Phoenicocoris vidali (Lindberg)
|
publication ID |
https://doi.org/10.1206/0003-0082(2004)464<0001:ROPRWD>2.0.CO;2 |
|
persistent identifier |
https://treatment.plazi.org/id/421687D2-BA3E-2B44-FF3D-08F379F0FA40 |
|
treatment provided by |
Carolina |
|
scientific name |
Phoenicocoris vidali (Lindberg) |
| status |
|
Phoenicocoris vidali (Lindberg) View in CoL
Sthenarus vidali Lindberg, 1940: 52 (n.sp.).
Phoenicocoris vidali: Wagner, 1966: 18 View in CoL (n.comb.); Schuh, 1995: 375 (catalog); Kerzhner and Josifov, 1999: 388 (catalog).
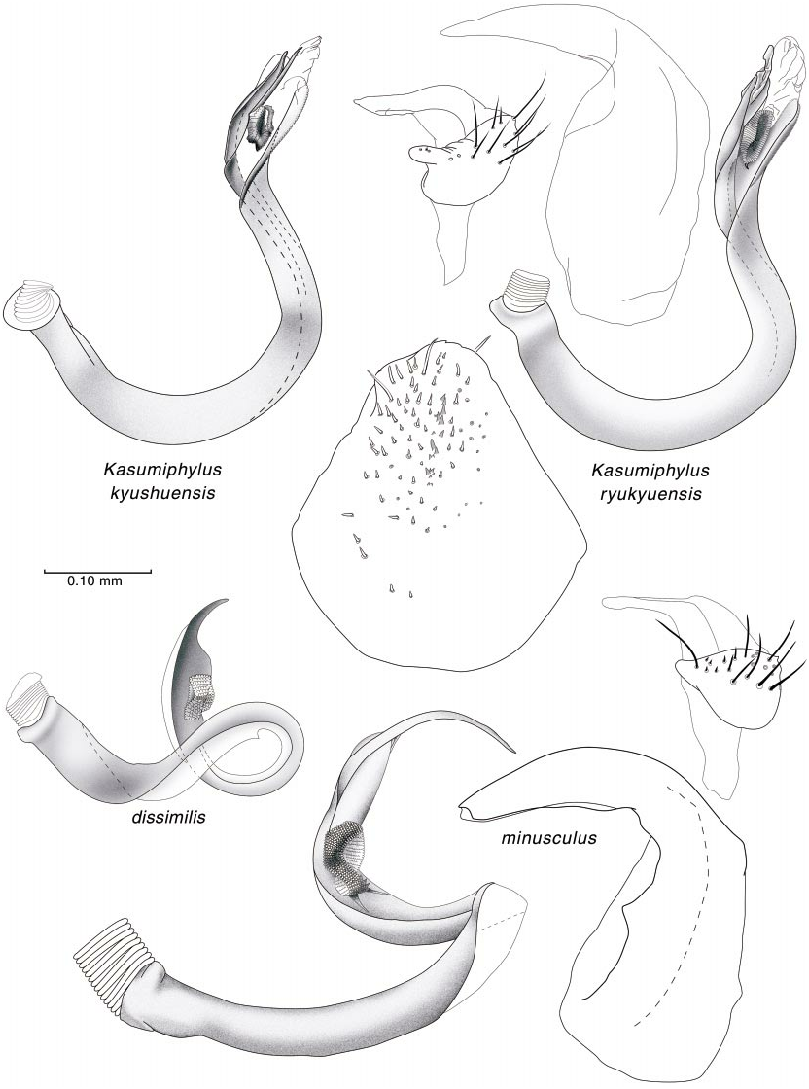
DISCUSSION: Lindberg (1940) compared P. vidali with P. dissimilis and commented that its body length was approximately 75% shorter. Wagner (1975: figs. 699, b 1–4) documented the male genitalia of P. vidali . Based on these illustrations it possesses a strongly twisted vesica, with the strap bifurcate apically, and the secondary gonopore situated mesially. Vesical form of this type is found in all species of Phoenicocoris except P. dissimilis . Based solely on Wagner’s illustrations, the vesica of P. vidali appears to be most similar to P. obscurellus . The known distribution of this species is RaselMa in the Middle Atlas Mountains of Morocco ( Lindberg, 1940). Host plants are unknown.
