurus are robust and rounded compared to the more elongate and slender lobes in all other Chalceus species.
Description
Morphometric data presented in Table 2 (some meristic data for holotype not recorded due to poor condition of specimen). Maximum size 213.5 mm SL. Body robust, relatively elongate, greatest body depth located slightly anterior to dorsal-fin origin. Dorsal profile of head straight from snout tip to end of supraoccipital spine in all specimens. Anterior profile of head distinctly rounded from dorsal view. Interorbital distance wide, proportionally wider relative to body size in larger specimens. Dorsal surface of head in interorbital region flat. Dorsal body profile straight to slightly convex from tip of supraoccipital spine to dorsal-fin origin. Dorsal body profile posteroventrally inclined along dorsal-fin base, straight to relatively convex to adipose fin and concave along dorsal profile of caudal peduncle to origin of procurrent caudal-fin rays. Overall dorsal body profile of juveniles up to 40 mm SL convex. Ventral profile of head strongly convex to pectoral-fin, slightly convex to somewhat straight to pelvic-fin insertion, convex to anal-fin origin. Body profile along anal-fin base posterodorsally inclined and slightly concave along ventral margin of caudal peduncle. Head robust. Dorsal surface of head with distinct median fontanel restricted to small region anterior to the epiphyseal bar between frontals and completely separating parietals. Fontanel wide in small specimens and progressively narrower in larger individuals. Fontanel almost completely closed in a 213.5-mm SL specimen but with small space remaining between contralateral parietals. Mouth large, terminal, slightly superior, upper and lower jaw of equal length. Maxilla not reaching vertical through anterior margin of orbit. Supramaxilla present.
Dorsal-fin rays (ii,10; ii, 11 in one specimen, n = 102). Dorsal-fin origin located posterior to vertical through insertion of innermost pelvic-fin rays. First basal dorsal-fin pterygiophore inserting behind neural spine of 12th vertebra 9 (n = 1). Distal margin of dorsal fin nearly straight to convex. Adipose fin present. Anal-fin rays (iii,9; iii, 8 in one specimen, n = 98). First basal anal-fin pterygiophore inserting behind haemal spine of 25th vertebra (n = 1). Distal margin of anal fin straight to emarginate with anterior branched rays approximately 3 times length of ultimate ray. Pectoral-fin rays (range i,15–18, mean 16.5, n = 104). Pectoral-fin pointed distally, with unbranched- and first branched rays longest and not reaching pelvic-fin insertion. Pelvic-fin rays i,8 (i,8; i, 7 in 3 specimens, i, 9 in one specimen, n = 102); fin pointed distally. Caudal fin forked, with lobes robust and rounded, lower fin lobe slightly more developed than upper lobe.
Premaxillary teeth in three rows. Outer row 8 (range 6–9, 10 in one specimen, mean 7.6, n = 104) with teeth tricuspid or pentacuspid in large specimens and smaller specimens with conical or tricuspid teeth, with medial cusp larger. Tooth close to premaxillary symphysis slightly larger than other teeth in series. Remaining teeth of similar size, but with one or two lateralmost teeth slightly smaller. Cusps slightly curved with concave portion facing mouth cavity. Inner row 5 (range 5–6, mean 5.1, n = 104) with largest, symphyseal tooth usually asymmetric with one cusp on medial margin and two on lateral margin of tooth. Remaining teeth penta- or heptacuspid in large specimens, with second tooth from symphysis larger. Teeth tricuspid in small specimens, with teeth gradually diminishing in size laterally. Cusps slightly curved with concave portion opposite of mouth cavity. Intermediate row 2 (2, n = 105) pentacuspid (rarely tricuspid) teeth more widely separated than those of other rows and of intermediate size. Cusps straight.
Maxillary teeth 9 (range 7–10, with one specimen each having 5, 11 and 12, mean 8.5, n = 93) with smaller specimens usually with higher numbers of teeth. First tooth pentacuspid followed by tricuspid and conical teeth distally. Teeth not extending along entire margin of ossification in large specimens. In small specimens teeth conical and extending along entire margin of ossification. Dentary teeth in two rows. Outer row 8 (range 7–11, 12, 13 and 16 in one specimen each, mean 9.4, n = 105). Teeth large and pentacuspid anteriorly, sometimes heptacuspid in large specimens, tricuspid in small specimens, gradually diminishing in size and number of cusps posteriorly. Posteriormost teeth conical. Cusps slightly curved with concave portion facing mouth cavity. Inner row consisting of large conical symphyseal tooth followed by series of minute conical teeth. First tooth originating behind fourth to fifth tooth of outer row with consequent gap between the symphyseal tooth and first minute conical tooth.
