Paradoris dubia, Bergh, 1904
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2006.00219.x |
|
persistent identifier |
https://treatment.plazi.org/id/575787C8-3B25-FFE2-FC1F-F91BDB440BE8 |
|
treatment provided by |
Felipe |
|
scientific name |
Paradoris dubia |
| status |
|
PARADORIS DUBIA View in CoL ( FIGS View Figure 5 View Figure 6 View Figure 7 View Figure 8 View Figure 9 View Figure 10 View Figure 11 View Figure 12 View Figure 13 View Figure 14 View Figure 15 View Figure 16 5–16)
Discodoris dubia Bergh, 1904: 50–51 View in CoL , plate III, figs 29, 30, plate IV, figs 1, 2. – Basedow & Hedley, 1905: 139. – Burn, 1969: 83–84, fig. 18. – Burn in Thompson,
1975: 515. – Coleman, 2001: 55–56, two unnumbered figures.
Discodoris dubia View in CoL (var.) Bergh, 1904: 52–53, plate IV, figs 3–6.
Paradoris dubia View in CoL . – Dayrat & Gosliner, 2005.
Discodoris View in CoL ? egena Bergh, 1904: 54–55 View in CoL , plate III, fig. 31, plate IV, figs 7–14. – Basedow & Hedley, 1905: 140.
Alloiodoris marmorata View in CoL . – Basedow & Hedley, 1905: 152, plate viii, figs 1, 2. – Burn, 1957: 19 (not Alloiodoris marmorata Bergh, 1904 View in CoL ).
Alloiodoris nivosus Burn, 1958: 29–30 View in CoL , fig. 6, plate 2, fig. 14. – Burn, 1962, 154–156, figs 4–6. – Burn, 1966: 274.
Paradoris leuca Miller, 1995: 901–907 View in CoL , figs 1–4. New synonym.
Type material: Holotype of dubia , by monotypy: northwestern coast of Tasmania, [no collecting date], one specimen 15/ 10 mm preserved, leg. miss Bodder ( ZMUC GAS-2063). Bergh, who dissected this specimen, only left the body wall and some pieces of the digestive gland in the jar. The body wall is largely destroyed, in particular in the oral area.
The two syntypes of the variety of dubia and the two syntypes of egena could not be found at the ZMUC; they are probably lost.
Holotype of nivosus , by original designation: Australia, Victoria, Shoreham , 18 September 1957, one specimen 25/ 18 mm preserved, leg. R. Burn ( NMV F19468 View Materials ). Externally, the holotype is well preserved and was not dissected.
Holotype of leuca , by original designation: New Zealand, Otago Harbour, Aquarium Point , 16 August 1962, one specimen 25/ 18 mm preserved, leg. M. Miller ( MNZ M.117832). One paratype: New Zealand, Otago Harbour, Aquarium Point, 19 August 1962, one specimen 15/ 7 mm preserved, leg. M. Miller ( MNZ M.117833). Externally, the holotype is well preserved. It was open dorsally, along the median line, certainly by Miller, but all the internal organs are still present inside the body. The paratype was dissected for the present study.
Additional material dissected: Australia, New South Wales, Bittangabee Bay , 37°13′S, 150°01.1′E, 25 February 1983, two specimens 15/9 (#1) and 11/8 (#2) mm preserved, leg. W. F. Ponder, identified as Discodoris by W. B. Rudman ( AM C137991 ) GoogleMaps ; Australia, Victoria, [Port Phillip Bay], off Mordialloc , 14 December 1958, one specimen 25/ 17 mm preserved, leg. R. Burn, identified as D. dubia by R. Burn ( NMV F20111) [R. Burn originally identified this specimen as Alloiodoris nivosus ]; Australia, Victoria, [Port Phillip Bay], Corio Bay , [no collecting date], one specimen 20/ 13 mm preserved, [leg. unknown, probably R. Burn], identified as D. dubia by R. Burn ( NMV F18237) [R. Burn originally identified this specimen as Alloiodoris nivosus ]; Australia, Victoria, [ Port Phillip Bay ], [no collecting date], one specimen 7/ 5 mm preserved, [leg. unknown, probably R. Burn], identified as D. dubia by R. Burn ( NMV F26015) [R. Burn originally identified this specimen as Alloiodoris nivosus ]; Australia, Victoria, Flinders , front beach, [no collecting date], two specimens 10/8 and 8/ 5 mm preserved, [leg. unknown, probably R. Burn], identified as D. dubia by R. Burn ( NMV F13684) ; Australia, Victoria, [Port Phillip Bay], Portarlington , [no collecting date], one specimen 20/ 10 mm preserved, [leg. unknown, probably R. Burn], identified as D. dubia by R. Burn ( NMV F20960) [this specimen was dissected prior to the present study, the radula was removed]; Australia, Victoria, Philip Island, Kitty Miller Bay , [no collecting date], one specimen 20/ 12 mm preserved, [leg. unknown, probably R. Burn], identified as D. dubia by R. Burn ( NMV F21247) [R. Burn originally identified this specimen as Alloiodoris nivosus ; this specimen was open prior to the present study]; Australia, Victoria, Philip Island, Sunderland Bay , 13 January 1957, six specimens 22/ 12 (#1), 15/11 (#2), 15/10 (#3), 10/6 (#4), 8/6 (#5), and 8/5 (#6) mm preserved, [leg. unknown, probably R. Burn], identified as D. dubia by R. Burn ( NMV F20962) [R. Burn originally identified these specimens as Alloiodoris nivosus ; the largest specimen was dissected and the radula was removed prior to the present study]; Australia, South Australia, Eyre Peninsula, Louth Bay, Point Warna , 34°32′S, 135°56′E, 11 February 1985, 13 specimens 20/12 (#1), 18/8 (#6), 16/ 10 (#7), 14/10 (#2), 14/7 (#9), 13/9 (#3), 13/9 (#8), 13/7 (#10), 11/8 (#11), 9/5 (#4), 6/4 (#5), and 6/5 (#12 and #13) mm preserved, leg. W. B. Rudman, I. Loch, and G. A. Avern, identified as D. dubia by W. B. Rudman ( AM C145110 ) GoogleMaps ; Australia, South Australia, Yorke Peninsula, Bluff Beach , 34°44′S, 137°29′E, 23 February 1985, four specimens 22/15 (#1), 17/12 (#2), 17/12 (#3), and 8/6 (#4) mm preserved, leg. W. B. Rudman, I. Loch, and G. A. Avern, identified as D. dubia by W. B. Rudman ( AM C145212 ) GoogleMaps ; Australia, South Australia, Gleesons Landing, Yorke Peninsula , 35°01′S, 136°58′E, February 1985, one specimen 14/ 8 mm preserved, leg. W. B. Rudman, I. Loch, and G. A. Avern, identified as D. dubia by W. B. Rudman ( AM C145197 ) GoogleMaps [specimen not dissected for the present study]; Australia, South Australia, Eyre Peninsula, south end Tumby Bay , 34°23′S, 136°07′E, 16 February 1985, three specimens 12/7, 11/6, and 11/ 5 mm preserved, leg. W. B. Rudman, I. Loch, and G. A. Avern, identified as D. dubia by W. B. Rudman ( AM C145160 ) GoogleMaps [specimens not dissected for the present study]; Australia, South Australia, Yorke Peninsula, Point Souttar , 34°54′S, 137°16′E, 19 February 1985, one specimen 10/ 6 mm preserved, leg. W. B. Rudman, I. Loch, and G. A. Avern, identified as D. dubia by W. B. Rudman ( AM C145174 ) GoogleMaps ; Australia, South Australia, Port Lincoln, Billy Lights Point , 34°45′S, 135°53′E, 15 February 1985, five specimens 20/12 (#1), 17/14 (#2), 14/12 (#3), 12/11 (#4), and 12/9 (#5) mm preserved, leg. W. B. Rudman, I. Loch, and G. A. Avern, identified as D. dubia by W. B. Rudman ( AM C145149 ) GoogleMaps ; Australia, Tasmania, [south-eastern coast of Tasmania], Frederick Henry Bay, Carlton Point, Red Ochre Beach , 23 November 1968, 13 specimens 25/20 (#1), 23/17 (#2), 20/15 (#3–8), 17/13 (#9) 14/12 (#10), 13/9 (#11), 10/8 (#12), and 8/5 (#13) mm preserved, leg. B. J. Smith, identified as D. dubia by R. Burn ( NVM F86424 View Materials ) [specimens 5–8 were not dissected for the present study]; Australia, Western Australia, Cockburn Sound, Palm Beach , 15 December 1971, 5 m depth, one specimen 15/ 12 mm preserved, leg. Neville Coleman, identified as Discodoris cf. dubia by Robert Burn ( NVM F86485 View Materials ) .
Type localities: North-western coast of Tasmania ( dubia , dubia var., egena ); Shoreham, Victoria, Australia ( nivosus ); Aquarium Point, Otago Harbour, New Zealand ( leuca ).
In the original description of nivosus, Burn (1958) mentioned ‘many’ additional specimens that were not part of the type material collected from two other localities, Portarlington and Phillip Island, both on the coasts of Victoria, Australia . Also, in the original description of leuca, Miller mentioned ten additional, nontype specimens: nine from the type locality, and one from Quarantine Island , also on the east coast of New Zealand .
Distribution: South-western Pacific Ocean (from New Zealand to South Australia) and south-eastern Indian Ocean (Western Australia): east coast of South Island, New Zealand ( Miller, 1995, as P. leuca ; present study), New South Wales (present study), Victoria ( Burn, 1957, 1958, 1966, as Alloiodoris nivosus ; Burn, 1969, as D. dubia ; present study), Tasmania ( Bergh, 1904, as D. dubia , D. dubia var., and D. egena ; present study), South Australia ( Basedow & Hedley, 1905, misidentified as Alloiodoris marmorata ; Burn, 1962, as Alloiodoris nivosus ; present study), Perth region, Western Australia ( Coleman, 2001; present study). In a checklist of Australian dorid species, Burn (in Thompson, 1975) mentioned the presence of dubia in Western Australia without providing material (he may have referred to the specimen collected by Coleman, which I dissected for the present study). In the future, one should expect to find dubia between Ceduna Bay, Great Australian Bight, South Australia ( Burn, 1962), and Perth, Western Australia.
Occurrence: The majority of the specimens that I found in museum collections or mentioned in the literature have been collected from Victoria and South Australia. This indicates that dubia might be more common in those waters than in Tasmania, New South Wales, New Zealand, or Western Australia, although one cannot fully exclude that sampling efforts have been biased. The only two specimens known from the New South Wales are held by the Australian Museum. The fact that I could borrow many other discodorids collected from New South Wales from the Australian Museum makes me think that dubia may really be more rare in New South Wales than in Victoria or South Australia. Miller (1995) only mentioned ‘few records (…) over the past 40 years’ of P. leuca on the east coast of South Island, New Zealand. In summary, Victoria and South Australia are probably the ‘heart’ of the geographical distribution of dubia ; other localities may represent the edges of its distribution.
Habitat: According to the literature and museum labels, dubia is found: under stones, between tides, usually in muddy positions ( Burn, 1958), under rock ( MNZ M.117832), on sponge under rock ( MNZ M.117833), nestling in sponge ( NMV F20111), on rock platform ( NMV F86424 View Materials ), on rocks ( AM C145110), limestone bench ( AM C145212), sheltered rocks ( AM C145160; AM C145174), rocky spit ( AM C137991), on muddy reef ( NMV F86485 View Materials ).
