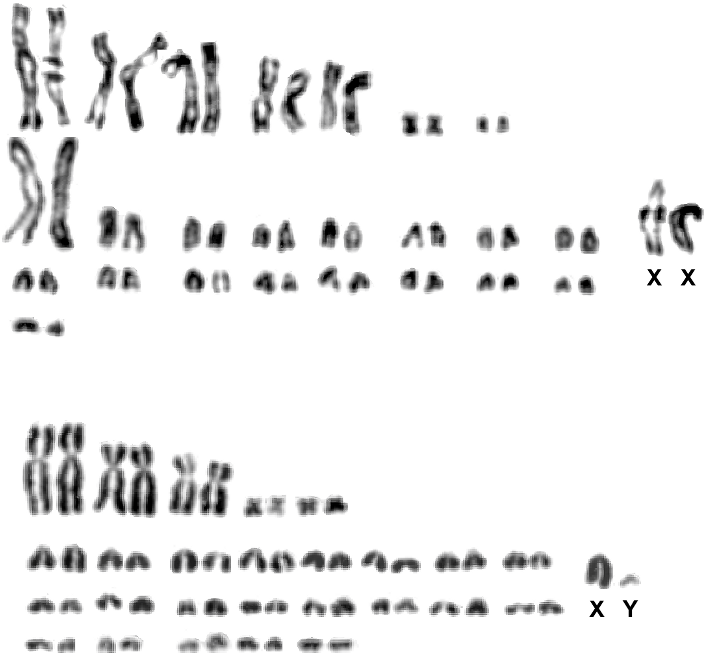Cerradomys vivoi, Percequillo & Hingst-Zaher & Bonvicino, 2008
|
publication ID |
https://doi.org/ 10.1206/495.1 |
|
persistent identifier |
https://treatment.plazi.org/id/403F2547-FFAD-FA11-FF58-CED5FBD9E338 |
|
treatment provided by |
Carolina |
|
scientific name |
Cerradomys vivoi |
| status |
sp. nov. |
Cerradomys vivoi View in CoL , new species
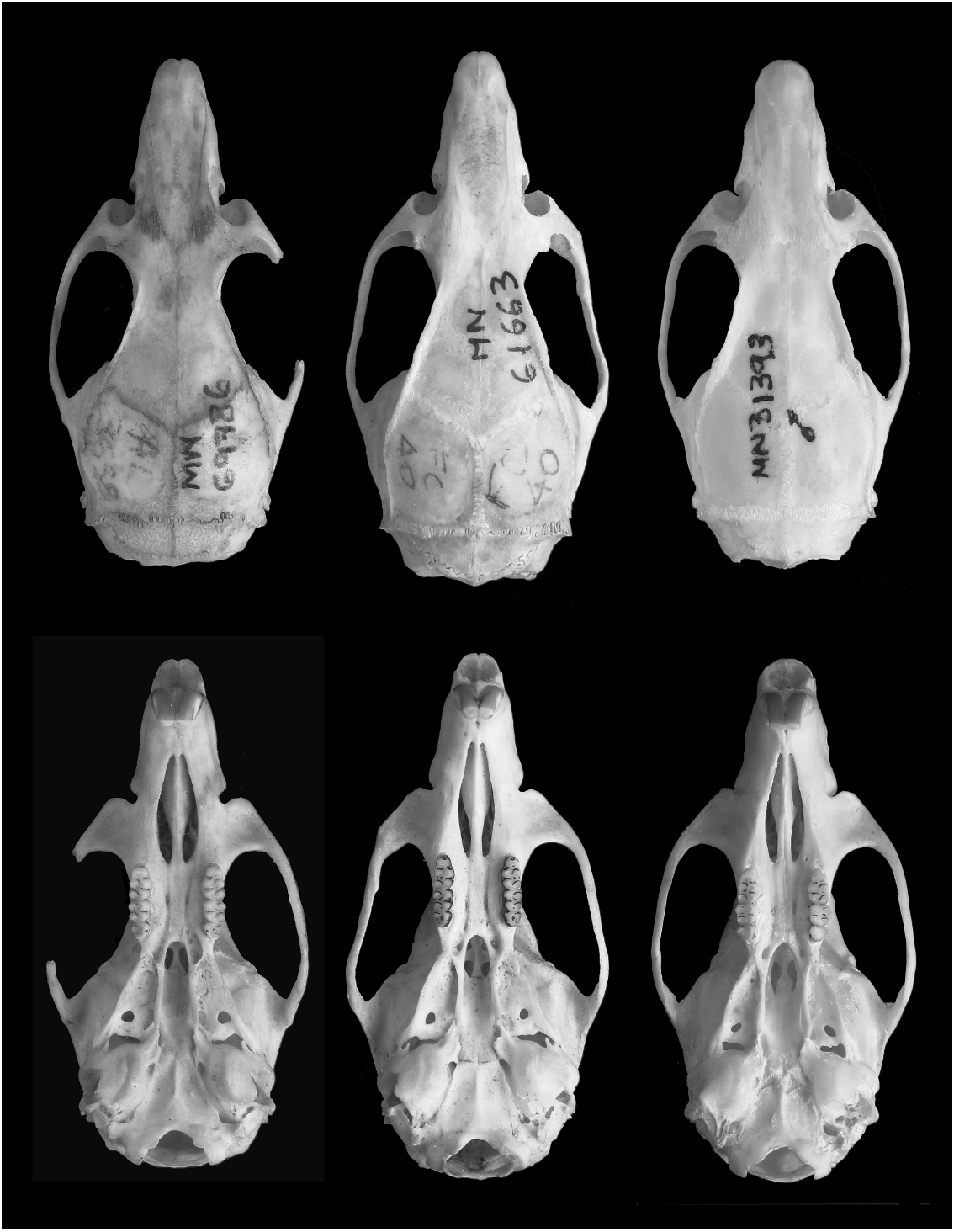
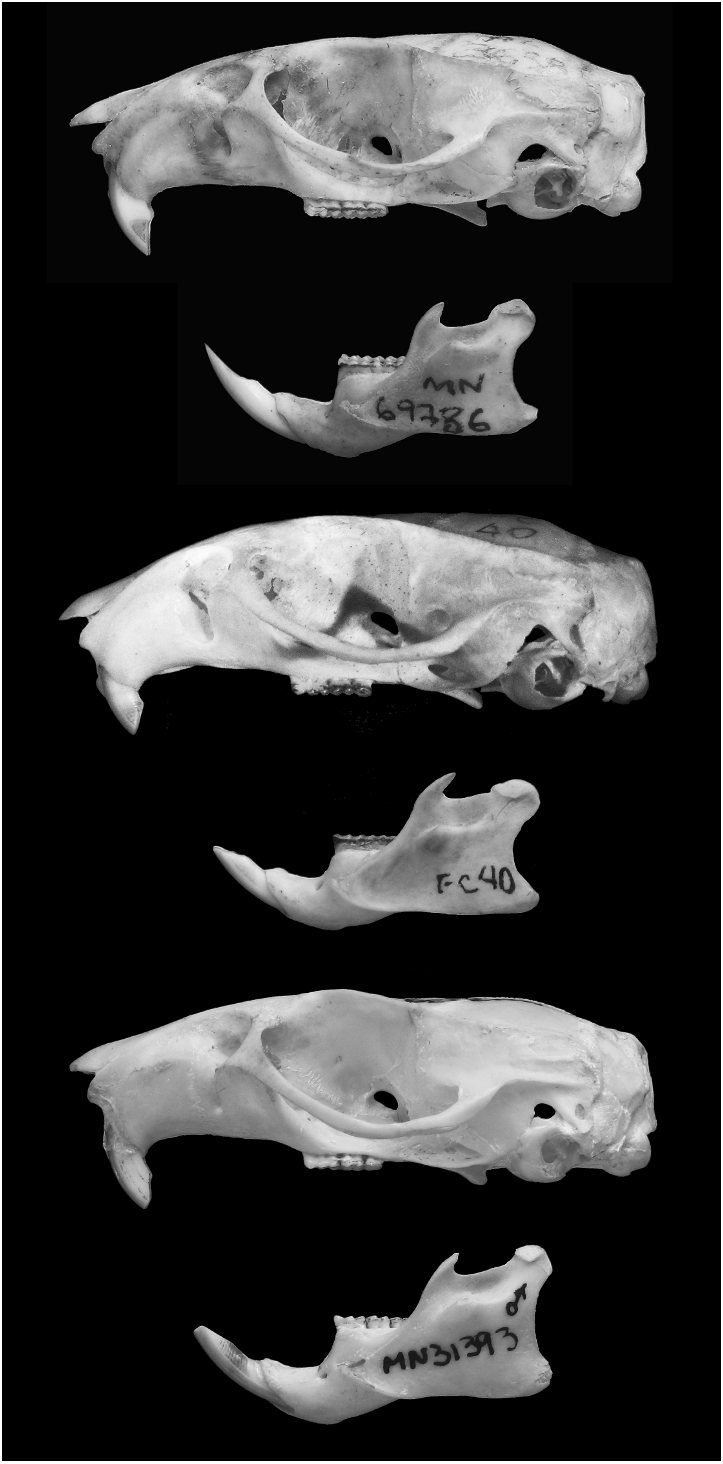
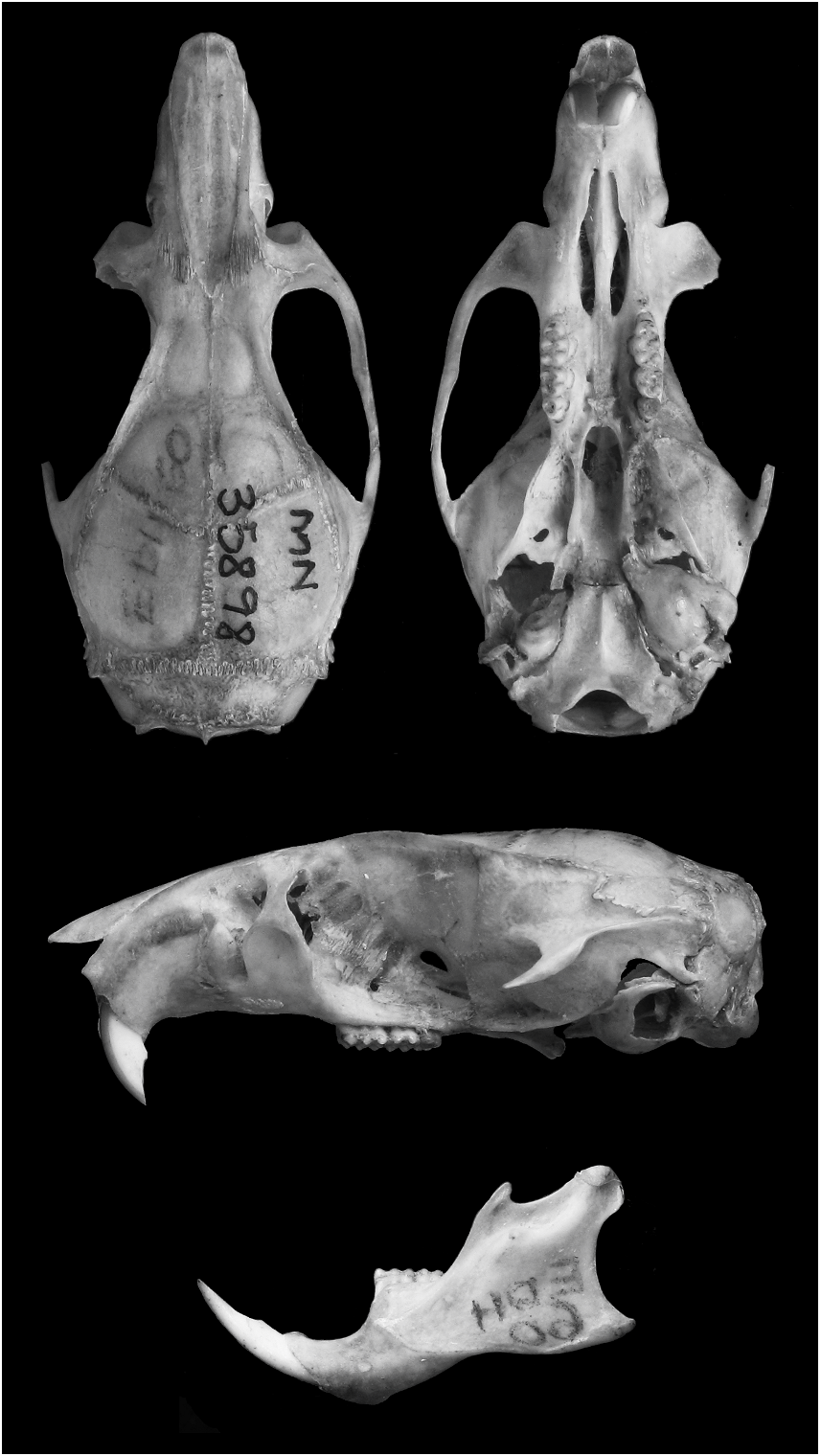
Figures 1–3 View Fig View Fig View Fig , 5 View Fig , 7 View Fig , 9–11 View Fig View Fig View Fig ; tables 1 and 2
HOLOTYPE: MN 35898 , an adult male collected by E. Hingst-Zaher and M. Lara (original field number EDH 60 ), on 29 December , 1992. The holotype consists of an undamaged skin, skull with fractured left zygomatic arch and right auditory bulla missing, and postcranial skeleton. 