Scales cycloid, large overall and approximately twice as large above lateral line as below it. Circuli on exposed portion of scales not concentric with those of anterior portion. Circuli on exposed portion of scales straight and extending to posterior margin of scale in small specimens; disorganized and with labyrinthic pattern in specimens around 200 mm SL. Radii originating on centre of scale and radiating anteriorly and posteriorly on scale surface.
Lateral line low on body sides, complete, with alternating large and small perforated scales from posterior margin of opercle to vertical through base of last anal-fin ray; scales smaller and of similar size from that point to end of caudal peduncle. Canals in large specimens with 3–7 elevated branches (usually 3 or 4), forming ridges on scale surface; ridges more evident on region of the caudal peduncle. Number of branches decreases towards caudal peduncle, with canals of posterior scales unbranched. Small specimens (less than 140 mm) with branching pattern less developed. Lateral-line scales (range 36–39, 35 in two specimens, mean 37.2, n = 97). Scale rows between dorsal-fin origin and lateral line 3; between lateral line and pelvicfin insertion 2. Scales around caudal peduncle 12. Vertebrae 37 (n = 1).
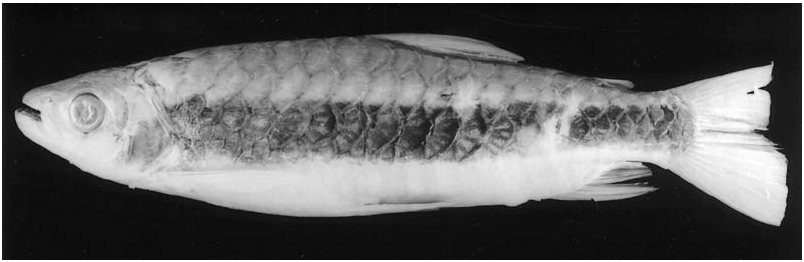
Colour in life. (Description based on photograph in Géry, 1977: 332, and personal observations of one specimen kept in aquarium). Overall coloration of head and body bright silver. Conspicuous humeral spot present on region of four first scales along longitudinal series just above lateral line. Overall shape of humeral spot rounded with notch on posterodorsal margin of spot. Dorsal portion of eye yellow. Dorsal profiles of head and body darker. Margins of scales on dorsal portions of body with strong concentration of dark chromatophores, forming well-defined reticulate pattern. Caudal fin bright red. Pelvic and anal fins distinctly yellow. Adipose and dorsal fins yellowish, both tinged with red. Pectoral fin hyaline.
Colour in alcohol. Few specimens that retain guanine on body and head have silvery ground coloration. Most specimens only retain silvery pigmentation on infraorbital and opercular regions. Ground coloration of head and body in specimens ≥ 50 mm SL, lacking guanine on scales, yellowish to tan, darker dorsally. Conspicuous humeral spot on region of first four scales of longitudinal series just above lateral line. Overall shape of spot rounded with notch on its posterodorsal margin. Margins of scales on dorsal portions of body with strong concentration of dark chromatophores, forming well-defined reticulate pattern, that may be present over entire dorsal region of body, or restricted to dorsal portion of lateral-line scales and on longitudinal series just above it. Dark chromatophores scattered over infraorbitals and opercular region. Reticulate pattern less evident in large specimens and sometimes restricted to series of longitudinal diffuse dark patches. Pelvic and anal fins dark. Dorsal, adipose and caudal fins dusky. Some specimens with dark tips on caudal-fin rays. Some specimens (AMNH 58440, INPA 16190 (4 of 5), INPA 17226, INPA 18620; MZUSP 13533, MZUSP 20385 (4 of 9) and MZUSP 77595) have dark and wide longitudinal band along body ( Fig. 11 View Figure 11 ) (see ‘Comments...’, below).
Ground coloration of specimens £ 50 mm pale yellowish ( Fig. 12 View Figure 12 ). Reticulate pattern on scales more conspicuous than in larger specimens and extending to longitudinal series of scales below lateral line. Humeral spot small, rounded and somewhat elongated vertically. Dark narrow longitudinal line from dorsal margin of humeral spot to end of caudal peduncle. Pelvic, dorsal, anal, adipose and lower lobe of caudal fin dark. Pectoral fin hyaline.
