Bolitoglossa paraensis ( Unterstein, 1930 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3686.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:301004F2-39C2-45D1-A145-F77AFE122A69 |
|
DOI |
https://doi.org/10.5281/zenodo.5621233 |
|
persistent identifier |
https://treatment.plazi.org/id/6154094F-565F-FE0B-CEDE-127F149C0628 |
|
treatment provided by |
Plazi |
|
scientific name |
Bolitoglossa paraensis ( Unterstein, 1930 ) |
| status |
|
Bolitoglossa paraensis ( Unterstein, 1930) View in CoL
( Figures 5 View FIGURE 5 A, 6)
Oedipus paraensis Unterstein, 1930: 271 View in CoL (holotype ZMB 32907); Myers & Carvalho 1945: 5; Schmidt & Inger 1951: 443.
Eladinea estheri Miranda-Ribeiro, 1937: 42 (syntypes MNRJ 68, four very young specimens).
Eladinea — Taylor 1944: 222.
Oedipus altamazonicus View in CoL — Parker 1939; Myers & Carvalho 1945: 7.
Bolitoglossa altamazonica View in CoL —Brame Jr. & Wake 1963: 13, Fig. 22 (in part); Wake & Brame Jr. 1966 (in part); Crump 1971; Wake & Lynch 1976: 41 (in part); Crump 1977; Duellman 1978: 81; Wake et al. 1982 (in part); Obst et al. 1984: 65 (in part); Frost 1985: 574 (in part); De la Riva et al. 2000: 52 (in part); Reichle et al. 2001; Bartlett & Bartlett 2003: 28 (in part); Schargel & Fuenmayor 2003: 94.
Bolitoglossa paraensis View in CoL — Taylor 1944: 219, 222; Brame Jr. & Wake 1962: 177; Avila-Pires et al. 2007; Estupiñán 2007; Galatti et al. 2007: 93; Neckel-Oliveira & Hoogmoed 2010 (in part); SEMA-PA 2008: [23]; Esqueda et al. 2009: 172; Peloso 2010: 669; Frost 2011; Neckel-Oliveira et al. 2011; Segalla et al. 2010 (in part); Correa et al. 2012.
“Nauta mushroom-tongue salamander Crump 2000: 43.
Bolitoglossa (Eladinea) paraensis View in CoL — Parra-Olea et al. 2004: 335 (in part); Raffaëlli 2007: 259.
Holotype. ZMB 32907, lost (Brame Jr. & Wake 1963; Bauer et al. 1993). The description of O. paraensis does not provide a clear diagnosis of the species, the holotype is lost (Brame Jr. & Wake 1963; Bauer et al. 1993) and the types of its junior synonym E. estheri are young and recently hatched, not showing the characters of adults from this species. Considering the confusing status of the taxonomy of South American bolitoglossines, and especially those from the Brazilian Amazon basin, we decided to designate a topotypical neotype for B. paraensis to serve as a reference in studies of Bolitoglossa from the Brazilian Amazonia .
Neotype designated herein. MPEG 31682, adult 3, Sítio Semente Etérea, 01° 12’ 10,49” S, 48° 18’ 02,43” W, Vila do Carapuru, Municipality of Santa Isabel do Pará, state of Pará, Brazil, 30 m elevation, 10-III-2010, leg. I.C. Brcko, F.S. Correa, L. Rodrigues and M.B. Silva, 20.00 h This specimen is from the same municipality (“Sta Isabel bei Para = Santa Isabel near Belém) where the holotype was collected, although it is impossible to say how far the original type locality and the present one are apart, but at least they are in the same vegetational zone and drainage basin.