Literature: Several publications provide information about the colour and the external morphology ( Burn, 1969). As far as I can discern, only two colour pictures were published ( Coleman, 2001; as dubia ). However, these pictures were not accompanied by an anatomical description. Anatomical data are provided by the original descriptions (see my remarks below) and in two additional contributions by Burn (1962, 1969). However, several mistakes have been made and several features have been overlooked, especially in the reproductive system. Burn discussed infra-specific variation for two features: the dorsal colour and the dorsal granulation. Character variation is discussed here for the entire anatomy.
Remarks on the original description of dubia ( Fig. 6J View Figure 6 ): Bergh (1904) described a whitish dorsal notum, although he indicated that the notum of the live animal was shining reddish-brown, probably based on notes from the collector, Miss M. Bodder. Currently, the holotype is creamish, with some faded, darker brown areas. The ventral surface is creamish. The dorsal notum is smooth, with no indecora -like tubercles. Bergh did not describe the oral tentacles. The right oral tentacle is missing and the left one is largely destroyed. It is digitiform and grooved ( Fig. 6J View Figure 6 ), although the groove is poorly preserved. According to Bergh, the rhinophores (with 20 lamellae) and the five branchial plumes are yellow. The jaws are supposed to be similar to the jaws of D. amboinensis Bergh, 1890 , which has only two lateral plates. The radular formula given by Bergh was 48 × (18-0-18) for a 15 mm long preserved specimen. Bergh (1904: plate IV, fig. 2) described that the hooks of the lateral teeth were ‘finely striated’, which certainly is what I refer to as ‘lateral groove’. Bergh’s original description of the reproductive system is not informative because of the lack of drawings. For example, the description of a ‘bent’ prostate is not precise and does not indicate whether Bergh saw that the prostate of dubia was cylindrical. However, Bergh described a large mucous gland, of a very bright white colour, and a spherical red-brown albumin gland.
Remarks on the original description of dubia var.: Bergh’s (1904) description of the variety of dubia is very similar to the description of dubia . Many characters are identical, such as the colour (yellowishwhite), the number of gills (five), the number of rhinophoral lamellae (20), a radular formula of 43 × (21-0- 21) for a 22 mm long preserved specimen, and the reproductive system. However, two features need to be commented on. First, Bergh described digitiform oral tentacles, but did not mention whether they were grooved longitudinally. Bergh probably missed the groove of the oral tentacles. Second, Bergh described and represented ( Bergh, 1904: plate IV, fig. 3) three jaw plates: two lateral plates and one additional, ventral, median plate.
Remarks on the original description of egena: Bergh (1904) described the colour of the dorsal notum of preserved specimens as bright dirty whitish with numerous irregular dirty grey spots and dots. Rhinophores and gills are red-brown in one specimen and yellow in the other. The ventral surface is yellowish in both specimens. Bergh described digitiform oral tentacles, but did not mention whether they were grooved longitudinally. Bergh probably missed the groove of the oral tentacles. According to Bergh, there are 20 rhinophoral lamellae and five gills. The labial cuticle bears three plates ( Bergh, 1904: plate IV, fig. 8). The radular formula given by Bergh was 43 × (17-0-17) for a 15 mm long preserved specimen (or perhaps a 10 mm long specimen, because Bergh did not indicate which individual he dissected). Bergh described ‘finely striated’ hooks, as in dubia . Bergh’s original description of the reproductive system is brief and not informative. Also, the reproductive system(s) that he observed was (were) either immature or incomplete because he could not find the prostate. Finally, note that Bergh did not observe a large white mucous gland in egena . As I demonstrate here with additional specimens, a large white mucous gland is only obvious in large individuals (c.> 15 mm length) with a fully mature reproductive system.
Remarks on the original description of nivosus: Burn (1958) described the dorsal notum of live animals as ‘pure white, sometimes margined with brown’, and the ventral surface (hyponotum and foot) as white with brown dots. Currently, the holotype is greyish, covered with indecora -like tubercles of various sizes. The preserved holotype of nivosus could not be distinguished from preserved specimens of indecora without the internal anatomy. Rhinophores were described as brown. The number of lamellae, not mentioned by Burn, could not be determined because the rhinophores are retracted within the body cavity. Burn described seven branchial plumes, white, tipped with brown. However, the gills are retracted and I could not count them. Burn simply described digitiform oral tentacles, but did not mention whether they were grooved longitudinally. The oral tentacles of the holotype, retracted and poorly fixed, could not be observed. However, the left oral tentacle seems to be grooved. Burn’s description of the jaws is confusing: ‘Labial armature weak, but there is a large, strong oral cuticle’. The radular formula given by Bergh was 37/ 40 × (18/19-0-18/19) for a maximum length of 30 mm. Burn did not describe the reproductive system.