Distribution
Rio Amazonas and Rio Solimões from Manaus to the Río Ucayali drainage in Peru ( Fig. 14 View Figure 14 ). Localities distributed mainly along main channel of Rio Amazonas and Rio Solimões and mouths of their tributaries.
Remarks
Fowler (1906: 444) described Pellegrinina heterolepis on the basis of one specimen that he believed originated from West Africa; as a consequence, he made comparisons of that nominal form with the African alestid genera Alestes , Brycinus and Brachyalestes in the diagnosis of the species. Subsequently the locality was shown to be incorrect and the fish was identified as a Chalceus species from South America ( Géry, 1977: 18). The holotype of Pellegrinina heterolepis (ANSP 8150) was examined in the present study ( Fig. 13 View Figure 13 ) and on the basis of the presence of a humeral spot with a notch along its posterodorsal margin it is readily identified as Chalceus erythrurus . Additional characters present on the specimen that also characterize C. erythrurus are the presence of a cranial fontanel, the dark pelvic and anal fins and the first small inner dentary row tooth originating behind the fifth tooth of the anterior outer tooth row with a gap between the symphyseal tooth and minute first conical tooth. Based on these features, Pellegrinina heterolepis is herein considered to be a junior synonym of Chalceus erythrurus .
Nakashima (1941: 76) described Chalceus macrolepidotus iquitensis from the surroundings of Iquitos, in the Peruvian Amazon, but did not provide any explanation as to why he recognized the material as a new subspecies. No information about type specimens was provided and no types are known to be extant. Based on his description, especially regarding the colour pattern and the shape of the humeral spot in the illustrated specimen, the species is most probably Chalceus erythrurus . Chalceus epakros also occurs in the Peruvian Amazon, but the humeral spot is usually absent in this species and when present it is less conspicuous than that of C. erythrurus and is, furthermore, more vertically elongate. In addition, C. epakros does not exhibit the reticulate pattern on the scales described by Nakashima (1941) which is, however, present in C. erythrurus . Chalceus macrolepidotus iquitensis Nakashima is therefore considered to be a synonym of C. erythrurus .
Material examined
Type material. ANSP 8032, 1, 44.5 mm SL; Peru, Río Ambyiacu (= Ampiyacu) at Pebas; holotype of Plethodectes erythrurus Cope, 1870 (specimen in very poor condition). – ANSP 8150, 1, 95.8 mm SL; Probably West Africa (?) [locality data incorrect, specimen from South America, see Géry, 1977: 18]; holotype of Pellegrinina heterolepis Fowler, 1906 .
Non-type material. BRAZIL: AMAZONAS: INPA 16928, 1, 195.0 mm SL; Rio Amazonas, Ilha do Careiro, Lago do Mingal.- INPA 16190(*) 5, 119.7– 158.7 mm SL; INPA 16929, 1, 189.0 mm SL; Rio Amazonas, Ilha do Careiro, Terra Nova.