Material examined. 166 individuals: Brazil, Pará state: Municipality of Barcarena: CZB 2012, Ƥ, 01°31’8, 93”S, 48° 36’59, 48”W, 12 m elevation, 15-XII-2010, leg. R.P. Silva; Municipality of Belém: MPEG 3721, Ƥ, Ilha de Mosqueiro, 12 m elevation, July 1971, leg. O.R. da Cunha; Parque Ambiental de Belém (Utinga), 01° 25’ 6” S, 48° 24’ 55” W, 10 m elevation: MPEG 7615, Ƥ, March 1966, leg. W. França; MPEG 7618, Ƥ, January 1967, leg. P. Martins; UFMT 7477, Ƥ, 24-VI-1969, leg. N.A. Rosa; MPEG 7617, Ƥ, 14-VII-1970, MPEG 7616, Ƥ, 21-VII- 1970, leg. M. Crump; MPEG 908–910, 2 3 and 1 Ƥ, leg. N.A. Rosa; UFMT 7476, Ƥ, 7-X-1985, leg. M. Rosa; MPEG 6073, Ƥ, 28-VI-1989, leg. F. Torres and J. Pena; MPEG 6072, 3, 14-IX-1989, leg. R. Constantino; MPEG 6074, 3, 17-II-1993, leg. A. Cardoso and N.V. Atzinger; MPEG 15856, 3, 14-I-1999, leg. U. Gallati et al.; Municipality of Benevides: Área Nova Amafrutas, 01° 17’ 55” S, 48° 09’ 38” W, 35 m elevation: MPEG 31641– 31652, 5 juveniles, 3 3 and 3 Ƥ, 20-XI-2003; MPEG 31653, Ƥ, 3-XII-2003, leg. R. da Silva and R.A. Estupiñán; Municipality of Bragrança: MPEG 28604, Ƥ, Colônia Benjamin Constant, 01° 04’ 13, 65” S, 46° 35’ 44, 30” W, 13 m elevation, 23-VI-2008, leg. J.B.F. Silva; Municipality of Mojú: MPEG 34630, Ƥ, Fazenda Experimental da EMBRAPA Amazônia Oriental , 02° 07’ 30” S, 48° 46’ 57” W, 24 m elevation, 13-I-11, leg. A. Palmeira; Municipality of Ourém: Parauateua, 01° 33’ 07” S, 47° 06’ 52” W, 14 m elevation: MPEG 28822, Ƥ, 20-X-2009, leg. D. Guimarães; Municipality of Primavera: Ponto MVP-P2, Futura Fábrica de Cimentos Votorantim, 00° 58’ 50,9” S, 47° 06’ 46,4” W, 26 m elevation: UFMT 11575–11576, 2 Ƥ, 6-X-2010, leg. L. Arruda; UFMT 11647– 11648, 1 juvenile and 1 Ƥ, 16-XII-2010, leg. R. Ávila; UFMT 11646, juvenile, 19-XII-2010, leg. N. Pinho; UFMT 12599–12600, 2 Ƥ, 25-IV-2011, leg. R. Ribeiro; UFMT 12598, Ƥ, 29-IV-2011, leg. E. Brito; Municipality of Santa Bárbara do Par: Parque Ecológico de GUNMA, 01° 12,48’ S, 48° 17,33’ W, 30 m elevation: MPEG 16622–16623, 2 Ƥ, January 2003, leg. S. Almeida; MPEG 19704, Ƥ, June 2005; MPEG 21310–21317, 2 juveniles, 2 3 and 4 Ƥ, 20-XII-2005; MPEG 29410–29411, 2 juveniles, 20-I-2006, leg. S. Neckel-Oliveira, R.R. Silva and R.B. Neto; MPEG 21318–21332, 6 juveniles, 3 3 and 6 Ƥ, 14-II-2006; MPEG 21333–21350, 6 juveniles, 6 3 and 6 Ƥ, 7-III- 2006; MPEG 21351–21360, 5 juveniles, 1 3 and 4 Ƥ, 14-IV-2006; MPEG 21361–21372, 4 juveniles, 3 3 and 5 Ƥ, 13-VII-2006; MPEG 21373–21380, 2 juveniles, 1 3 and 5 Ƥ, 29-III-2006, all leg. S. Neckel-Oliveira, J.F. Sarmento, U. Galatti, P. Suárez, A. Lima and C. Lima; MPEG 31654–31665, 1 juvenile, 4 3 and 7 Ƥ, 20-III-2007, leg. P. Suárez; MPEG 21381–21390, 2 juveniles, 3 3 and 5 Ƥ, 11-IV-2007, leg. S. Neckel-Oliveira, J.F. Sarmento, U. Galatti, P. Suárez, A. Lima and C. Lima; Municipality of Santa Isabel do Pará: MPEG 31666–31681; 6 juveniles, 5 3 and 5 Ƥ; MPEG 31683–31685, 1 juvenile and 2 Ƥ, same locality data and collectors as neotype; Municipality of Tailândia: Empresa Agropalma, 02° 33’ 3” S 48° 47’ 27” W, 30 m elevation: AGP 046–053, 1 3 and 7 Ƥ; AGP 134, Ƥ; AGP 142–145, 4 juveniles, April 2012, all leg. F.S. Correa, L. Rodrigues and H. Figueira.