3 The cell suspension of bone marrow in Carnoy’s fixative (methanol:acetic acid) and the livertissue aliquot preserved in ethanol are housed at Laboratório de Biologia e Parasitologia de Mamíferos Reservatórios Silvestres , Instituto Osvaldo Cruz –FIOCRUZ, under original field number EDH 60. Cytochrome b DNA data are available in GenBank under the accession number AF181275 View Materials .
External and selected skull dimensions of holotype are: HBL, 152; LT, 180; HF, 34 (including claw); Ear, 21; Wt, 82.15; CIL, 32.23; LD, 9.62; LM, 4.88; BM1, 1.40; LIF, 6.82; PB, 5.45; BR, 6.56; LN, 13.79; LPB, 5.89; HB, 10.36; LIB, 5.19; CZL, 25.10; OL, 12.62.
PARATYPES: We assign all specimens herein examined (see below) as paratypes of C. vivoi .
TYPE LOCALITY: The Cerradomys vivoi holotype was captured in coastal Atlantic Forest , near Itabuna in the Brazilian state of Bahia. The type locality is situated at Parque Zoobotânico da Comissão Executiva do Plano da Lavoura Cacaueira ( CEPLAC), situated 6 km E of Itabuna by road, state of Bahia, Brazil, at 14 ° 489S, 39 ° 169W, on sea level (figs. 1, 2) .
DISTRIBUTION: Known collection localities of C. vivoi are distributed in the Brazilian states of Minas Gerais, Bahia, and Sergipe. In the state of Minas Gerais, distribution records are restricted to the northern portion of this state, around Rio Jequitinhonha, and Rio São Francisco and its right bank tributaries. In Bahia, sampling localities of C. vivoi extend from the coastal region to the eastern bank of Rio São Francisco. In Sergipe, the only recorded site of collection is in the coastal
3 The specimen MN 35898 was selected as holotype, because it is the only available specimen with karyotype and tissue sample .
region, near the mouth of Rio São Francisco (figs. 1, 2).
ETYMOLOGY: This species is named after Dr. Mario de Vivo, mammal curator of Museu de Zoologia da Universidade de São Paulo, for his outstanding contribution to the development of mammalogy in Brazil.