SPECIES REMOVED FROM PHOENICOCORIS Phoenicocoris dissimilis (Reuter) incertae sedis Figures 1A, D, 2 View Fig , 20 View Fig , 21 View Fig , 25 View Fig
Sthenarus dissimilis Reuter, 1878: 174 (n.sp.); Henry and Wheeler, 1974: 217 (biology); Kerzhner and Matocq, 1994: 59 ( lectotype designation).
Phoenicocoris dissimilis: Andersen and Gaun, 1974: 119 View in CoL , 126, 131 (n.comb., distribution); Schuh, 1995: 374 (catalog); Kerzhner and Josifov, 1999: 387 (catalog).
Sthenarus carbonarius Horváth, 1888: 185 (n.sp.); Kerzhner, 1996: 100 ( lectotype designation). NEW SYNONYM.
Phoenicocoris carbonarius: Wagner, 1975: 104 View in CoL (n.comb.); Schuh, 1995: 374 (catalog); Kerzhner and Josifov, 1999: 387 (catalog).
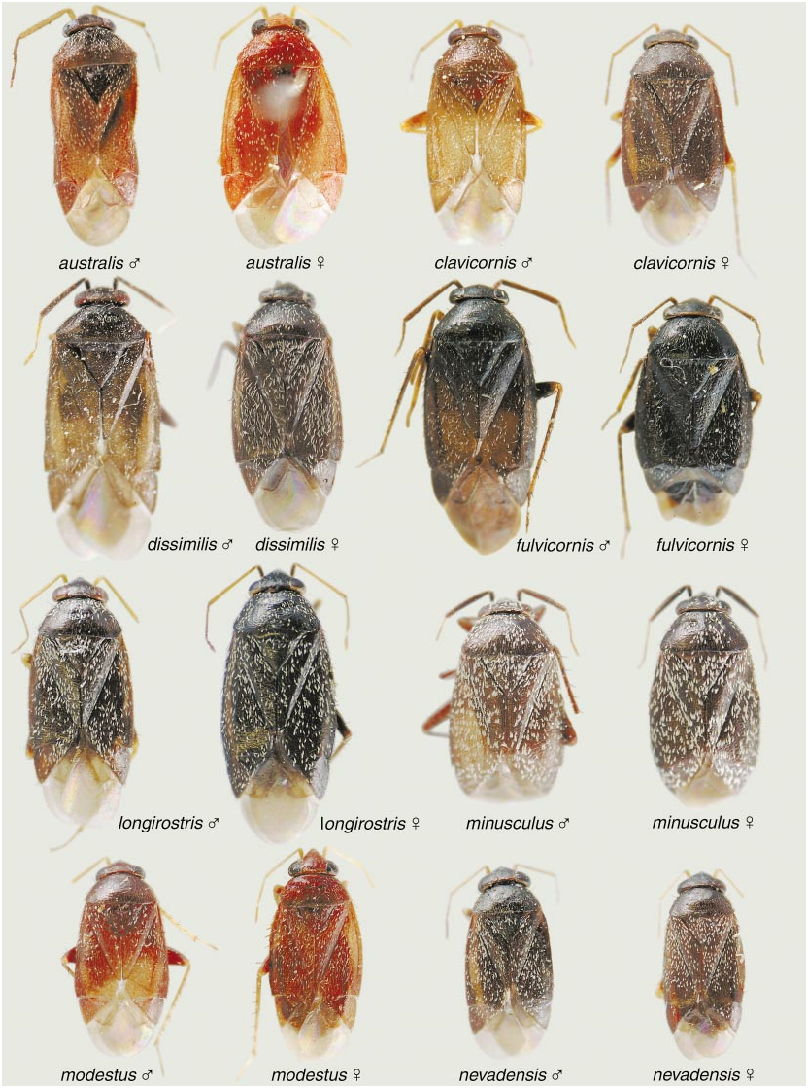
DIAGNOSIS: Recognized by the large, elongate body; dorsum uniformly dark reddish brown to nearly black, with vestiture of narrow, lanceolate, somewhat woolly setae and longer, suberect, black simple setae; relatively short labium; antennal segments 1 and 2 fuscous, 3 and 4 dusky yellow; pretarsus with relatively short, wide parempodium; and by the structure of the male vesica (fig. 25). Distribution of scalelike setae as in australis , claricornis , longirostris , and pallidicornis . The labium of dissimilis , which only reaches as far as the middle coxa, is much shorter than the labium of these other species, which at the least reaches the hind coxae and sometimes reaches the middle of the abdominal sternum.