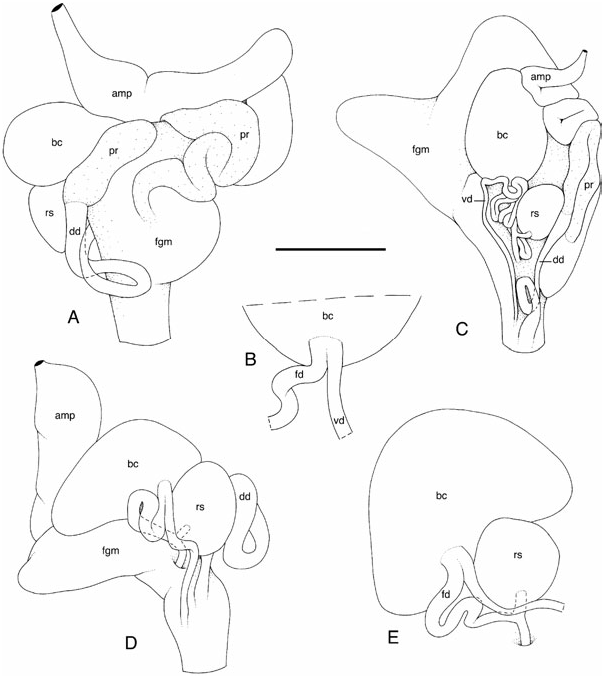
Remarks on the original description of leuca ( Figs 5 View Figure 5 , 6G, H View Figure 6 , 9A View Figure 9 , 15B, C View Figure 15 ): The original description of leuca by Miller (1995) is largely complete and accurate. All characters described by Miller were checked and a few additional characters were found in the paratype. The colour of live animals was described thoroughly by Miller: ‘White, mantle translucent with a subepidermal reticulum of opaque white, rhinophores, gills and foot translucent, viscera showing through sole of foot as grey. Rhinophore laminae, inner edges of tertiary pinnae of gills and dorsal surface of mantle speckled with dark reddish-brown pigment though it looks chocolate brown or black to the naked eye. Reddishbrown pigment very dense on rhinophoral laminae, less so on the gills and on the mantle consists of a few largish particles scattered irregularly and diffuse groups of evenly scattered very small particles (a dusting of pigment giving the appearance of light grey spots) on the tips of the tubercles.’ Currently, both the paratype and the holotype are homogeneously creamish. Miller described ‘low, hummock-like tubercles’ that are still distinguishable. Those indecora -like tubercles give a granular texture to the notum. Miller did not notice the presence of wide holes on the dorsal surface of the notum. Those holes are abundant on the margins of the notum ( Fig. 6H View Figure 6 ). According to Miller, there are up to 14 rhinophoral lamellae and up to six tripinnate gills. Miller described longitudinally grooved oral tentacles ( Fig. 6G View Figure 6 ). There are two lateral plates, and a third, ventral, median plate. Jaw plates can be easily observed on the holotype because its oral tube is devaginated. Miller drew pointed jaw rodlets. The latter are actually irregularly shaped, as usual: pointed and roundish ( Fig. 5B, C View Figure 5 ). The radular formula given by Miller was up to 48 × (21-0-21) for a specimen of a maximum length of 36 mm, alive. Miller carefully drew a groove on the outer edge of the tooth hooks, although he did not describe it. The radular formula of the paratype dissected for the present study was: 38 × (17-0-17) for a 15 mm long preserved specimen ( Fig. 5D–F View Figure 5 ). Miller’s drawing of the reproductive system is too schematic and does not give a good idea of the genital organization ( Fig. 15B, C View Figure 15 ). Also, Miller drew three features without pointing out that they were very unusual within Paradoris and Discodorididae : the huge mucous gland, the tubular prostate, and the fact that the vaginal and fertilization ducts are fused near the bursa copulatrix.
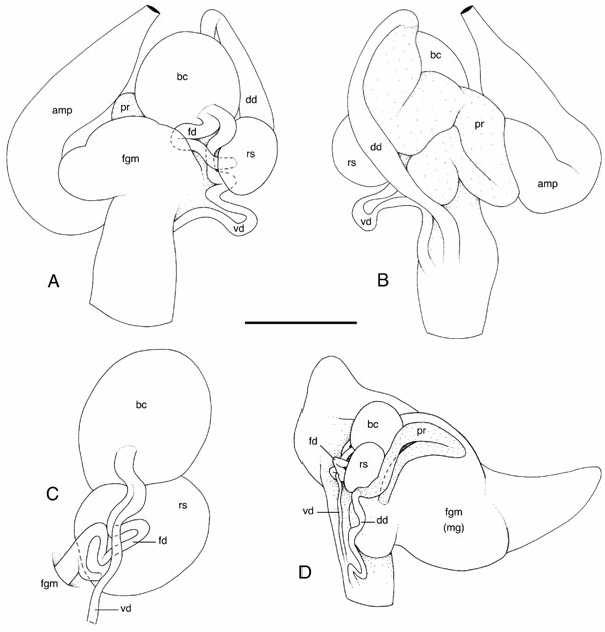
Description of new specimens ( Figs 6A–F, I View Figure 6 , 7 View Figure 7 , 8 View Figure 8 , 9B–E View Figure 9 , 10–14 View Figure 10 View Figure 11 View Figure 12 View Figure 13 View Figure 14 , 15A, B, D, E View Figure 15 , 16 View Figure 16 ): Unfortunately, no information about the colour of the live animals was available. Most specimens (e.g. Fig. 6A, B View Figure 6 ) have a creamish ground colour with traces of brown spots and minute brown dots on the dorsal surface (e.g. NMV F13684, NMV F20960, NMV F20961, NMV F20962, NMV F21247, NMV F86424 View Materials , AM C137991, AM C145110, AM C145160, AM C145174, AM C145212). The same specimens have a creamish ventral surface with ( NMV F20960, NMV F86424 View Materials , AM C137991, AM C145110, AM C145160, AM C145174, AM C145212) or without (e.g. NMV F13684, NMV F20961, NMV F20962, NMV F21247, NMV F86424 View Materials , AM C137991, AM C145110) brown dots. Some specimens are homogeneously greyish on the dorsal surface and creamish on the ventral surface, with ( NMV F18237) or without ( NMV F20111, NMV F26015, NMV F86424 View Materials ) brown dots. Finally, some specimens are homogeneously creamish on both the ventral and the dorsal surface ( NMV F20961, NMV F20962, AM C145197). The colour of preserved specimens is surely different from the colour of live animals. However, the fact that the preserved colour varies among specimens from the same locality suggests that the colour also varies among live animals. The rhinophores and gills vary from yellowish to brown.