DIAGNOSIS: Cerradomys vivoi is characterized by intermediate body size, short and dense dorsal pelage, dorsal body color orange grizzled with brown, head color grayish, ventral body color grayish or slightly yellowish, skull with long and wide sphenopalatine vacuities, alisphenoid strut absent, deep palatal fossae (complex posterolateral palatal pits), and a unique chromosomal formula (2n 5 50, FN 5 62–64).
MORPHOLOGICAL DESCRIPTION: Head and body medium sized (table 2); tail length longer than head and body (127%–150% of head and body length); hind feet moderately narrow and long (21%–24% of head and body length), with large and fleshy interdigital, thenar, and hypothenar pads; pinnae rounded and small (12.5%–16% of head and body length). Dorsal pelage short and dense (table 1), consisting of short and dense underfur (wool hairs; thin, wavy, short) and longer and lax overfur (guard and cover hairs; thick, long). Dorsal body color buffy orange densely grizzled with black; wool hairs (range: 6–11 mm) with basal part grayish and distal part (1/10 of total length) orange or brown; cover hairs long (range: 9–15 mm), with distal 1/4 dark brown with a subterminal orange band; guard hairs sparse and long (range: 14–20 mm), with distal half entirely black or dark brown. Anterior half of head (until eyes) covered with gray-based and white- or buffy-tipped hairs, clearly distinct from color of posterior half of head and dorsal body fur. Ventral pelage composed of wool, cover and guard hairs, with individual hairs gray at the base and tipped with white, buff, or yellow; general ventral color grayish, buffy, or yellowish, slightly grizzled, and distinctively lighter than dorsal pelage. Flanks bright orange; banded cover hairs and dark guard hairs rare. Mystacial vibrissae long, reaching but not surpassing pinnae when laid back. Tail slightly bicolored, covered with short, sparse brown hairs and scales on dorsal surface and
32.2 mm).
unpigmented hairs and scales on ventral surface. Dorsal surface of hind foot predominantly white, covered with short hairs, with 3/ 4 distal portion white and basal 1/4 grayish or washed brown; ungual tufts sparse, shorter than claws especially on digit I; ventral surface naked, unpigmented, with four interdigital pads and two tarsal pads (thenar and hypothenar). Pinnae covered internally with short orange hairs and externally with orange, brown-tipped hairs.
Skull size intermediate (tables 1, 2; figs. 3, 5). Rostrum long, broad, tapering, with inflated capsular projection of nasolacrimal foramen, and flanked by deeply excavated zygomatic notches; interobital region long and narrow (table 2), converging anteriorly, with dorsolateral margins with sharp and welldeveloped supraorbital crests; braincase oblong, with prominent temporal crests. Zygomatic plate (in lateral view) projected forward, with dorsal free margin rounded and anterior margin straight or slightly concave, and zygomatic spine absent. Incisive foramina long (averaging about 74% of length of diastema), with lateral margins concave and diverging posteriorly, wider posteriorly; posterior margins extending or not between the alveolus of upper first molars. Palate long and wide (sensu Hershkovitz, 1962); posterolateral palatal pits numerous and complex, recessed in moderately deep palatal fossae; palatal excrescences rarely present. Mesopterygoid fossa narrow, with anterior margin rounded or slightly acute, not reaching the alveolus of M3; bony roof of mesopterygoid fossa perforated by long and wide sphenopalatine vacuities, exposing the orbitosphenoid. Alisphenoid strut absent (buccinator-masticatory foramen and ovale foramen confluent). Postglenoid foramen large and nearly semicircular in shape separated from small subsquamosal fenestra (absent in specimens with moderate to heavy tooth wear), by a wide hamular process of squamosal. Tegmen tympani weakly overlapping squamosal; posterior suspensory process of squamosal absent. Ectotympanic bullae globose; eustachian tube short, with distinct medial laminae in a few specimens; stapedial process short and wide, overlapping squamosal; bony process dorsal to stapedial process present, overlapping squamosal.
Mandible long and deep (fig. 9); coronoid process large, falciform or triangular, nearly equal to condyloid process; superior notch shallow; angular process short, not surpassing the condyloid process posteriorly; inferior notch shallow; capsular process of lower incisor well developed.
Incisors, upper and lower molars as for the genus (no mesoloph/mesolophid reduction was observed in C. vivoi ). Mammary counts and soft anatomy (stomach and glans penis) as described for the genus.
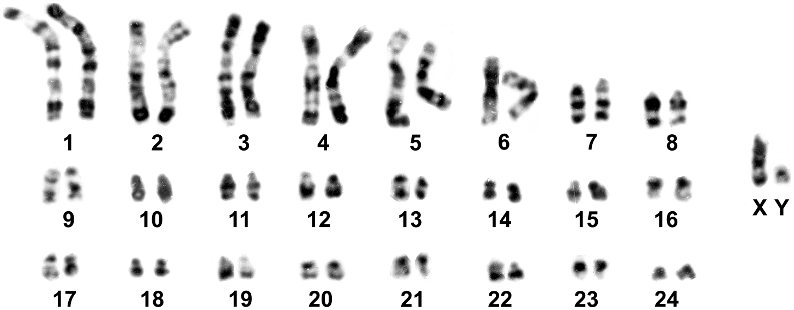
KARYOLOGY: The holotype of Cerradomys vivoi ( MN 35898 ), as well as seven specimens collected in Caetite´, Bahia, and Juramento, Minas Gerais, showed a karyotype with 2n 5 50 and FN 5 62–63 (table 7, figs. 10, 11). The autosomal complement comprises seven biarmed pairs (1 large, 4 medium, and 2 small pairs) and 17 acrocentric pairs (1 large and 16 small pairs). The X chromosome is a mediumsized acrocentric and the Y, small-sized. Variation in autosome fundamental number is due to pericentric inversion affecting a medium acrocentric. The G-banding pattern of C. vivoi allowed the unequivocal identification of homologues in autosome pairs and sex chromosomes (fig. 11). One male specimen ( LV-FC22 ) and one female specimen ( MN 61661 ) showed 2n 5 50, FN 5 63, due to a pericentric inversion involving one chromosome of a medium autosome pair .