REDESCRIPTION: GENERAL ASPECT: elongate; total length male 3.57 (3.20–3.90), female 3.28 (3.10–3.40); length to cuneal fracture male 2.44 (2.26–2.58), female 2.34 (2.20–2.48); coloration dark brown to black; distal antennal segments, apices of femora slightly, and basal segment of tarsi dusky yellowish brown; dorsum with moderately to densely distributed, broad, flattened, apically pointed, silvery scalelike setae and densely distributed, suberect to reclining, black simple setae; thoracic pleura and venter without scalelike setae. HEAD: width male 0.73 (0.69–0.76), female 0.74 (0.72–0.76); vertex width male 0.32 (0.31–0.34), female 0.37 (0.36–0.38); region anterior to antennal insertion relatively short, anteocular length male 0.18 (0.18–0.19), female 0.20 (0.18– 0.21); eyes large, about 90% of head height, ventral margin of antennal insertion dorsal to ventral margin of eye, only slightly ventral to midline of eye; antennal measurements male 0.20 (0.19–0.21): 0.86 (0.78–0.93): 0.42 (0.38–0.46): 0.32 (0.30–0.34), female 0.20 (0.18–0.21): 0.80 (0.74 –0.86): 0.43 (0.39–0.45): 0.33 (0.31–0.36); antennal segment 2 is 20% longer than head width across eyes, evenly thickened slightly, throughout length, in both sexes; labium dark brown to black, reaching middle of middle coxae in both sexes; labial length male 0.98 (0.91– 1.01), female 1.02 (1.00–1.04); length of segment 4 male 0.29 (0.26–0.30), female 0.28 (0.26–0.33). THORAX: width male 1.12 (0.98–1.18), female 1.13 (1.06–1.20); length male 0.53 (0.48–0.56), female 0.51 (0.48–0.54); mesoscutum moderately exposed. HEMELYTRA: maximum width male 1.46 (1.25–1.59), female 1.46 (1.38– 1.55); male subparallel laterally, female slightly ovate.
BIOLOGY: Breeds on Abies spp. ( Henry and Wheeler, 1974). Wheeler and Henry (1992) reported populations in the eastern United States on balsam fir ( A. balsamea (Linnaeus) Miller ), Grecian fir ( A. cephalonica Loudon ), Cilician fir ( A. cilicica (Ant.et Kotschy) , white fir ( A. concolor (Gordon and Glendinning) Hildebrand ), and Caucasian fir, A. nordmanniana (Steven) Spach. Wheeler (2001) reported that P. dissimilis is a predator of balsam twig aphid ( Mindarus abietinus (Koch) ( Mindaridae )) on white fir in Pennsylvania.
DISTRIBUTION: In Europe, Kerzhner and Josifov (1999) reported this species from Denmark, France, Germany, Poland, Romania, Slovakia, and Ukraine (Carpathians). Presumably introduced to eastern United States on ornamental firs in the early nineteenth century ( Wheeler and Henry, 1992). Henry and Wheeler (1974) first reported it from New York and Pennsylvania, and later ( Wheeler and Henry, 1992) added Connecticut, Delaware, Massachusetts, Maryland, and Rhode Island.
DISCUSSION: Wagner (1975) distinguished P. carbonarius from P. dissimilis based on the black tibiae, slightly larger (up to 4 mm) oval body, and slightly wider vertex of the former species as opposed to the yellowish brown tibiae with black base, shorter (up to 3.6 mm) more elongate body, and slightly more narrow vertex of the latter species. Kerzhner (1962) suggested that P. carbonarius is a dark colored variation of P. dissimilis . We concur with Kerzhner and consider S. carbonarius the junior subjective synonym of S. dissimilis . The distribution of the junior synonym, described as endemic to the Carpathians ( Romania and Ukraine), is subsumed by the range of P. dissimilis .
