Vipera walser Ghielmi, Menegon, Marsden, Laddaga & Ursenbacher
|
publication ID |
https://doi.org/10.1111/jzs.12138 |
|
DOI |
https://doi.org/10.5281/zenodo.4386624 |
|
persistent identifier |
https://treatment.plazi.org/id/3C548B6A-FF8C-6B02-FF97-F8C5FC7BFD11 |
|
treatment provided by |
Plazi |
|
scientific name |
Vipera walser Ghielmi, Menegon, Marsden, Laddaga & Ursenbacher |
| status |
sp. nov. |
Vipera walser Ghielmi, Menegon, Marsden, Laddaga & Ursenbacher sp. nov. ( Figs 1–4 View Fig. 1 View Fig. 2 View Fig. 3 View Fig. 4 ).
Holotype
Adult female: MSNG34485 , collected in S. Giovanni d’Andorno, on the road to Oropa in the Biella prealps , at about 1300 m a.s.l. by A. Rosazza in the summer of 1930 ( Fig. 5 View Fig. 5 ).
Paratypes
One adult male: MSNG 33638M collected at Monte Rosso del Croso, on 30 August 1933. One juvenile male: MSNG 33637B and one subadult male: MSNG 30818C collected at Alpe Finestre by Felice Capra, respectively, on 28 July 1930 and 15 August 1928. One adult female: MSNG 30818A, one subadult female: MSNG 30818B, and two juvenile females: MSNG 33637C and MSNG 33637D collected by Felice Capra at Alpe Finestre between August 1928 and August 1939. One juvenile female: MSNG 30286 collected by F. Capra at Monte Rosso del Croso on 12 September 1934; one adult female MSNG 33637A collected by F. Capra at Alpe le Piane on 5 August 1937; one adult female MSNG 41663 collected by A. Margiocco at Piedicavallo in September 1967.
Type locality
San Giovanni d’Andorno, strada per Oropa at 1300 m a.s.l. in the Alps north of town of Biella, a subrange of the Pennine Alps, north-western Italy.
Differential diagnosis
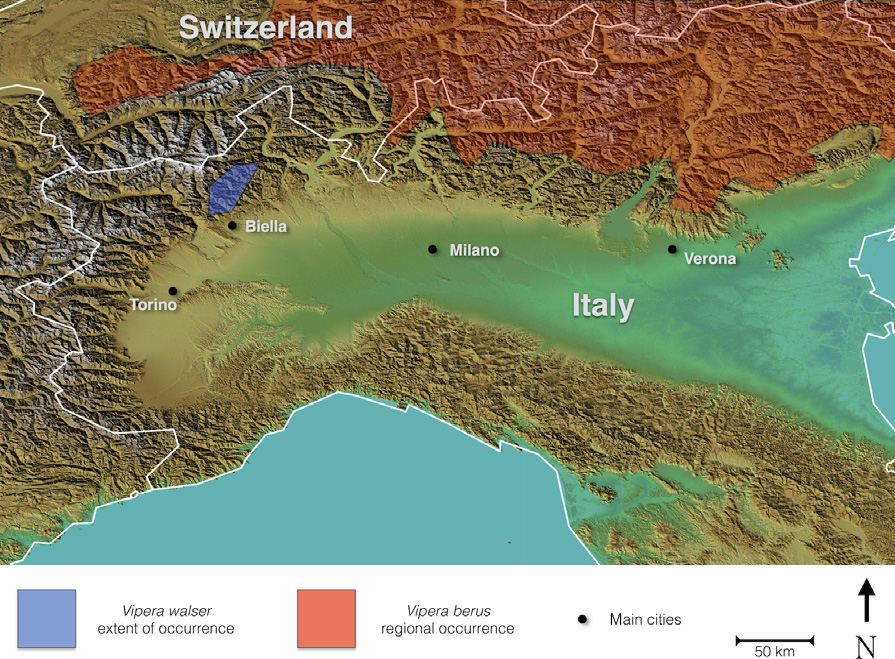
Vipera walser sp. nov. is generally similar to the species of the subgenus Pelias and can be confused with V. berus, which co-occurs on the Alps in allopatry ( Fig. 6 View Fig. 6 , Table 2 View Table 2 ). The species differs in a generalized higher count of cephalic scales, in particular the ones listed below ( V. berus in parentheses): higher number of crown scales: 7–30, mean 17.4 (versus 4–22, mean 13.0); loreals: 4–15, mean 9.36 (versus 2–12, mean 6.72); and, to a lesser extent, perioculars: 16–23, mean 19.8 (versus 13–23, mean 18.4) (see Table 2 View Table 2 ). V. walser , in contrast to V. berus , also shows a marked tendency towards fragmentation of the cephalic large shields: the parietal scales are often completely broken down into several smaller scales: 2–14, mean 6.3 (versus 2–10, mean 2.4; see also Fig. 7 View Fig. 7 ). Less commonly, also the frontal scale is fragmented into smaller scales. Some individuals exhibit a dorsum of the head covered in small, irregular scales, like in V. aspis . V. walser has between 1.5 and 2 rows of subocular scales on both sides of the head in 85% of the analysed specimens (V. berus has typically one row of suboculars, with the exception of some populations in the southern Alps). The dorsal zigzag is often broken down into separate bars as in Vipera aspis (Linnaeus, 1758) or Vipera berus bosniensis (see Fig. 6 View Fig. 6 ). Despite the lack of a strictly diagnostic morphological character, V. walser can be readily distinguished from populations of V. berus from Central and northern Europe by a combination of several characters (e.g. the number of subocular scales, fragmentation of parietals and number of apicals). Identification based solely on observation of external morphology is less obvious if individuals of V. berus from southern Alps are considered. Despite this, discriminant analysis correctly identified individuals to species in 94% of females and 88% of males, based on a set of analysed characters (see Figs 2 View Fig. 2 and 3 View Fig. 3 ). The mean p-distance, based on a combined dataset of about 3000 base pairs of mitochondrial genes, between V. berus and. walser is 5.36%. Based on our current knowledge of its distribution, Vipera walser is restricted to the Alps north of town of Biella, a subrange of the Pennine Alps, west of the river Ticino, north-western Italy ( Fig. 8 View Fig. 8 ).