NATURAL HISTORY: In the Cerrado- Caatinga transitional areas, Cerradomys vivoi inhabits secondary semideciduous and gallery forests, as well as arboreal Caatinga (all information on the natural history of this species is based on Hingst et al., 1997, except where noted). In the same region this species was trapped along with Gracilinanus agilis , Marmosops incanus , Monodelphis domestica , Calomys expulsus , Oligoryzomys fornesi , Oligoryzomys nigripes , Oligoryzomys stramineus , Nectomys rattus , Rhipidomys sp. , Wiedomys pyrrhorhinos , and Thrichomys apereoides . The diet of C. vivoi consists mainly of vegetal material and arthropods. The only ectoparasite species found in C. vivoi is the mite Gigantolaelaps vitzhumi (Acari: Laelapidae ).
In the Atlantic Forest, at Una Biological Reserve located in southern Bahia ( Pardini, 2004), Cerradomys vivoi was captured by R. Pardini (unpubl.; in litt., A.R. Percequillo archives), who provided the following account on this species abundance and habitat preference:
I had performed 36,288 trap nights employing Sherman traps and 10,368 pitfall nights using 35 l. [5 liters] buckets, an effort equally divided through six habitats (‘‘cabrucas’’ [disturbed canopy forests, mixed with cacao plantations: the understory is removed and replaced by cacao plants], ‘‘capoeiras em estadio inicial a medio-inicial de regeneracao’’ [disturbed forests in initial and inicial/ advanced stage of regeneration], and interior and edge of mature forest with more than 1,000 ha and interior and edge of mature forest less than 100 ha). I captured 9 O [ryzomys]. subflavus [5 Cerradomys vivoi ], 1 in ‘‘cabruca’’ and 8 in ‘‘capoeiras’’. Moreover, in preliminary un-standardized sampling,... I captured few individuals... of subflavus [5 C. vivoi ] in open abandoned pasture areas.… Considering the smaller effort of this sampling, the number of individuals captured (2 or 3) was comparatively larger. Considering these numbers, I believe that subflavus [5 C. vivoi ] is absent or very rare in mature forests, including the edges, occurs in disturbed forests in initial stages of regeneration or other disturbed forests (as cabrucas) and that is probably more common in open areas. It is noteworthy that even in ‘‘capoeiras’’ or ‘‘cabrucas’’ they are relatively rare, since only 8 individuals were captured in 350 captures in ‘‘capoeiras’’ and only 1 specimen was captured in 299 captures in ‘‘cabrucas’’....
In the same geographical area, Cerradomys vivoi was captured along with Didelphis aurita , Gracilinanus microtarsus , Marmosa murina , Marmosa sp. , Marmosops incanus , Metachirus nudicaudatus , Micoureus demerarae , Monodelphis americana , Sciurus ingrami , Akodon cursor , Blarinomys breviceps , Euryoryzomys russatus , Hylaeamys laticeps , Nectomys squamipes , Oecomys sp. , Oligoryzomys sp. , Rhipidomys mastacalis , Thaptomys sp. , and Phyllomys sp. ( Pardini, 2004) .
SPECIMENS EXAMINED: BRAZIL: BAHIA: Andaraí: F: UFPB 2376 View Materials . Caetite´: MN 63377 , 63381 . CEPLAC, E de Itabuna : M: MN 35898 (holotype of C. vivoi ). Fazenda Bolandeira, 10 km S Una: M: UFMG MAS 1 View Materials , MAS 31 View Materials , MAS 46 View Materials , 2854, 2856, YL 21 ; F: UFMG LPC 104 , 2215 View Materials , 2855 View Materials , YL 103 . Fazenda Lagoa D’Água, Serrinha, Feira de Santana : I: BMNH 1986.1596 . Fazenda Massapeˆ, SW Serrinha: F: UFMG YL 208 . Fazenda Santa Rita, E Andaraí: M: UFMG 2859–61 View Materials , 2863–5 View Materials , LPC 221 , 225 ; F: UFMG 2862 View Materials , 2866 View Materials , LPC 224 . Gandú: F: UFPB 2063 View Materials . Lagoa de Itaparica : F: MZUSP 28889 View Materials , 28890 View Materials . Mirorós: M: UnB 137–8 . Mucujeˆ: M: MZUSP 27403 View Materials . Reserva Biológica de Una : M: MZUSP RP 1785 , 2954 View Materials . Rio Unamirim , 14 km W Valença: M: UFPB 540 View Materials , 541 View Materials . Sebastião Laranjeiras: F: UnB 1403 . Una : M: UFMG MAS 32 View Materials . MINAS GERAIS: Fazenda Canoas, Juramento: M: MN 61662 , 61664 , LV – FC 22 ; F: MN 61661 , 61663 , LV – FC 148 ; I: MN 61665 . Itaobin: F: UFMG 907 View Materials . Itinga: M: UFMG 1453–4 View Materials . Jequitinhonha: M: UFMG 1458 View Materials . Riacho Mocambinho, Jaíba: M: MN 34433 , 34436 ; F: MN 34432 , 34434 . Ribeirão Contendas, Cristália: F: UFMG 58 View Materials . SERGIPE: Fazenda Capivara, 7 km SE Brejo Grande : M: MN 30694 ; F: MN 30587–8 .