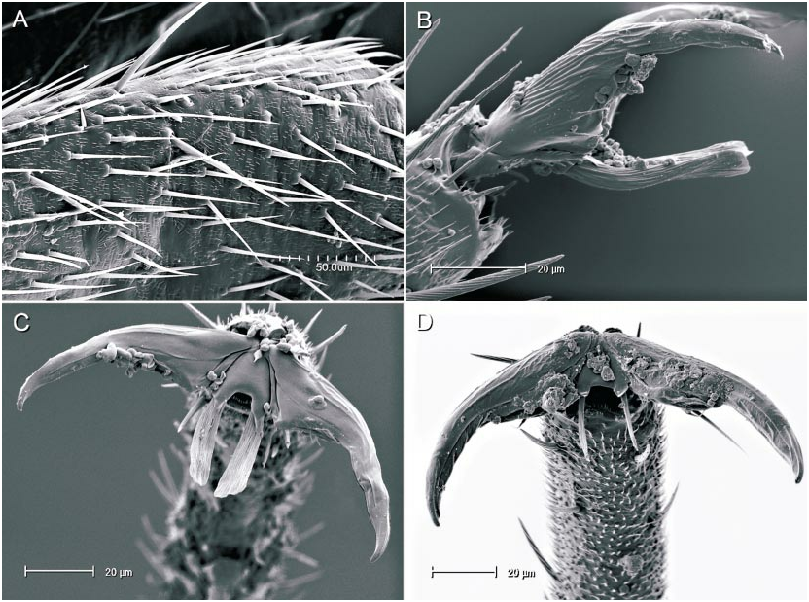
The illustrations of the vesicae for both nominal species, carbonarius and dissimilis , presented by Wagner (1975) are erroneous. Based on our examination of dissimilis the vesical strap has an undivided apex and not the bifurcate apices as in all other species of Phoenicocoris . The basal edge of the secondary gonopore in all species of Phoenicocoris has at least a short subtending gonopore sclerite (figs. 22, 23). The secondary gonopore of dissimilis does not have a subtending gonopore sclerite (fig. 25). The parempodia of all Phoenicocoris species are long, narrow, and with flattened apices (figs. 8C, 9C, 11A, 12C, 13C, 14D, 15C, 16D, 18C, 19E). Our micrographs of the pretarsus of dissimilis document that the parempodium of this species does not have a flattened apex, but is unusually wide throughout the entire length (fig. 21B, 21C). Although the scalelike setae of dissimilis are relatively flattened as other Phoenicocoris , the distal edge is pointed as opposed to the truncate distal edge of all other Phoenicocoris except for obscurellus and strobicola (compare figs. 1A, D, M, N). All species of Phoenicocoris , except minusculus , utilize species of Pinus for their host plants. The known hosts for dissimilis in both the Palearctic and Nearctic regions are species of Abies .
SPECIMENS EXAMINED: ROMANIA: Bucarest, A. L. Montandon, 73, 4♀ (AMNH, USNM). USA: Connecticut: Tolland Co.: University of Connecticut, Storrs, 29.V.1983, A. G. Wheeler, Jr., Abies sp. , 5th instar, 23, 1♀ (PDA). Delaware: Kent Co.: Dover, 28.V.1984, A. G. Wheeler, Jr., Picea abies , 33 (PDA). Maryland: Washington Co.: Hagerstown, 23.V.1986, A. G. Wheeler, Jr., P. abies , 23 (PDA). New York: Nassau Co.: Roslyn, Fine Arts Museum and Gardens on Rte 25A, 13.VI.1986, M. D. Schwartz, Abies balsamea , 2♀ (AMNH). Pennsylvania: Bradford Co.: E of Athens, Shelan Gardens, A. G. Wheeler, Jr., P. abies , 1♀ (PDA). Dauphin Co.: Hershey Hotel grounds, 19, 30.V.1973, 1.VI.1974, T. J. Henry, A. G. Wheeler, Jr., A. balsamea , 83, 18♀ (PDA). Luzerne Co.: Huntsville Nursery, Huntsville, 4.VI.1975, A. G. Wheeler, Jr., A. concolor , 23, 7♀ (PDA). Montgomery Co.: Barnes Arboretum, Merion Station, 2.VI.1982, A. G. Wheeler, Jr., A. cilicica , A. nordmanniana , P. glauca , 13, 4♀ (PDA); Manufacturers Golf and Country Club, 31.V.1973, A. G. Wheeler, Jr., J. L. Stimmel, A. concolor , 13, 1♀ (PDA); Philadelphia, Forest Hills Cemetery, J. L. Stimmel, Tsuga canadensis , 1♀ (PDA). Northumberland Co.: Turbotville, nr, 15.V.1986, A. G. Wheeler, Jr., A. concolor , 5th instar, 43, 1♀ (PDA). Philadelphia Co.: Chestnut Hills, Morris Arboretum, 11.V.1982, A. G. Wheeler, Jr., A. cephalonica , 5th instar, 43, 4♀ (PDA). York Co.: Strathmeyer Forest Nursery, nr Wellsville: 7, 17.V.1973, T. J. Henry, A. concolor , 33, 8♀ (PDA); 16.V.1974, T. J. Henry, A. G. Wheeler, Jr., A. concolor , 23, 7♀ (PDA).