- INPA 18619, 1, 92.1 mm SL; Mamirauá Lake system, Paraná Maiana station A, 2.5 km from Comunidade Boca do Mamirauá; INPA 18620(*), 3, 102.9–176.0 mm SL; Rio Solimões, Lago Capivara, Costa das Capivaras. - MZUSP 6881, 12, 95–166.7 mm SL; MZUSP 64203, 1, 106.8 mm SL; USNM 308376, 2, 38.3–39.1 mm SL; USNM 308820, 7, 28.7–37.9 mm SL; USNM 308371, 1, 1, 106.7 mm SL; USNM 308596, 1, 50.1 mm SL; Lago Janauari, right margin of Rio Negro, Manaus. – INPA 16949, 3, 75.6–88.6 mm SL; Janauari. – MZUSP 75610, 1, 89.4 mm SL; Lago Janauari, near Canta Galo. – MZUSP 75611, 2, 86.9– 85.4 mm SL; Lago Janauari, near its mouth. – MZUSP 75612, 2, 79.6–88.1 mm SL; Lago Janaurai, first brick factory. – MZUSP 6707, 35, 94.7–136.3 mm SL; Rio Negro, vicinity of Manaus. – MZUSP 6462, 3, 160.7– 213.5 mm SL; Lago Jacaré, right margin of Rio Solimões, above Manacapuru. – MZUSP 20053, 1, 161.7 mm SL; Lago do Rei, Ilha Canini, in front of Santo Antonio do Içá. – MZUSP 27297, 2, 95.5–104.2 mm SL; Paranã do Lago Amanã, lower Rio Japurá. – MZUSP 27296, 6, 84.4–112.5 mm SL (1 C & S); Costa Japão, Ressaca do Japão, lower Rio Japurá. – MZUSP 27298, 4, 140.0– 145.9 mm SL; Lago Mamirauá, mouth of Rio Japurá. MZUSP 13533(*), 1, 195.9 mm SL; Lago do Miguel, below Itacoatiara. – MZUSP 20385(*) 1, 109.6 mm SL; Lago Janauacá. – CAS 139277, 5, 158.0–166.0 mm SL; Rio Solimões, Lago Coari. – CAS 69083, 2, 174.3– 187.7 mm SL; Manaus. – INPA 17226(*), 2, 157.0–190.0 mm SL; MZUSP 77595, 3, 158– 178 mm SL; Rio Purus, Paraná do Lago Jacaré. – INPA 17235, 1, 186.0 mm SL; Rio Purus, Igarapé do Sacado. – INPA 17240, 1, 177.0 mm SL; Rio Purus, Beruri, at mouth of lake. – INPA 17257, 1, 84.0 mm SL; Rio Purus, Beruri, Paraná do Seixo. – INPA 17294, 1, 172.0 mm SL; Rio Purus, Beabá. – AMNH 12556, 1, 82.2 mm SL; vicinity of mouth of Rio Embira, tributary of Rio Tarauacá, Rio Juruá. – MZUSP 75614, 1, 118.8 mm SL; São José, Lago do Castanho, Janauacá. – USNM 229093, 1, 95.8 mm SL; Paraná do Lago Janauacá, entrance of Castanho. – USNM 308821, 3, 72.8–101.1 mm SL; Lago Murumuru, Lago Janauacá, near INPA stable. – USNM 308807, 2, 87.2–90.6 mm SL; MZUSP 75613, 6, 84.8– 100.4 mm SL; Lago Murumuru, Lago Janauacá, at stable, near Manaus. – USNM 229083, 2, 86.4– 98.2 mm SL; Lago Murumuru, at stable, near Manaus. – USNM 119947, 18, 92.6–126.0 mm SL; Rio Solimões, Codajás. – BMNH, 1929.11.18.3, 1, 189, 6 mm SL; Manaus, Rio Amazonas. – BMNH, 1925.10.28.85–84, 5, 87.8–195.3 mm SL; Rio Solimões, Manacapuru.
PERU: LORETO: CAS 69082, 4, 147.7–172.0 mm SL; Río Amazonas drainage, Iquitos. – CAS 69074, 6, 92.2–128.1 mm SL; Río Amazonas drainage, Yarinacocha, lake connected to Río Pacaya. – CAS 136871, 6, 66.3–87.7 mm SL; Caño Tuye, Pebas. – CAS 17272, 2, 86.8–141.5 mm SL; Caño Chancho, near Pebas. – MZUSP 78064, 1, 139.0 mm SL; Río Yarapa, tributary of Río Ucayali, tributary of Río Amazonas, several sites along a 10-km stretch of river. – AMNH 218032, 1, 79.9 mm SL; Río Itaya, near Iquitos. – USNM 167798, 4, 113.7– 124.9 mm SL; Yarinacocha. – USNM 280437, 1, 99.5 mm SL; Río Itaya, main river channel and lower portion of caños, 5–20 km upstream of Belen, Iquitos. – USNM 280441, 3, 74.4–81.7 mm SL; green water caño on leftbank of Río Manite, about 8 km upriver of junction of Río Manite and Río Amazonas. – FMNH 70229, 3, 90.3–116.8 mm SL; Río Maniti, Santa Cecilia 20. – FMNH 100438, 1, 105.4 mm SL; Río Airico, 5 km above mouth in Río Chambira.
AMNH 58440 (*), 1, 105.9 mm SL; South America, no locality data.
CHALCEUS SPILOGYROS SP. NOV.