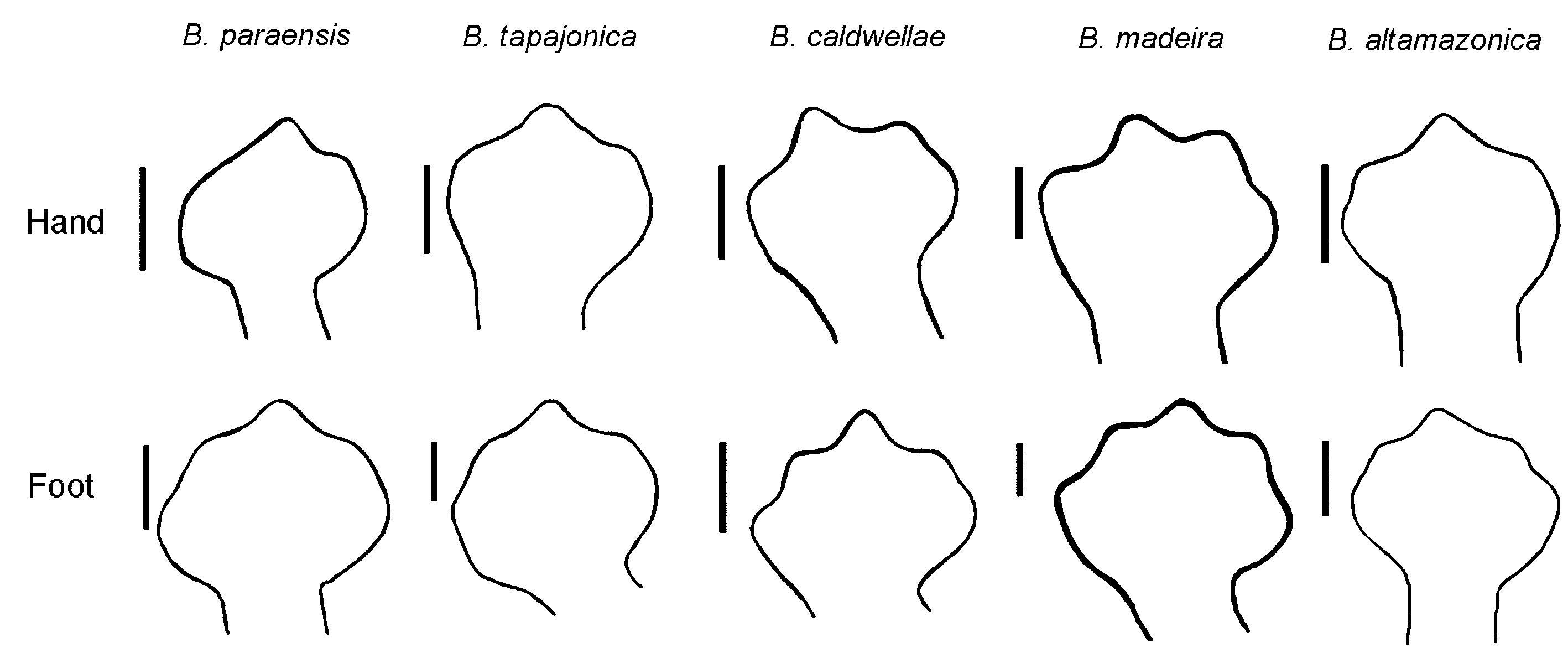
Comparisons with other species. Distinguished from all other genera of Neotropical salamanders by extensive digital webbing, 13 costal grooves between the limbs and the absence of a sublingual fold. A moderately small and relatively slender species of Bolitoglossa , with digits completely webbed, morphologically similar to other species of Bolitoglossa of the Brazilian Amazonia . It can be distinguished from other Amazonian species of Bolitoglossa by the following characteristics (condition for B. paraensis in parentheses): B. altamazonica : hands and feet extensively webbed, tips of all digits of hands and feet visible (extensively webbed but only the tips of third finger and third toe visible, Figure 2 View FIGURE 2 ); SL in adult males 33.1–46.8 mm, mean 39.0 mm (28.9–41.7 mm, mean 35.6 mm) and in adult females 43.3–54.1 mm, mean 47.0 mm (31.4–48.2 mm, mean 38.8 mm); FW/ HLL ≥ 40% in adult males and females (≤ 40% in adult males and females); TL/SL in adult males 70–120% (80–100%). B. peruviana : SL in adult males 25.2–32.1 mm (28.9–41.7 mm) and in adult females 30.1–42.5 mm (32.8–48.2 mm); MT/SL in adult females 0.3–1.4 teeth per mm (0.3–1.0); SL/HW in adult male 5.2–6.3 times (6.7–7.6 times) and in adult females 5.9–7.0 times (6.3–8.9 times); SL/FW in adult males 9.4–10.3 times (10.2–13.3 times) and in adult females 10.0–12.0 times (11.2–16.5 times). Bolitoglossa tapajonica sp. nov.: SNL/HL ≤ 44% in adult males and females (≥ 40% in adult males and females); LWS/HL ≤ 76% in adult males (>70%); tail becoming thinner at about one-third of its length (tail becoming thinner at about half of its length); PT in adult males 3–5 (1–3); DT in adult males and females> 31, mean 48 (18–68, mean 41). B. caldwellae sp. nov.: ventral surface light brown with very distinct irregular, broad, cream blotches (light brown or gray with tiny cream spots); wide head (narrow head), SL/ HW 