SPECIES LIMITS AND COMPARISONS
MORPHOLOGIC VARIATION: Cerradomys vivoi differs from C. maracajuensis and C. marinhus by its shorter, rich orange-brown dorsal pelage, when compared to the longer and denser yellow-brown dorsal pelage of the latter two taxa (table 1). Additionally, the anterior half of dorsal head pelage is distinctively grayish to yellow gray in C. vivoi , whereas in C. maracajuensis and C. marinhus its color is the same as the dorsal body pelage. The ventral pelage in C. vivoi varies from grayish white to grayish yellow; conversely, in both C. maracajuensis and C. marinhus this region exhibits an intense yellow or buffy color. Cerradomys vivoi also differs from C. maracajuensis and C. marinhus by the presence of posterolateral palatal pits recessed in deeply excavated fossae, whereas in the latter species pits are recessed in shallow palatal fossae. Another cranial feature that discriminates C. vivoi from C. marinhus and C. maracajuensis is the roof of mesopterygopid fossa, which is perforated by long and wide sphenopalatine vacuities, largely exposing the orbitosphenoid in C. vivoi . On the other hand, both C. maracajuensis and C. marinhus are characterized by shorter vacuities restricted to presphenoid (in all 92 Bolivian specimens of C. maracajuensis surveyed for this trait) or by a completely ossified roof of mesopterygoid fossa (in 77% of 31 Paraguayan and Brazilian specimens of C. maracajuensis ).
Cerradomys vivoi can be easily distinguished from C. scotti and from the holotype of C. andersoni (CBF 6151) by dorsal body color, which is buffy yellow with brown in the latter two species. Cerradomys vivoi can also be recognized from C. scotti and C. andersoni in some tail traits (table 1): C. vivoi exhibits a moderately hirsute tail, ranging from uniform to bicolored dorsoventrally, whereas both C. andersoni and C. scotti present strongly hirsute and sharply bicolored tails. Cranial differences are also conspicuous: C. scotti , as well the holotype of C. andersoni , are characterized by the presence of alisphenoid strut in 84% of specimens (n 5 92), while this trait is consistently absent in C. vivoi . Palatal excrescences are also present in C. scotti and absent in C. vivoi (small in the young specimen of C. andersoni ). Dental morphological traits also are distinctive regarding C. vivoi and C. scotti : the mesoloph is reduced in M2 (22% of 78 examined specimens) and the mesolophid is consistently absent from m1 and m2 (in all surveyed specimens for this trait) in C. scotti (also in C. andersoni ); conversely, both mesoloph and mesolphid are present in all specimens of C. vivoi .
The cranium of C. vivoi is readily distinguished from C. subflavus and C. langguthi by its long and wide sphenopalatine vacuities, largely exposing the orbitosphenoid, while C. subflavus shows long and narrow vacuities, barely exposing the orbitosphenoid and C. langguthi exhibits short and narrow sphenopalatine vacuities.
MORPHOMETRIC VARIATION: Cerradomys scotti , C. marinhus , and C. maracajuensis can be readily and unambiguously distinguished from C. vivoi by discrete qualitative traits (see also table 3 for external and cranial measurements). In contrast, C. vivoi is most similar to C. subflavus and C. langguthi , with more subtle cranial differences. Therefore, all our subsequent analyses (morphometric, karyologic, and molecular) were restricted to these three forms in order to emphasize the differences among them.
Sexual dimorphism in species of genus Cerradomys is not an important component of variation in cranial morphometrics ( Brandt and Pêssoa, 1994; Percequillo, 1998), a pattern recurrent in oryzomyine ( Goldman, 1918; Musser and Williams, 1985) and other sigmodontine rodents ( Voss, 1988: 362; Carleton and Musser, 1989; Voss and Marcus, 1992). Consequently, we combined males and females for all subsequent univariate and multivariate statistical analyses herein performed.
Among external measurements, C. vivoi and C. langguthi differ significantly from C. subflavus in body length (one-way ANOVA, df 5 160, F 5 40.05, p,0.001) and weight (df 5 134, F 5 71.16, p,0.001). With respect to cranial measurements, 7 of 15 variables significantly discriminated the three species: CIL (one-way ANOVA, df 5 159, F 5 84.73, p,0.001), HB (df 5 155, F 5 51.03,
TABLE 4 Results of Principal Components Analysis Employing the Adults of Cerradomys Specimens included: C. subflavus (n 5 38), C. vivoi (n 5 31) and C. langguthi (n 5 32).
p,0.001), LIB (df 5 179, F 5 84.08, p,0.001), ZB (df 5 152, F 5 65.87, p,0.001), CZL (df 5 163, F 5 91.87, p,0.001), OFL (df 5 179, F 5 123.31, p,0.001), and BB (df 5 162, F 5 104.18, p,0.001). All cranial measurements exhibited differences among these three species, with C. subflavus significantly larger, and the two other species of similar size (table 2). For those variables significantly different among the three species, C. vivoi is of intermediate size, while C. subflavus is consistently the most robust, and C. langguthi is always the smallest (table 2).