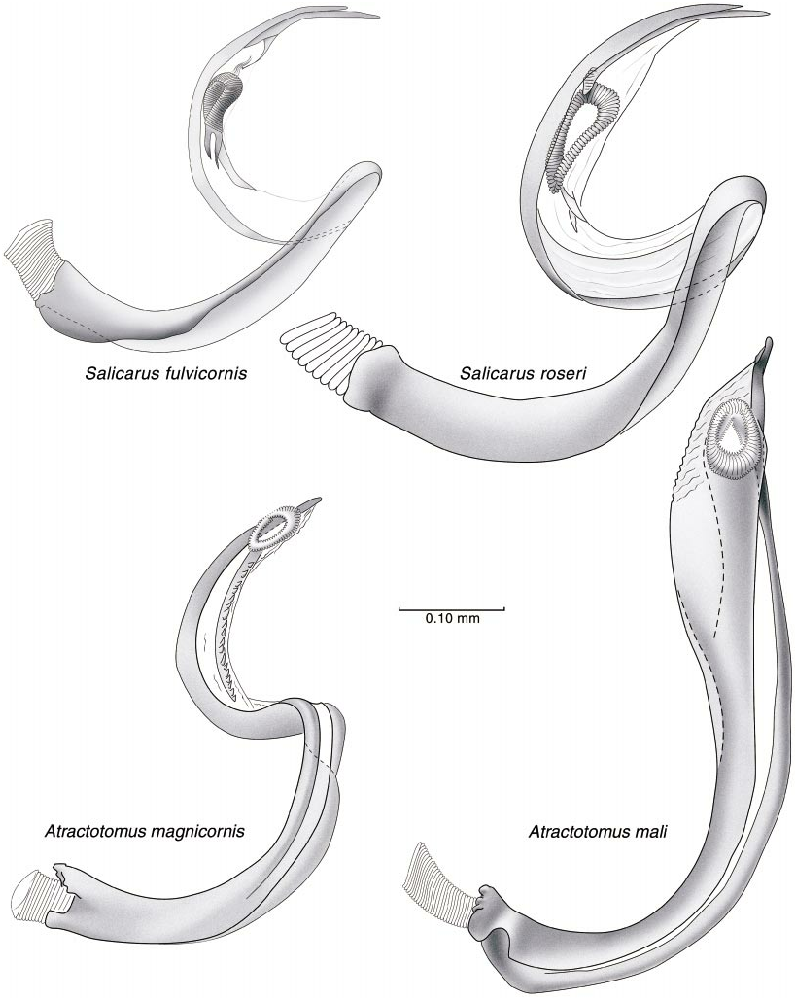
Salicarus fulvicornis Jakovlev
Figures 2 View Fig , 26 View Fig
Agalliastes fulvicornis Jakovlev, 1889: 348 View in CoL (n.sp.); Kerzhner, 1997: 247 (n.comb.).
Chlamydatus fulvicornis: Carvalho, 1958: 32 View in CoL (catalog); Schuh, 1995: 290 (catalog).
Phoenicocoris flagellatus Wagner, 1967: 71 View in CoL (n.sp.); Schuh, 1995: 374 (catalog).
Salicarus flagellatus: Vinokurov and Kanyukova, 1995: 58 View in CoL (n.comb.); Kerzhner, 1997: 247 (n.syn.). Kerzhner and Josifov, 1999: 421 (catalog).