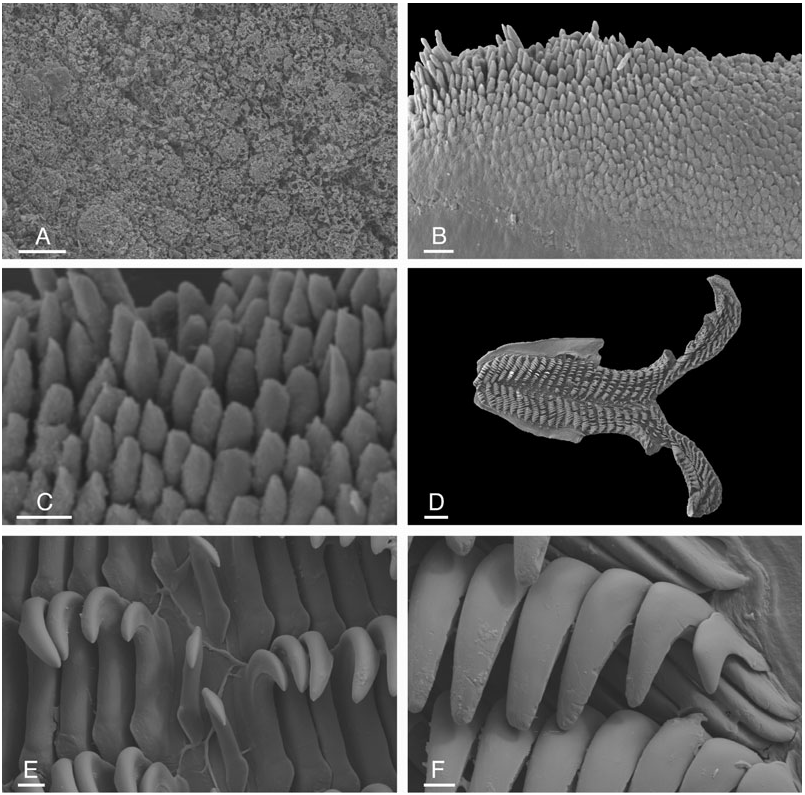
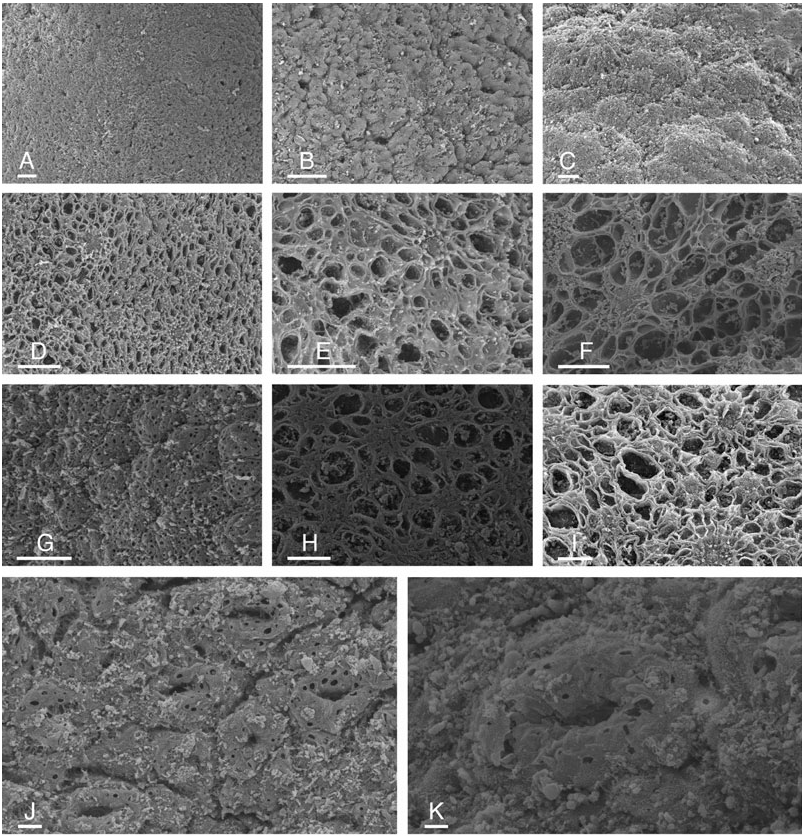
The body is oval, not significantly elongated. The largest specimens were 25 mm long preserved, i.e. approximately 35 mm long alive ( NMV F20111, NMV F86424 View Materials ). The foot is rounded posteriorly and anteriorly. The width of the foot equals approximately onethird or one-half the width of the dorsal notum (in preserved specimens). The anterior margin of the foot is bilabiate and the upper lip is notched ( Fig. 6B, D View Figure 6 ). The two oral tentacles are digitiform and grooved ( Figs 6B, D View Figure 6 , 8 View Figure 8 ), although the groove may be difficult to observe in cases where the tentacles are retracted and poorly fixed. The dorsal notum is granulated with tubercles of various sizes, some of which are indecora -like ( Fig. 6C, F View Figure 6 ). In some preserved specimens, the notum is almost smooth. Many small holes (diameter <10 µm) and many tufts of cilia are found on the surface of the dorsal notum, including gills and rhinophores. However, cilia are not clearly distinguishable, certainly due to the poor condition. Wide holes (diameter> 20 µm) are clearly present in a few specimens ( Figs 6I View Figure 6 , 7 View Figure 7 ). A network of hollows and ridges on the dorsal notum has obscured these wide holes in several specimens ( Fig. 7D–F, H, I View Figure 7 ). In preserved specimens, the margins of the rhinophoral and branchial sheaths are smooth or loosely crenulate ( Fig. 6A, C, F View Figure 6 ). There are between four and eight tripinnate branchial plumes arranged in a circle around the anus. The rhinophores have from ten to 20 lamellae, depending on the size of the specimens, but most of them have from 14 to 17 lamellae.