6.3–6.9 times in adult males (6.7–7.6 times) and 6.1–7.0 times in adult females (6.3–8.9 times); extensively webbed hands and feet with the tip of the fingers and toes visible (extensively webbed but just de tip of third finger and third toe visible); SNL/HL ≤ 40% in adult males and females (≥ 40%), TL/SL in adult males 60–90%, mean 80% (80–100%, mean 90%) and in adult females 60–90%, mean 70% (70–100%, mean 90%); PT in adult males 2– 4, mean 3 (1–3, mean 2). B. madeira sp. nov.: extensively webbed hands and feet with the tip of fingers and toes visible (extensively webbed but just the tip of third finger and third toe visible); SL in adult females 49.8–58.9 mm (31.4–48.2 mm); DT in adult females 53–78, mean 65 (18–61, mean 41); VT in adult females 20–33, mean 26 (8– 28, mean 15). For comparison of selected morphometric and dentition characters for B. altamazonica , B. paraensis , B. peruviana , B. tapajonica sp. nov., B. caldwellae sp. nov. and B. madeira sp. nov. see Table 3.
Measurements (in mm) and counts of neotype (MPEG 31682). Total length 72.4; SL 37.7; SVL 35.2; HL 6.0; HW 5.8; HD 3.3; SGF 2.5; EYW 1.4; EYL 2.5; SNL 2.8; SP 1.2; SWS 4.8; LWS 3.0; EN 1.8; OD 1.9; DBE 3.8; IDE 1.8; WMG 2.7; LMG 3.0; NGGF 2.5; SA 12.3; AG 20.0; PECW 4.3; interval between adpressed fore- and hind limbs 5.5 costal folds; 13 costal grooves; FL 7.1; HLL 8.1; HDW 2.3; FW 3.3; LIIIF 2.5; LIIIT 2.8; LVT 2.5; TL 34.7; TW 3.1; TD 3.4; VL 2.5; number of teeth: PT 2, MT 13–14, VT 7–7, DT 33–29.
Coloration of the neotype in life. Dorsal surface of body gray with blotches in different degrees of light or dark brown. Some white spots along the body. Flanks with whitish spots. Ventral surface dark gray with evenly distributed white spots. Snout with cream blotches between nostrils and above tip of nasolabial protuberances. Tail lighter than back, brownish with white spots on dorsal and lateral surfaces. Iris pale golden. Mental gland whitish.