( FIGS 14–17 View Figure 14 View Figure 15 View Figure 16 View Figure 17 ; TABLE 3)
Diagnosis
The presence of a dark rounded humeral spot readily distinguishes C. spilogyros ( Figs 15–17 View Figure 15 View Figure 16 View Figure 17 ) from C. macrolepidotus and C. epakros . Chalceus spilogyros shares with C. erythrurus the presence of a humeral spot formed by dark chromatophores located superficially on the skin. However, the spot of C. spilogyros is rounded and relatively small compared with the larger spot of C. erythrurus ; in the latter, there is also a notch along its posterodorsal margin. In addition, the caudal-fin lobes in C. spilogyros are relatively elongate and slender compared to the robust and rounded lobes of C. erythrurus . A humeral spot is present in C. guaporensis and sometimes in C. epakros ; however, in these species it is usually rounded to vertically elongate, located deeper in the skin and thus somewhat less conspicuous than that of C. spilogyros . In addition, C. spilogyros lacks the longitudinal dark stripe characteristic of C. epakros and C. guaporensis , although in some specimens a wide dark longitudinal band is present. However, it differs from the band of C. epakros and C. guaporensis in the pattern of distribution of the chromatophores on the skin (see ‘Comments...’, below).
Description
Morphometric data presented in Table 3. Maximum size 223.2 mm SL. Body robust, relatively elongate, greatest body depth located slightly anterior to dorsalfin origin. Dorsal profile of head distinctly convex anteriorly in snout region, posterodorsally inclined to convex from anterior end of snout to tip of supraoccipital spine and continuous with dorsal body profile. Anterior profile of head somewhat acute from dorsal view. Interorbital distance wide, proportionally wider relative to body size in larger specimens. Dorsal body profile somewhat convex to straight from tip of supraoccipital spine to dorsal-fin origin. Dorsal body profile posteroventrally inclined along dorsal-fin base, straight to relatively convex to adipose fin and concave along dorsal profile of caudal peduncle to origin of procurrent caudal-fin rays. Ventral profile of head distinctly convex along lower jaw. Ventral body profile gently convex from posterior limit of isthmus to analfin origin. Body profile posterodorsally inclined along anal-fin base, slightly concave along ventral margin of caudal peduncle. Head robust in specimens larger than 120 mm SL. Smaller specimens with relatively longer heads and more acute snout. Dorsal surface of head with distinct fontanel restricted to a small portion anterior to the epiphyseal bar between frontals and completely separating contralateral parietals. Fontanel wide in small specimens and progressively narrower in larger individuals. Small fontanel still present in largest examined specimen (223.2 mm SL). Mouth terminal, large, upper and lower jaw equally long. Maxilla extending approximately to vertical through anterior margin of orbit. Supramaxilla present.
Dorsal-fin rays ii,10 (ii,10; ii, 9 in one specimen, ii, 11 in three specimens, n = 88). Dorsal-fin origin located posterior to vertical through insertion of innermost pelvic-fin rays. First basal dorsal-fin pterygiophore inserting behind neural spine of 13th vertebra (n = 1). Distal margin of dorsal fin nearly straight to convex. Adipose fin present. Anal-fin rays iii,9 (iii,9; iii, 8 in one; iii, 10 in two specimens, n = 84). First basal anal-fin pterygiophore inserting behind haemal spine of 26th vertebra (n = 1). Distal margin of anal fin straight to emarginate with anterior branched rays approximately 3 times length of ultimate ray. Pectoral-fin rays i,15 (range 13–17, 18 in two specimens, mean 16.1, n = 88), pointed distally, with unbranched- and first branched rays longest, but not reaching pelvic-fin insertion. Pelvic-fin rays i,8 (i,8 n = 88), fin pointed distally. Caudal fin forked, with lobes slender particularly in specimens up to 120 mm SL, lower fin lobe slightly more developed than upper lobe.
Premaxillary teeth in three rows. Outer row 8 (range 8–11, 7 in four specimens, mean 9.0, n = 88) either tricuspid or pentacuspid, with medial cusp larger. Tooth close to premaxillary symphysis slightly larger than other teeth of series. Remaining teeth of similar size with one or two lateralmost teeth slightly smaller. Cusps slightly curved with concave portion facing mouth cavity. Inner row 7 (range 6–7, 5 in four specimens, mean 6.2, n = 88), with largest, symphyseal tooth usually asymmetric with one cusp on medial margin and two on lateral margin of tooth. Remaining teeth, penta- or heptacuspid in large specimens, with second tooth from symphysis larger and remaining teeth gradually diminishing in size laterally. Cusps slightly curved with concave portion opposite of mouth cavity. Intermediate row, with 2 (2; 1 in one specimen, n = 88) pentacuspid teeth more widely spaced than teeth of other rows and of intermediate size. Cusps straight.