Principal component analysis confirmed that size was an important characteristic separating the three species. The first principal component is responsible for more than 50% of the observed variance (table 4). Moreover, all eigenvectors were positively related to the greatest variation axis. Some overlapping can be observed along the first axis (fig. 12), especially between the two smaller species, C. vivoi and C. langguthi .
Discriminant analysis using log-transformed cranial measurements (fig. 13) separated the three species along the first and second canonical axes. OL, BB, and CIL were the variables that mostly contributed to separation on first and second axis, respectively (table 5). Mahalanobis D 2 distances between species were all significant (table 6).
KARYOLOGIC VARIATION: Karyologic data reinforced the uniqueness of Cerradomys vivoi with respect to other species of the genus (table 7). When compared to C. subflavus , karyotypes were similar, since both species shared the same fundamental number. However, this resemblance is only apparent because they differed in diploid number and chromosome morphology. Complex rearrangements were responsible for these differences (fig. 10): C. subflavus has three large and two small biarmed chromosome pairs, while C. vivoi has two extra medium-sized biarmed chromosome pairs. Furthermore, C. vivoi exhibits a large acrocentric pair, the largest of its autosomal complement, without a recognizable counterpart in the C. subflavus complement, composed only of small-sized acrocentric pairs.
A similar 2n 5 50 and FN 5 64 karyotype was previously reported for specimens collect- ed within the distribution range of Cerradomys vivoi : specimens from localities from central Bahia, namely Jacobina, Andaraí, Morro do Chapéu, and Mucugê ( Percequillo, 1998) and from Sergipe ( Andrades-Miranda et al., 2002). Moreover, a 2n 5 50 and FN 5 62 karyotype was found in specimens from northern Minas Gerais, Mocambinho (J. A. Oliveira, personal commun.). Quantitative and qualitative analyses of voucher specimens from Mucugê (MZUSP 27403) and Mocambinho (MN 34432–4, 34436) and non-karyotyped specimens from Andaraí (UFPB 2376) allowed us to identify them as C. vivoi .
Some karyologic similarities were observed between C. vivoi and C. langguthi , which are characterized by a 2n 5 48–50 and FN 5 56. This intrapopulational polymorphism overlaps with the known diploid number of C. vivoi (2n 5 50), but not with its fundamental autosome number (FN 5 64). However, C. vivoi was not variable in diploid number in samples from Bahia and Minas Gerais, south of Rio São Francisco (there is no available information of samples from Sergipe). Moreover, the morphologic distinctiveness of the autosomal complement between the samples from north of Rio São Francisco (herein C. langguthi ) and C. vivoi allows for the recognition of both forms as separate species.
MOLECULAR VARIATION: Genetic distance estimates between C. vivoi and C. subflavus were smaller (1.7%–2.0%) than between other species pairs of Cerradomys (table 8). Maximumparsimony analyses placed Scotinomys and Neotoma apart from the oryzomyine species (fig. 14). The oryzomyines formed a trichotomy consisting of Nectomys genus, Sooretamys angouya , and a well-supported monophyletic group corresponding to Cerradomys species.
Within Cerradomys , the clade formed by C. maracajuensis and C. marinhus is the sister group to the remaining species of the genus: a
TABLE 5 Results of Discriminant Analysis Employing Only Adults of Cerradomys Specimens included: C. subflavus (n 5 38), C. vivoi (n 5 31) and C. langguthi (n 5 32).
clade joining C. scotti and a clade grouping Cerradomys langguthi , Cerradomys vivoi , and C. subflavus . The topology of this latter clade is well resolved, with Cerradomys langguthi emerging as a sister taxon to a monophyletic group formed by C. subflavus and Cerradomys vivoi .
SUMMARY, COMPARISONS, AND DISCUSSION
Unlike other Brazilian species of Cerradomys ( C. scotti , C. maracajuensis , and C. marinhus ), C. vivoi , C. subflavus , and C. langguthi do not exhibit discrete autapomorphic diagnostic traits. On the contrary, as several sigmodontine rodents (see Patton et al., 2000; Bonvicino, 2003; D’Elía and Pardiñas, 2004; Gonçalves et al., 2005; Emmons and Patton, 2005; Percequillo et al., 2005) these species are diagnosed by a unique combination of character states, together with karyologic, morphometric, and molecular evidence.
Cerradomys species are characterized by a remarkable karyologic divergence (see Langguth and Bonvicino, 2002; Bonvicino, 2003); current recognized species, including C. vivoi View in CoL and C. langguthi View in CoL are clearly diagnosed by diploid and/or fundamental numbers, and by the morphology of autosomes and the sex chromosomes.
The combination of uni- and multivariate analyses confirms the existence of differences in the size and shape of cranial measurements among the valid species of Cerradomys View in CoL : C. maracajuensis View in CoL , C. marinhus View in CoL , C. scotti View in CoL , C. subflavus View in CoL , C. vivoi View in CoL , and Cerradomys langguthi View in CoL . Regarding Cerradomys subflavus View in CoL , C. vivoi View in CoL , and C. langguthi View in CoL , skull dimensions contributing significantly to this differentiation were relat- ed to the orbital region, auditory bulla, and cranial length and height. External measurements also separated the three species and, combined with skull dimensions, indicated a pattern in which C. subflavus View in CoL is always larger and more robust than C. vivoi View in CoL , whereas C. langguthi View in CoL is consistently smaller.