Coloration of the neotype in preservative ( Figure 6 View FIGURE 6 ). Dorsal surface of head and body dark gray with some reddish brown blotches. Ventral surface light brown with evenly distributed tiny cream spots. Snout with cream blotches between nostrils and above the tip of nasolabial protuberances. Mental gland apparent and cream.
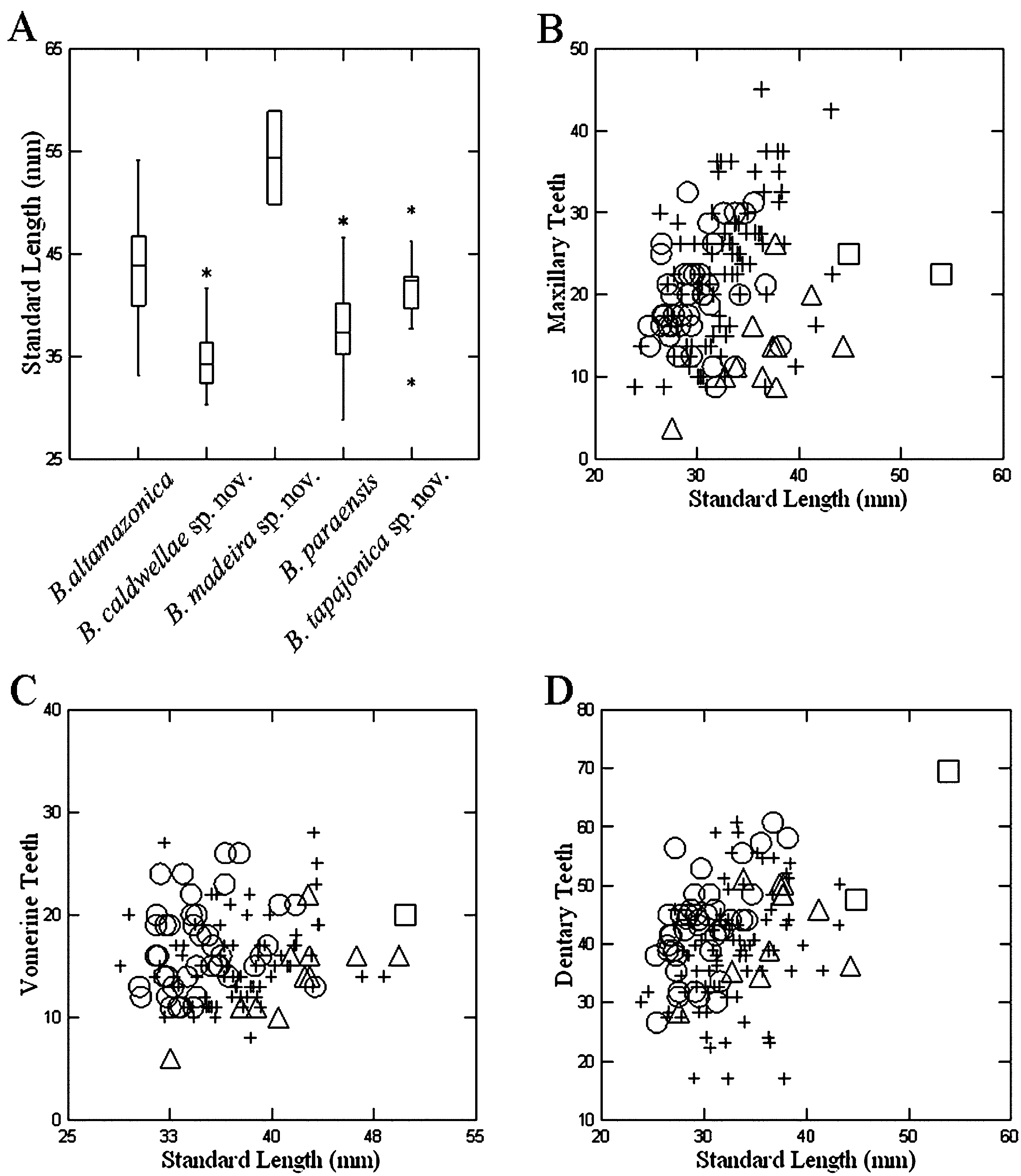
Description. Relatively slender species, small to moderate size. Maximum total length 95.1 mm. SL paratypes 28.9–41.7 mm (mean 35.6 mm) in 28 adult males, 31.4–48.2 mm (mean 38.8 mm) in 57 adult females. Adult females are significantly larger than adult males (SL) (p<0.000; t=3.985; df=57.877). Species with moderately slender head; SL/HW 6.7–7.6 times (mean 7.1 times) in males, 6.3–8.9 times (mean 7.2 times) in females; head flattened, about 0.9–1.2 times as long as wide, head slightly wider than neck. Eyes prominent, horizontal orbit diameter on average 85% of the snout length. Eyes protrude slightly beyond lateral margins of head. Nasolabial protuberances evident and modestly developed in males and females. Snout short, SNL/HL 40–50% (mean 40%), broad, SWS/HL 45–70% (mean 60%) in males, 40–60% (mean 50%) in females. Snout slightly rounded or truncate in dorsal and lateral views. Canthus rostralis not distinct. Nostrils small, located near tip of snout. Mental gland present only in males, oval; WMG 1.6–3.5 mm (mean 2.5 mm), LMG 1.6–3.0 mm (mean 2.2 mm). Body cylindrical, 13 shallow costal grooves. Limbs slender and short, costal interspaces between adpressed fore- and hind limbs 2.5–5.5 (mean 2.5). Hands and feet moderately broad, completely webbed with distal phalanges of longest digits free. Fingers in order of decreasing length 3–2–4–1, toes 3–2–4–5–1. Original tail round in crosssection, becoming thinner at about half its length; moderate length, TL/SL 75–103% (mean 90%) in 25 males, 