Maxillary teeth 8 (range 7–11, 6 in two specimens, 12 in three specimens, mean 9.2, n = 88) with smaller specimens usually having more teeth. First teeth pentacuspid, followed by tricuspid and conical teeth distally. Teeth not extending along entire margin of ossification in large specimens. In small specimens conical and extending almost along entire margin of ossification. Dentary teeth in two rows. Outer row 12 (range 9–14, 8 and 17 in one specimen each, mean 11.0, n = 87), with teeth large and pentacuspid anteriorly, sometimes heptacuspid in large specimens and tricuspid in small specimens. Teeth gradually diminishing in size and number of cusps posteriorly. Posteriormost teeth conical. Cusps slightly curved with concave portion facing mouth cavity. Inner dentary row with large, conical, symphyseal tooth (tricuspid in a few larger specimens) followed by series of minute conical teeth. First small tooth usually situated behind fourth tooth of anterior row with gap between symphyseal tooth and first small conical tooth of rest of series.
Scales cycloid, large overall and approximately twice as large above lateral line as below it. Circuli on exposed portion of scales not concentric with those of anterior portion. Circuli on exposed portion of scales straight and extending to posterior margin of scale in small specimens; disorganized and labyrinthic pattern in specimens around 200 mm SL. Radii originating on centre of scale and radiating anteriorly and posteriorly on scale surface.
Lateral line low on body sides, complete, with alternating large and small perforated scales from posterior margin of opercle to vertical through base of last anal-fin ray. Lateral-line scales smaller and of similar size from that point to end of caudal peduncle. Canals in large specimens with 3–6 (usually 3 or 4) elevated branches, forming ridges on scale surface. Ridges more evident on region of caudal peduncle. Number of branches decreases toward caudal peduncle with posterior scales unbranched. Smaller specimens (£ 140 mm) with branching pattern less developed. Lateral-line scales 38 (range 36–39, 34 and 40 in one specimen each, mean 38.2, n = 79). Scale rows between dorsal-fin origin and lateral line 3; between lateral line and pelvic-fin insertion 2. Scales around caudal peduncle 12. Vertebrae 38 (n = 1).
Colour in alcohol. All available specimens lack guanine on body except for few individuals that retain silvery pigmentation on infraorbital and opercular regions. Ground coloration of head and body yellowish to tan, darker dorsally. Conspicuous, small, rounded humeral spot on region of three first scales of longitudinal series just above lateral line. Humeral spot formed by dark chromatophores located superficially on skin (see ‘Comments...’, below). Dark chromatophores scattered over infraorbitals and opercular region. Some specimens with dark blotch present on lower half of opercle. Scales of dorsal portion of body with chromatophores concentrated along posterior margin, more so in central portion and forming reticulate pattern, more conspicuous on two longitudinal series of scales above lateral line. Reticulate pattern more evident in specimens £ 150 mm SL ( Fig. 16 View Figure 16 ). All fins hyaline. Distal portion of longer caudal-fin rays darkened. Some specimens (MZUSP 7053 (5 of 7), MZUSP 20314 (1 of 55) and MZUSP 54568 (1 of 5)) with dark, wide longitudinal band along body ( Fig. 17 View Figure 17 ) (see ‘Comments...’, below).
Distribution
Occurs only in Rio Trombetas, lower Rio Tapajós and Rio Canumã in the Rio Madeira drainage ( Fig. 14 View Figure 14 ) .
Etymology
Spilogyros , from the Greek spilos meaning spot and gyros, meaning circle, round, in reference to the rounded humeral spot of the species.
Material examined
Type material. HOLOTYPE: MZUSP 20314 View Materials , 1 View Materials , 208.0 mm SL; Brazil, Pará, Igarapé Jacaré, right margin of Rio Tapajós , near Boim. (3∞0¢S; 55∞15¢W). Collector Expedição Permanente à Amazônia, 27/Oct/ 1970 . PARATYPES: MZUSP 76069 View Materials 42, 1C & S 86.2 – 214.0 mm SL ; INPA 18589 View Materials , 3 View Materials , 100.3 View Materials –201.0 mm SL; USNM 368278, 3 View Materials , 107.1 View Materials –196.0 mm SL; UMMZ 239930, 3 View Materials , 106.8 View Materials –183.0 mm SL; same data as holotype .