Maximum-parsimony analysis clearly supported the monophyly of Cerradomys . This analysis also demonstrated the close relationship between C. maracajuensis and C. marinhus , and C. scotti as a sister branch with respect to the clade formed by C. subflavus , C. vivoi , and C. langguthi . This analysis also suggested that the ancestor of C. subflavus and Cerradomys vivoi was more recent than their common ancestor with C. langguthi .
Species of the genus Cerradomys inhabit open and drier biomes in South America, namely the Caatinga, Cerrado, and Chaco. Nevertheless, species of this genus are predominantly associated with the more mesic habitats of these biomes, such as gallery forests; humid forests formed by orographic rainfall, observed in hills and mountain ranges in the biome Caatinga ( Mares et al., 1981),
TABLE 7 Summary of Karyotypic Variation from Samples of the Six Species of Cerradomys 2n 5 diploid number; FN 5 autosomal fundamental number. See fig. 1 for acronyms of Brazilian states.
TABLE 8 Genetic Distance Estimates between Species of Cerradomys locally known as brejos de altitude; patches of more humid, closed canopy forest to drier, open-canopy woodland, locally known as cerradão ( Eiten, 1972, 1992); and semideciduous forest. Exceptions to this pattern are the species that inhabit eastern Brazil: C. subflavus , C. vivoi , and C. langguthi . C. vivoi is a Caatinga-dwelling species that penetrates on Atlantic coastal rainforest in southern Bahia. The occurrence of C. vivoi in the latter habitat could be interpreted as a response to longterm, anthropic alteration of the habitat in this region (for example, extensive cacao plantations), since this species is found in low densities only in secondary and more open forest fragments, that occur marginally to more preserved fragments of Atlantic Forest (R. Pardini, unpublished data). A similar situation might be postulated for explaining the presence of Cerradomys langguthi (in this case, probably due to sugar-cane cultivation) and C. subflavus (probably due to extensive Eucalyptus plantations) in coastal Atlantic Forest in northeastern Brazil.
As the core species of the group in terms of geographic distribution (figs. 1, 2), C. scotti is sympatric with almost all described species of the group: C. maracajuensis in Mato Grosso and Mato Grosso do Sul, Brazil, Sapucay, Paraguay, and Santa Cruz, Bolivia; C. marinhus , at the type locality of this species in Bahia; and samples of C. subflavus in Minas Gerais and São Paulo. However, there are some important differences among these species regarding habitat preferences in the Cerrado: C. scotti is associated more with the open vegetation of the Cerrado biome, namely the open and closed scrubs, both with scattered trees, locally known as campo cerrado and cerrado sensu stricto, respectively ( Eiten, 1992), whereas C. maracajuensis and C. subflavus are associated more often with gallery forest and C. marinhus with grass marshes with buriti palms, called veredas ( Eiten and Goodland, 1979), and flooded forests. At present, there is no evidence of sympatry between C. vivoi , C langguthi , and other congeneric forms: the known distributional records of the first species are restricted to the habitats of Caatinga and Atlantic Forest biomes south and east of the Rio São Francisco; the available collecting localities for the second species are distributed on the Caatinga and Atlantic Forest habitats north to the Rio São Francisco.
On the basis of the evidence herein present- ed, the evolutionary differentiation of the genus Cerradomys can be someway inferred. Species from eastern Brazil, C. subflavus , C. vivoi and C. langguthi , exhibit a clear trend of clinal variation, observed both in quantitative morphologic and karyologic traits. Samples of C. subflavus , the species with the southernmost distribution, present the largest dimensions in most external and cranial measurements, while samples of northernmost Cerradomys langguthi are characterized by the smallest values (table 2). Although some overlap is observed, average body and cranial dimensions diminish from south to north: geographic samples of C. subflavus are larger than those of C. vivoi , which are larger that those of C. langguthi . The same pattern of variation is observed regarding the diploid number (table 7), which decreases from south to north. In addition Cerradomys subflavus , C. vivoi , and C. langguthi also share a common ancestor. If this were accepted as indicative of sequence events since divergence, this clade is the most recent evolutionary branch of this group, a likely explanation for the striking morphologic resemblance among these species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cerradomys vivoi
| Percequillo, Alexandre R., Hingst-Zaher, Erika & Bonvicino, Cibele R. 2008 |
C. vivoi
| Percequillo & Hingst-Zaher & Bonvicino 2008 |
C. langguthi
| Percequillo & Hingst-Zaher & Bonvicino 2008 |
C. vivoi
| Percequillo & Hingst-Zaher & Bonvicino 2008 |
Cerradomys langguthi
| Percequillo & Hingst-Zaher & Bonvicino 2008 |
C. vivoi
| Percequillo & Hingst-Zaher & Bonvicino 2008 |
C. langguthi
| Percequillo & Hingst-Zaher & Bonvicino 2008 |
C. vivoi
| Percequillo & Hingst-Zaher & Bonvicino 2008 |
C. langguthi
| Percequillo & Hingst-Zaher & Bonvicino 2008 |
C. scotti
| Langguth and Bonvicino 2002 |