71– 99% (mean 86%) in 56 females. Number of teeth in adults: PT 1–3 (mean 2) in males, 0–4 (mean 1) in females. The number of maxillary teeth increases with length (r2 = 0.22; p<0.05), MT 12–34 (mean 23) in 27 males, 12–41 (mean 24) in 56 females ( Figure 7 View FIGURE 7. A B). No relation between the number of vomerine teeth and length (r2 = 0.03; p=0.10), VT 10–27 (mean 16) in 26 males, 8–28 (mean 15) in 51 females ( Figure 7 View FIGURE 7. A C). The number of dentary teeth increases with length (r2 = 0.10; p<0.05), DT 18–68 (mean 46) in 27 males, 18–61 (mean 41) in 54 females ( Figure 7 View FIGURE 7. A D).
Color variation in preservative. Dorsally always darker than ventrally. Some specimens with uniform dorsal coloration, whereas others are mottled or with a mid-dorsal stripe, either lighter or darker than the ground color. Dorsum from dark gray to reddish brown. Most individuals have a triangular dark brown marking between the eyes, with apex extending posteriorly, following mid-dorsal line. Venter from dark gray to light brown, often flecked with white spots. Snout cream in many individuals, others have cream spots mainly on the nasolabial protuberances. Some specimens have white blotches on the limbs, flanks and on the lateral surfaces of tail. Mental gland cream.
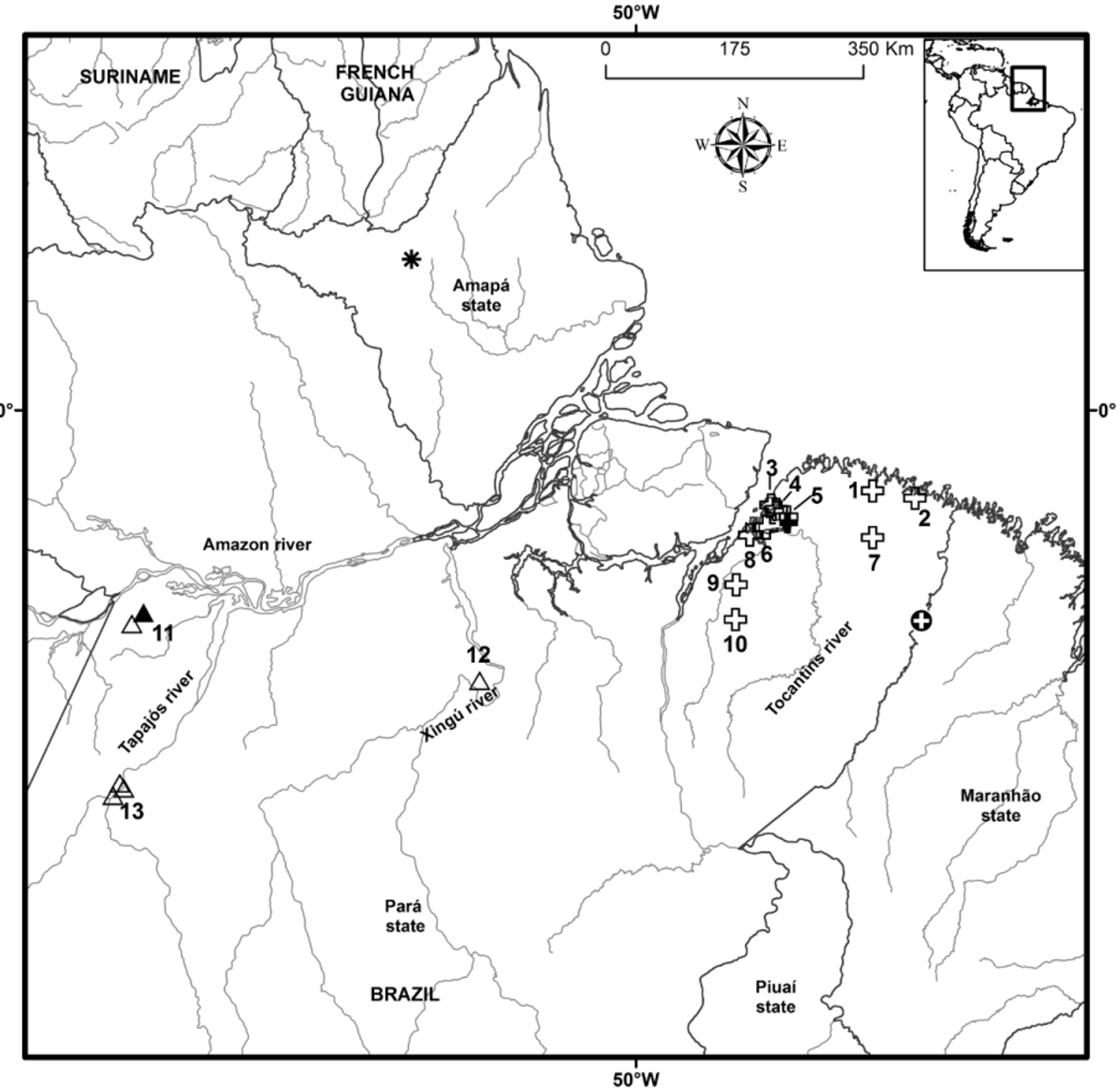
Habitat and range. Known from the eastern part of the state of Pará, Brazil, in the west from the Pará river, and localities on both sides of the Guamá River, both southern affluents of the Amazon River, in the east close to the Atlantic coast and to the border with Maranhão, in the municipalities Barcarena, Belém, Bragança, Benevides, Canindé, Mojú, Ourém, Primavera, Santa Bárbara do Pará, Santa Isabel do Pará, and Tailândia ( Figure 8 View FIGURE 8 ). Localities are from 10 to 40 m above sea level. Specimens were found active at night between 0.3 and 