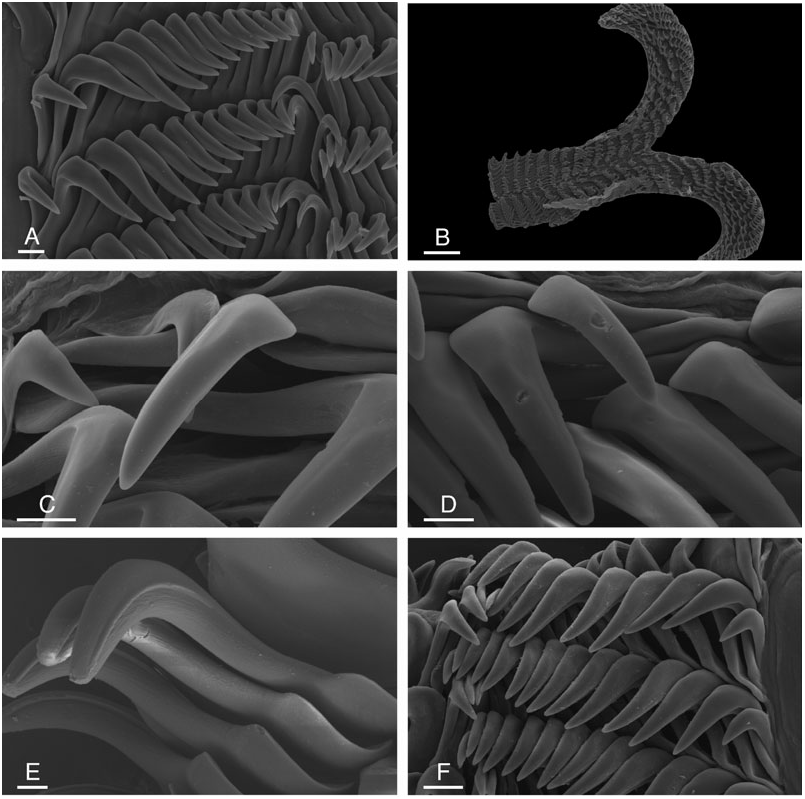
The stomach is large and free, on the left anterior side of the digestive gland, narrow and median, or intermediary ( Fig. 9B, C, E View Figure 9 ). A small caecum is located on the left posterior side of the stomach. The intestine is straight and dorsal. The labial cuticle is armed with a pair of lateral jaw plates, and an additional, ventral, median plate ( Fig. 10A, B View Figure 10 ). The rodlets are perpendicular to the surface of the labial cuticle, and seem to be ‘vertical’ ( Fig. 10C View Figure 10 ). Rodlet tips are irregular, pointed or rounded ( Fig. 10C–E View Figure 10 ). Some rodlet tips are significantly curved ( Fig. 10C View Figure 10 ). However, they do not bear a posterior pointed spur. The radula is elongated ( Figs 11 View Figure 11 , 12 View Figure 12 ): its length equals more than twice its width. The radular sac can be seen by dorsal dissection ( Fig. 9B, E View Figure 9 ). Radular formulae were: 28 × (14-0- 13) in an 8 mm long specimen ( NMV F86424 View Materials #13), 28 × (15-0-15) in an 8 mm long specimen ( NMV F20962 #5), 29 × (15-0-15) in a 7 mm long specimen ( NMV F26015), 30 × (16-0-16) in a 14 mm long specimen ( NMV F86424 View Materials #10), 34 × (14-0-13) in a 6 mm long specimen ( AM C145110 #5), 36 × (18-0-17) in a 15 mm long specimen ( NMV F20962 #2), 37 × (15-0-14) in a 9 mm long specimen ( AM C145110, #4), 37 × (17-0-16) in a 15 mm long specimen ( NMV F86485 View Materials ), 37 × (16-0- 16) in a 15 mm long specimen ( AM C137991 #1), 37 × (19-0-19) in a 25 mm long specimen ( NMV F20111), 39 × (20-0-20) in a 23 mm long specimen ( NMV F86424 View Materials #2), 38 × (20-0-20) in a 13 mm long specimen ( AM C145110 #3), 40 × (20-0-20) in a 22 mm long specimen ( AM C145212 #1), 42 × (21-0-21) in a 25 mm long specimen ( NMV F86424 View Materials #1), 42 × (20-0- 20) in a 14 mm long specimen ( AM C145110, #2), 50 × (20-0-20) in a 20 mm long specimen ( AM C145110, #1), 38 × (17-0-17) in a 15 mm long specimen ( MNZ M.117833). The rachidian teeth are absent and the rachidian space is narrow. The rows of lateral teeth are at an angle of approximately 45 degrees with the rachidian axis. The size of the lateral teeth increases gradually towards the edges, and the size of the four or five outermost teeth decreases gradually. All teeth are hamate; the outermost tooth may be vestigial. The hook of all lateral teeth is grooved on its outer edge. Teeth have no denticles.
The cerebral and pleural ganglia are fused ( Fig. 9D View Figure 9 ). The length of the circum-oesophageal nerve ring equals one to three times the width of the cerebropleural ganglia. They may or may not be fused with the pedal ganglia. Their surface is smooth.