The differences in cephalic scale count between Vipera walser and. berus are shown in Table 2 View Table 2 : Crown scales (females: t 45,49 = 4.81, p <0.0001; males: t 28,71 = 5.20, p <0.0001); loreals (females: t 94,59 = -7.52, p <0.0001; males: t 62,67 = -4.43, p <0.0001); and, in females only, perioculars (female: t 64,16 = 5.33, p <0.0001; males: t 17,25 = -0.16, p = 0.87) and apicals (females: t 32,86 = 2.14, p = 0.04; males: t 18,0 8 = -0.12, p = 0.91); the number of scales between the eyes and the supralabials are higher (females: t 66,40 = 5.85, p <0.0001; males: t 37,93 = 7.90, p <0.0001).
Paratype variations
Details and meristics for the analysed individuals, including the type series, are summarized in Table 3 View Table 3 .
Description of the holotype
Adult female conserved in 70% EtOH in rather good condition, with the body slightly swollen probably due to preservation. Snout-vent length (SVL) 515.2 mm, tail 55.0 mm, ratio of tail proportion (/SVL) 0.107. Two apical scales in contact with the rostral. Head oval shaped, wider in the temporal region, neck not very distinct, snout rounded. Frontal single, and larger than any other scale on head, five parietals. Rostral slightly higher than broader; nasal roundish, nostril circular and approximately in the centre of the nasal; one internasal on left side of the head and two on right side; perioculars 11–10; two rows of suboculars on both sides of the head; circumoculars separated from nasals by six and five loreal scales, respectively, on right and left side; supralabials 9–9, the fourth and the fifth below the eye; 147 ventrals; 31 divided subcaudals (excluding spine); anal entire; 21 scale rows at midbody. Dorsum is brown in colour with a continuous and regular darker brown zigzag. Head is reddish-brown with scattered, faint darker markings, and a more obvious inverted V-shaped ornamentation just before the neck. Labials are paler with black markings bordering the edges. A wide black band is present on both sides of the head between the postoculars and the neck. Ventrals are black, with white, scattered speckling along the lower margin of the scales and, more consistently, on both scale extremes by the first row of dorsals.
Etymology
Vipera walser sp. nov. is named after, and dedicated to, the Walser people with whom it shares an extraordinary beautiful and wild area of the south-western Alps.
Discussion
Delineating species boundaries correctly is crucial for the discovery of life’s diversity because it determines whether or not different individual organisms are members of the same entity ( Dayrat 2005). Most evolutionary biologists now agree that species are separately evolving lineages of populations or meta-populations, with disagreements remaining only about where along the divergence continuum separate lineages should be recognized as distinct species ( Padial et al. 2010). The Mitochondrial Tree Morphological Character Congruence (MTMC) approach has been formalized by Miralles and Vences (2013) and represents the most common practice in zootaxonomic studies, combining evidence from DNA sequences and morphological data. Integrative taxonomy has been also proposed as a framework to bring together conceptual and methodological developments aimed to describe, classify and name new taxa ( Padial et al. 2010). The integration by congruence approach of integrative taxonomy follows the principle that different lines of evidence should be combined to delimit species, such as genetic (mtDNA and nuclear), morphological, distributional and ecological data. The genetic differentiation between. walser and. berus, both on mitochondrial and nuclear DNA, is beyond known values between well-established species within the same subgenus. The status of full species is further confirmed by the bPTP analysis and as a morphological line of evidence by the discriminant analysis. Furthermore, there is no evidence of introgression from, for example,. berus as confirmed by the numerous individuals analysed for mtDNA, and the strong difference between these two species on the two nuclear genes sequenced. The species, within the alpine context, inhabits an ecologically peculiar area, characterized by some the highest rainfall of the whole alpine region ( Mercalli et al. 2008).