DISCUSSION: Vinokurov and Kanyukova (1995) transferred flagellatus from Phoenicocoris to Salicarus based on the head structure, particularly the elevated transverse basal carina, the relatively strongly curved claws, the silvery vestiture on the dorsum and lateral portion of the prothorax, and the form of the vesica and parameres. Kerzhner (1997) placed flagellatus in synonymy with fulvicornis after designating a lectotype for the latter nominal taxon. We cannot comment on the synonymy; however, our examination of the specimens listed below allow us to support the combination, S. flagellatus . The posterior margin of the vertex in flagellatus is conspicuously pale (fig. 2); such coloration is not found in Phoenicocoris spp. , as all have unicolorous black heads. The vesica of flagellatus (fig. 26) is of a form seen in species of Salicarus (ref. fig. 26, roseri ), not those of Phoenicocoris spp. The vesica of flagellatus has several features which are unlike those of Phoenicocoris spp. (figs. 23– 25): The secondary gonopore is situated more distally on the vesical strap, the anterior and posterior apices of the vesical strap are weakly diverging, and the vesical strap is not tightly coiled.
Although not indicative of unequivocal generic affiliation (as apparently both Phoenicocoris and Salicarus spp. have long parempodia with weakly spatulate apices), flagellatus does have parempodia similar to that of S. roseri (fig. 6A). The distribution of scalelike setae is also inconclusive for determining the generic placement of flagellatus . There are Phoenicocoris and Salicarus spp. with scalelike setae present on both dorsal and lateral aspects of the body.
SPECIMENS EXAMINED: [in Cyrillic characters] Mongolia: SuheBator Aimak , Ongonels, sandy desert, 15 km SSE of Hongor, 5–6. VI .1971, Kerzhner , Caragana sp. 33, 3♀ ( AMNH) .
Salicarus qiliananus (Zheng) , new combination
Phoenicocoris qiliananus Zheng in Zheng and Li, 1996: 101, 103 (n.sp.); Kerzhner and Josifov, 1999: 388 (catalog); Qi et al., 2003: 428 (list).
DISCUSSION: The following characters argue for transferring qiliananus from Phoenicocoris to Salicarus : the minute pulvillus; the only slightly curved claw; the Sshaped, but not tightly coiled, vesica; the large secondary gonopore, placed near the apex of the vesica and without a gonopore sclerite; and the apical processes of the vesica fused throughout their entire length, giving the appearance of a single process.
The coloration and general distribution of qiliananus , from Gansu Province in northwestern China, is similar to that of S. halimodendri Putshkov, 1977 (Kerzhner, personal commun.). The latter is variably colored (dark brown or black to pale brown to yellow) and is distributed in Central Asia on the widely distributed legume, Russian saltree ( Halimodendron halodendron (Pallas) Voss ) ( Fabaceae ) ( Putshkov, 1977). However, we hesitate to place the former species in synonymy pending examination of additional Palearctic material.
| VI |
Mykotektet, National Veterinary Institute |
| AMNH |
American Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Phoenicocoris vidali (Lindberg)
| SCHWARTZ, MICHAEL D. & STONEDAHL, GARY M. 2004 |
Phoenicocoris vidali
| Kerzhner, I. M. & M. Josifov 1999: 388 |
Phoenicocoris qiliananus
| Qi, B. & C. W. Schaefer & N. Bai & Z. Zheng 2003: 428 |
| Kerzhner, I. M. & M. Josifov 1999: 388 |
| Zheng, L. Y. & X. M. Li 1996: 101 |
Salicarus flagellatus : Vinokurov and Kanyukova, 1995: 58
| Kerzhner, I. M. & M. Josifov 1999: 421 |
| Kerzhner, I. M. 1997: 247 |
| Vinokurov, N. N. & E. V. Kanyukova 1995: 58 |
Phoenicocoris carbonarius : Wagner, 1975: 104
| Kerzhner, I. M. & M. Josifov 1999: 387 |
| Wagner, E. 1975: 104 |
Phoenicocoris dissimilis : Andersen and Gaun, 1974: 119
| Kerzhner, I. M. & M. Josifov 1999: 387 |
| Andersen & N. Moller & S. Gaun 1974: 119 |
Chlamydatus fulvicornis : Carvalho, 1958: 32
| Carvalho, J. C. M. 1958: 32 |
Sthenarus vidali
| Lindberg, H. 1940: 52 |
Agalliastes fulvicornis
| Kerzhner, I. M. 1997: 247 |
| Jakovlev, B. 1889: 348 |
Sthenarus carbonarius Horváth, 1888: 185
| Kerzhner, I. M. 1996: 100 |
| Horvath, G. 1888: 185 |
Sthenarus dissimilis
| Kerzhner, I. M. & A. Matocq 1994: 59 |
| Henry, T. J. & A. G. Wheeler, Jr. 1974: 217 |
| Reuter, O. M. 1878: 174 |