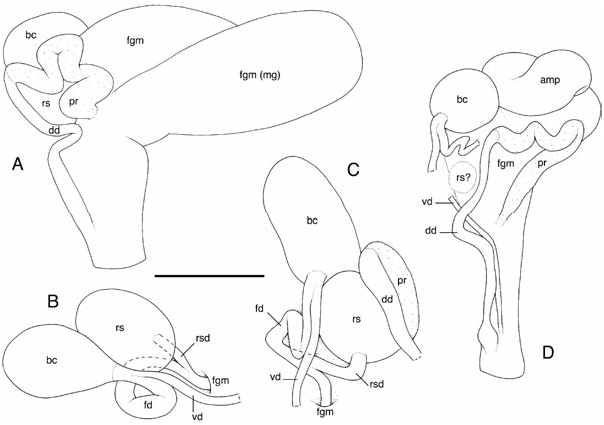
The reproductive system is located on the right side of the body, between the buccal mass and the digestive gland ( Figs 9B, C, E View Figure 9 , 13 View Figure 13 , 14 View Figure 14 , 15A, D, E View Figure 15 , 16 View Figure 16 ). The white ampulla is convoluted, with one or several loops before it disappears into the female gland mass. The division between male and female ducts could not be seen by dissection (it is hidden within the female gland mass). The prostate is homogeneously yellow and tubular ( Figs 13A, D View Figure 13 , 14B, C, E View Figure 14 , 15A View Figure 15 , 16B, D View Figure 16 ). In large specimens, the prostate tends to be U-shaped, with a long and tight loop. In younger specimens, it is more convoluted (up to six or seven loose loops). The white deferent duct can be straight or loosely convoluted. There is no penial papilla. The vaginal duct is more or less straight or very loosely convoluted, but significantly narrow. The fertilization duct is tightly convoluted, with at least three or four loops. The vaginal and fertilization ducts are fused near the bursa copulatrix ( Figs 13B–D View Figure 13 , 14A View Figure 14 , 15D, E View Figure 15 , 16A, C View Figure 16 ). The duct of the receptaculum seminis is short and straight, but distinct. The connection between the fertilization duct and the female gland mass is marked by a short duct. The deferent duct and the vaginal duct join together and form a vestibule that may or may not remain separate (at least externally) from the female duct. The disappearance of the fertilization duct into the female gland mass (where it connects to the fertilization chamber) is hidden by the receptaculum seminis in most cases; it is seldom distinguishable without moving the receptaculum seminis. The size of the bursa copulatrix equals from one to three times the size of the receptaculum seminis. On occasion, it can also be smaller. However, in most cases, the bursa copulatrix is slightly larger than the bursa copulatrix. Both pouches are spherical-ovate. The surface of the spermatic pouches is smooth. Finally, in fully mature specimens, the white, mucous gland is huge, not very well delimited, and can almost cover the rest of the reproductive system ( Fig. 14B View Figure 14 ).
Infra-specific character variation: Burn (1969) discussed the infra-specific variation within dubia . Concerning the variation in the colour of the dorsal notum, Burn (1969) rightly pointed out that: ‘This is a variable species where specimens range in colour from pure white (nivosa) sometimes with a brown notal edge, to dark pinkish grey with brown rosettes on the notum ( marmorata of Basedow and Hedley)’. Indeed, dubia presents the same range of variation as indecora for the background colour and dark dots. The variation that I observed for the granulation of the dorsal notum of preserved specimens was also mentioned by Burn: the notum can be almost smooth or distinctly granulated with indecora -like tubercles of various sizes.
Burn thought that strongly armed (e.g. Bergh, 1904) or smooth (e.g. Burn, 1958) labial cuticles could be the ‘result of differing methods of preservation’. I obtained smooth labial cuticle for two specimens ( AM C145110 #1 and #3). However, I have always considered that this was suspicious and certainly not natural. A possible explanation is that I mounted these two labial cuticles on the wrong side (and the rodlets could not be seen). I know that I made this regrettable mistake in a few preparations for other discodorids. Of course, the fact that some specimens could have an unarmed labial cuticle cannot be excluded. Clearly, the presence of three jaw plates in dubia is the rule; if the absence of jaws is natural in some cases, it shall be a rare exception. Finally, I observed three jaw plates in all specimens with an armed labial cuticle: I think that the presence of only two jaw plates in the original description of dubia was a mistake.
In total, seven radular formulae have been provided prior to the present study. First, let us repeat the radular formulae from the original descriptions: 48 × (18- 0-18) for a 15 mm long specimen ( dubia ), 43 × (21-0- 21) for a 22 mm long specimen ( dubia var.), 43 × (17-0- 17) for a 10 or 15 mm long specimen ( egena ), 37/ 40 × (18/19-0-18/19) for a maximum 30 mm long, probably alive, specimen ( nivosus ), and 48 × (21-0-21) for a maximum 36 mm long live specimen ( leuca ). Burn (1958) also gave an additional radular formula for two specimens from South Australia: 33/44 × (19/21-0-19/ 21) for a maximum length of 22.5 mm. The radular formula that Burn (1969) gave was probably a synthesis of his observations and the literature: 33/48 × (17/23- 0-17/23). In summary, when we consider the 17 radulae dissected here, the radular formula of dubia ranges from 28 × (14-0-13) in an 8 mm long specimen ( NMV F86424 View Materials #13, present study) to 42 × (21-0-21) in a 25 mm long specimen ( NMV F86424 View Materials #1, present study), 50 × (20-0-20) in a 20 mm long specimen ( AM C145110 #1, present study), and 48 × (21-0-21) for a maximum 36 mm long specimen, alive (original description of leuca ). Concerning the shape of the lateral teeth, my observations are not contradicted by the few drawings published prior to the present study: the hook of the outermost teeth has never been represented with a dorsal spur.
The descriptions of the reproductive system published so far are unclear or erroneous. Contrary to what Burn (1962) asserted, the hermaphroditic gland is not a discrete mass, independent from the liver. This feature is important because it led Burn to allocate nivosus to Alloiodoris (see Discussion). I believe that Burn confused the huge, white mucous gland with the hermaphroditic gland. Also, contrary to what Burn (1962) asserted, the reproductive system of dubia does not lack a prostate: the latter is narrow and tubular. Miller (1995: fig. 4A) represented a tubular prostate, but did not point out that a tubular prostate was a unique feature among Paradoris species. I could not observe any distinct, permanent penis. Burn (1969) admitted that the ‘four vertical rows of 4–6 minute and simple hooks’ he had described ( Burn, 1962) in the penial sheath were absent. I could not observe either the ‘minute wart-like papillae’ that Burn (1962) mentioned. I believe that if there is a copulatory organ in dubia , it is simply the evaginable, distal part of the deferent duct. Finally, note that the fertilization duct of the specimen from New Zealand ( MNZ M.117833) is more convoluted than the Australian specimens, but the topology and relative position of the ducts are exactly the same.