Non-type material. BRAZIL. AMAZONAS: MZUSP 7054, 10, 92.3–134.8 mm SL; MZUSP 7053(*), 7, 175.9–198.0 mm SL; Rio Canumã. PARÁ: MZUSP 15801–802, 2, 92.7–174.0 mm SL; MZUSP 15791–92, 2, 145.4– 223.2 mm SL; Igapó do Lago Farias, Rio Trombetas, Reserva Biológica Trombetas. – MZUSP
54566, 2, 205.1–206.0 mm SL; MZUSP 54568(*), 5, 149.4– 195.7 mm SL; Lago Mussurá, left margin of Rio Trombetas, Porto Trombetas, Oriximiná. – MZUSP 54569, 2, 184.4– 200.4 mm SL; MZUSP 54570, 1, 188.9 mm SL; Lago Batata, right margin of Rio Trombetas, Porto Trombetas, Oriximiná. – MZUSP 19698, 18, 77.7–148.6 mm SL; Lagoa Jacaré, Rio Trombetas. – INPA 16930, 2, 193.0–203.0 mm SL; Lago Cruz Alta, Rio Trombetas. – INPA 16943, 2, 165.0–210.0 mm SL; Lago Corusca, Igarapé do Braço, Rio Trombetas drainage. – INPA 16944, 4, 166.0–190.0 mm SL; Lago Jamari, Rio Trombetas drainage.
| MNHN |
Museum National d'Histoire Naturelle |
| ANSP |
Academy of Natural Sciences of Philadelphia |
| FMNH |
Field Museum of Natural History |
| AMNH |
American Museum of Natural History |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| CAS |
California Academy of Sciences |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| UMMZ |
University of Michigan, Museum of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Chalceus
| Zanata, Angela M. & Toledo-Piza, Mônica 2004 |
Creagrutus pellegrini
| Vari RP & Harold AS 2001: 2 |
| Gery J 1977: 654 |
| Myers GS 1960: 211 |
| Puyo J 1949: 128 |
| Puyo J 1943: 143 |
Chalceus macrolepidotus
| La Monte FR 1935: 7 |
Pellegrinina
| Fowler HW 1906: 442 |
| Fowler HW 1906: 442 |
Pellegrinina heterolepis
| Fowler HW 1906: 442 |
Plethodectes erythrinus:
| Eigenmann CH & Eigenmann RS 1891: 51 |
Chalceus erythrurus
| Ortega H & Vari RP 1986: 7 |
| Gery J 1977: 332 |
| Regan CT 1912: 388 |
| Eigenmann CH & Eigenmann RS 1891: 55 |
| Cope ED 1872: 262 |
Plethodectes
| Cope ED 1870: 563 |
| Cope ED 1870: 563 |
Plethodectes erythrurus
| Fowler HW 1950: 365 |
| Eigenmann CH & Allen WR 1942: 278 |
| Eigenmann CH 1910: 439 |
| Fowler HW 1906: 441 |
| Cope ED 1870: 563 |
Chalceus ararapeera
| Bertin L 1948: 9 |
| Cuvier G & Valenciennes A 1850: 244 |
Chalceus
| Cuvier G 1817: 454 |
| Cuvier G 1817: 454 |
Chalceus macrolepidotus
| Taphorn D & Royero R & Machado-Allison A & Mago-Leccia F 1997: 70 |
| Planquette P & Keith P & Le Bail PY 1996: 230 |
| Gery J & Planquette P & Le Bail PY 1991: 43 |
| Gery J & Planquette P 1982: 73 |
| Lauder GV 1981: 162 |
| Azuma H 1979: 58 |
| Cala P 1977: 7 |
| Gery J 1977: 342 |
| Heyer HC 1975: 343 |
| Boeseman M 1952: 189 |
| Fowler HW 1950: 364 |
| Puyo J 1949: 129 |
| Bertin L 1948: 9 |
| Eigenmann CH & Allen WR 1942: 277 |
| Cockerel TDA 1914: 107 |
| Regan CT 1912: 388 |
| Eigenmann CH 1910: 439 |
| Regan CT 1905: 190 |
| Eigenmann CH & Eigenmann RS 1891: 55 |
| Cope ED 1872: 262 |
| Cuvier G & Valenciennes A 1850: 240 |
| Schomburgk RH 1841: 216 |
| Cuvier G 1817: 454 |