2.6 m above the ground on leaves of small shrubs (about 60% of individuals), but also can be observed on leaf litter on the ground ( Neckel-Oliveira et al. 2011) or using cavities in or under the bark of trees ( Correa et al. 2012). The neotype was collected active at night on a leaf of a bush at 0.6 m above the ground, near a permanent pond in forest. The species was not observed in open areas and occurs in different types of vegetation: primary terra firme forest, secondary forest, floodplain forest, igapó, capoeira ( Crump 1971) or near monocultures of passion fruit ( Passiflora edulis ), cocoa ( Theobroma cacao ) or African oil palm ( Elaeis guineensis ) (Estupiñán 2007). The abundance of the species in terra firme and floodplain forest and in capoeira is greater than in transitional areas between terra firme and floodplain forests ( Crump 1971). In places where it occurs it is abundant and easy to observe from December to March, which is the period of higher rainfall and humidity in the region. In this season, in nine days of observations realized in an area of forest in Benevides 15 individuals were found (Estupiñán 2007), and in Santa Bárbara do Pará 104 individuals were observed in eight days during a two year period, whereas on four other days no specimens were recorded ( Neckel-Oliveira et al. 2011). In Santa Isabel do Pará 30 specimens were observed by the senior author in just over two hours in a single night. Bolitoglossa paraensis is listed as Vulnerable in the list of threatened species in the state of Pará ( SEMA-PA 2008) and Subir et al. (2012) in an evaluation of all Brazilian amphibians based on four workshops in 2011 and 2012 with specialists considered it endangered [EN B1ab(iii)]. However, these categorizations might need to be reviewed, since this study provides new, positive data about distribution, habitat and abundance of the species.