Diagnostic features: Two genital features are exclusively present in dubia and distinguish it efficiently from the other species of Paradoris . The first feature is easy to observe: the prostate is tubular ( Figs 13A, D View Figure 13 , 14B, C, E View Figure 14 , 15A, C View Figure 15 , 16B, D View Figure 16 ). The second character is more difficult to see, but distinctly present: the vaginal and fertilization ducts form a single duct (at least externally) near the bursa copulatrix ( Figs 13B–D View Figure 13 , 14A View Figure 14 , 15B, D, E View Figure 15 , 16A, C View Figure 16 ). In all the discodorids that I have dissected, the two ducts always enter (vaginal duct) and exit (fertilization duct) the bursa copulatrix separately.
Discussion: The first question we need to address is why dubia has been described under four species names allocated to three genera – Discodoris , Alloiodoris , and Paradoris . The fact that Bergh (1904) could describe the same species under two different names ( dubia and egena ) should not surprise the reader. There are several similar cases in Bergh’s work. He is not alone, however.
According to Bergh, egena differed from dubia because egena was supposedly lacking a prostate. For the same reason, he thought that egena was probably not a Discodoris (he described dubia as D. dubia , and egena as D.? egena ). However, Bergh certainly missed the prostate in egena : he made the same mistake in Geitodoris , a genus that he erroneously diagnosed by the lack of a prostate; Burn (1962) also missed the prostate in nivosus . Bergh also thought that dubia differed from egena because they supposedly did not have the same number of jaw plates. However, the fact that Bergh described three jaw plates in the variety of dubia suggests that he missed the third jaw in dubia . If one admits that Bergh missed the prostate in egena and the third jaw plate in dubia , both species then become equivalent. Of course, another explanation is that the holotype of dubia really lacked a third, ventral jaw plate, and that the two syntypes of egena really lacked a prostate. I personally see this as another argument against the fact that authors could describe and name new species based on a small number of specimens: all the specimens of dubia that I dissected had three jaw plates and a prostate; Bergh could have corrected his mistakes if he had observed additional specimens.
Bergh allocated dubia and egena to Discodoris rather than Paradoris because they lacked two important features that he had included in the diagnosis of Paradoris : grooved oral tentacles, and accessory glands and stylet sacs in the reproductive system. He obviously did not consider that the presence of three jaw plates, which he also cited in the diagnosis of Paradoris , could have been a reason to place egena in Paradoris (he described only two plates in dubia ). Also, the radular formulae did not influence his choice because he had not included the presence of an elongated radula in the diagnosis of Paradoris . One thing is clear: Bergh missed the grooved oral tentacles in the holotype of dubia . He probably missed the three jaws in dubia . Finally, the present cladistic analysis demonstrates that Paradoris species can lack accessory glands and stylet sacs.
The description of Alloiodoris nivosus by Burn (1958) originated from a misidentification of South Australian specimens by Basedow & Hedley (1905). The absence of ‘denticles on the lateral teeth’ indicated that these specimens were not part of Alloiodoris marmorata Bergh, 1904 . Also, the granulated dorsal notum indicated that Basedow & Hedley had certainly collected specimens of dubia . Then Burn (1957) misidentified new specimens from Victoria as Alloiodoris marmorata . However, a year later, he described them as a new species, Alloiodoris nivosus . Burn (1958) argued that nivosus differed from both Alloiodoris marmorata Bergh, 1904 , the type species of Alloiodoris under the ICZN, and Alloiodoris hedleyi O’Donoghue, 1924 because nivosus had a significantly elongated radula. I re-examined the types of both marmorata and hedleyi . Alloiodoris marmorata has caryophyllidia on the dorsal notum and has a hermaphroditic gland that is independent from the digestive gland. Bergh mentioned an independent hermaphroditic gland in his original description of marmorata ; Valdés & Gosliner (2001) overlooked this critical character when they re-described the reproductive system of a syntype. I think that hedleyi is simply one of the many synonyms of D. fragilis Alder & Hancock, 1864 : the hermaphroditic gland of hedleyi is not independent from the digestive gland.
Originally, Burn (1958) did not explain why he had classified nivosus within Alloiodoris . He justified his taxonomic decision when he described a hermaphroditic gland independent from the digestive gland and a penial sheath with a few hooks in the reproductive system of nivosus 4 years later ( Burn, 1962). The problem is that both observations were mistakes. Burn (1969) admitted that there were no hooks in the penial sheath of nivosus . Also, all the specimens that Burn originally identified as nivosus , and that I dissected for the present study, had a hermaphroditic gland that was not independent from the digestive gland. Finally, Burn (1969) acknowledged that nivosus was just a synonym of D. dubia , and therefore had no reason to be classified in Alloiodoris .
The most recent episode of the nomenclatural history of dubia is the description of P. leuca by Miller (1995). Miller’s description is largely correct, except for a few details (see my remarks on the original description). The main problem, however, is that Miller overlooked the existence of dubia . Miller’s original description and my re-description of the paratype of leuca demonstrate that dubia and leuca are very similar, especially for genital anatomy.
I wish to conclude this discussion with a few comments on the photographs of two specimens identified as dubia from Western Australia ( Coleman, 2001). The fact that the specimen that I dissected from Western Australia is clearly part of dubia indicates that Coleman’s identification is probably correct. However, this would need to be checked by dissection, especially for the specimen with a smooth notum ( Coleman, 2001: 56).
| ZMUC |
Zoological Museum, University of Copenhagen |
| NMV |
Museum Victoria |
| AM |
Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Paradoris dubia
| Dayrat, Benoît 2006 |
Paradoris leuca Miller, 1995: 901–907
| Miller MC 1995: 907 |
Alloiodoris nivosus Burn, 1958: 29–30
| Burn RF 1966: 274 |
| Burn RF 1958: 30 |
Alloiodoris marmorata
| Burn RF 1957: 19 |
| Basedow H & Hedley C 1905: 152 |
Discodoris dubia
| Burn RF 1969: 83 |
| Basedow H & Hedley C 1905: 139 |
| Bergh LSR 1904: 51 |
Discodoris dubia
| Bergh LSR 1904: 52 |
Discodoris
| Basedow H & Hedley C 1905: 140 |
| Bergh LSR 1904: 55 |