Remarks. Based on two specimens of Bolitoglossa (male and female, DZSP 22463–64) collected in Canindé near the Gurupi river—boundary between Par and Maranhão states—about 230 km southeast of Belém, Wake and Brame Jr. (1966) mentioned that the last location [= Canindé] establishes the presence of the genus in eastern Brazil beyond the Amazon drainage. This notice is generally correct, although it should be noted that this area still belongs to the Amazon region with Amazonian vegetation, although the Gurupi drains directly in the Atlantic Ocean and as such is not part of the Amazon drainage proper. Unfortunately, it was not possible to study these specimens. Recently specimens of Bolitoglossa were collected in two localities in the state of Pará (PA), Ourém and Bragança, respectively about 150 and 170 km from the type locality of B. paraensis . These individuals were analyzed, but due to poor preservation and low number of specimens collected we only tentatively identify them as B. paraensis because of some similarities and geographic proximity to the area of occurrence of that species. In 2006 an expedition to the Serra do Tumucumaque (=Tumuc Humac Mountains), Amapá state, Brazil, north of the Amazon river, reported the occurrence of a specimen identified as Bolitoglossa cf. paraensis by Lima (2008). We examined the specimen (IEPA TQ 849), but because it is juvenile we could not confirm its specific identity beyond doubt, so we decided not to include its morphometric and meristic data in the morphological variation of B. paraensis . The record of Bolitoglossa in Amapá is remarkable because it is the first record for the Guiana region of a species of Bolitoglossa ( Señaris & MacCullough 2005; Lima 2008). In countries such as Suriname or French Guiana, where the amphibian fauna is relatively well studied, this genus has not been recorded ( Lescure & Marty 2000; Hoogmoed 2013, in press). Thus, it is necessary to confFirm this record by new fieldwork in the Tumucumaque area and to collect more (adult) specimens, in order to confirm the relationship of the Amapá population with B. paraensis . Assuming that the specimen reported by Lima (2008) is a specimen of B. paraensis , its distribution pattern would coincide with that known for some other species (eg. Plica plica Linneaus, 1758; Caecilia gracilis Shaw, 1802 ; Siphonops annulatus Mikan, 1820 ; Norops fuscoauratus Duméril & Bibron, 1837 ; Potomotyphlus kaupii Berthold, 1859 ; Gonatodes nascimentoi Sturaro & Avila-Pires, 2011 ), which occur both in the Guiana region, north of the Amazon river, and in the Belém area of endemism, south of the Amazon river ( Avila-Pires 1995; Maciel & Hoogmoed 2011, Sturaro & Avila-Pires 2011).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Bolitoglossa paraensis ( Unterstein, 1930 )
| Brcko, Isabela Carvalho, Hoogmoed, Marinus Steven & Neckel-Oliveira, Selvino 2013 |
Bolitoglossa (Eladinea) paraensis
| Parra-Olea 2004: 335 |
Bolitoglossa altamazonica
| Bartlett 2003: 28 |
| Schargel 2003: 94 |
| Riva 2000: 52 |
| Frost 1985: 574 |
| Duellman 1978: 81 |
| Wake 1976: 41 |
Oedipus altamazonicus
| Myers 1945: 7 |
Bolitoglossa paraensis
| Peloso 2010: 669 |
| Esqueda 2009: 172 |
| Galatti 2007: 93 |
| Taylor 1944: 219 |
estheri
| Miranda-Ribeiro 1937: 42 |
Oedipus paraensis
| Schmidt 1951: 443 |
| Myers 1945: 5 |
| Unterstein 1930: 271 |