The discovery of the. walser lineage was particularly unexpected, especially in this biologically well-known and densely sampled region of western Europe. The species shows closer genetic affinities with, on one hand,. darevskii and. kaznakovi, species occurring in the Caucasus and, on the other, with the. ursinii complex (see Table 1 View Table 1 ), than with the. berus complex. Limited phylogenetic support suggests a simultaneous split between. ursinii complex,. kaznakovi ( Georgia) complex and. walser (possible trichotomy). Moreover, the ML phylogenetic reconstruction regrouped. walser with the. kaznakovi ( Georgia) complex, whereas the genetic distance displayed more affinities with the. ursinii complex.
Until now, it was believed that western Europe was colonized from the Pelias subgenus only by. berus (including. seoanei Lataste, 1879 , restricted to the Iberian peninsula), and the. ursinii group, which occupy distinct habitats (cold forest for. berus and steppe areas for. ursinii; Saint Girons 1978). The presence of a new distinct lineage, more related to the Caucasian vipers, strongly suggests an additional, more recent, colonization of western Europe (from the. kazankovi complex or during the split between the. kaznakovi complex and. ursinii complex) than the one involving the. berus group, and possibly one that was concurrent with that of. ursinii (Early Pliocene; Ferchaud et al. 2012).
Given that the European viper species tend to exclude each other geographically, resulting in limited portions of overlapped ranges ( Saint Girons 1978), we can assume that. walser found refugial areas different from those of. berus during the numerous glaciations of the Pleistocene. Currently, both. berus and. walser seem to occupy very similar habitats, suggesting a possible competition (or ecological differentiation as that between. aspis and. berus; Guillon et al. 2014). It is, however, possible that, like V. kaznakovi ,. walser can tolerate warmer temperatures than can. berus so long as sufficient humidity is present. Yet, this possibility needs to be investigated as it could have important implications for future conservation programmes.
Near-future threats and conservation
Vipera walser appears to occur only in a very limited area in the Alps north of Biella ( Fig. 8 View Fig. 8 ). It is very likely that all native populations of adder south of the Alps and west of the river Ticino belong to the species herein described. Based on the Italian Atlas of Amphibians and Reptiles ( Sindaco et al. 2006), the current distribution area (‘extent of occurrence’) is almost certainly < 1000 km 2. Consequently,. walser should be classified as ‘endangered’ according to (2014) Red List criteria B1a/B2a. If we consider that the population is strongly fragmented, or that the actual area of occupancy is probably < 500 km 2 and fragmented (Red List Categories and Criteria: Version 3.1. Second edition), then. walser appears to be among the most threatened vipers in the world. The new taxon’s sister species. darevskii, with area of occupancy < 10 km 2, is now listed as ‘critically endangered’ ( Tuniyev et al. 2009), whereas. kaznakovi (related to. darevskii and thus to. walser) is considered ‘endangered’, meaning that the entire clade is highly threatened with extinction.
Within its restricted range,. walser appears to be quite common in suitable habitat. However, to date, no systematic survey has been undertaken, either to estimate its population density or identify its habitat requirements. Such surveys are clearly a priority for the future research. Estimates of current abundance, using mark–recapture or distance sampling (e.g. Mazerolle et al. 2007), would be useful to determine total population size and trends, and to more precisely assign the species to a Red List category. Occupancy modelling ( Larson 2014) might also be suitable to determine areas of occupancy at appropriate scales.
Perhaps more important would be detailed studies of the species’ precise habitat requirements, to determine how past and current land use changes have affected the species, and how they might be altered to benefit the species in the near future. Based on our preliminary observations, this species inhabits open areas, often with rocky outcrops ( Fig. 9 View Fig. 9 ), and may not tolerate woodland unless it is very sparse. European mountains experienced a long period of agricultural/agropastoral expansion from the Late Middle Ages to the 19th century, with large areas of the Alps converted to upland grasslands and heathlands (e.g. Vives et al. 2014). These open landscapes were presumably beneficial for. walser. However, the decline in agropastoral activities in the last 100 years and associated afforestation ( Carlson et al. 2014; Garbarino et al. 2014) is probably the greatest threat to the species, and it is an urgent priority to assess such changes within the range of. walser. More immediate and major threats in the short term are culling and collection. Indeed, the description of several new vipers species (e.g.. kaznakovi and Montivipera wagneri (Nilson & Andŕen, 1984)) , as well as the attraction of being peculiar and rare (e.g. Macrovipera schweizeri ( Werner, 1935) , has led to the illegal collection of numerous individuals for the international pet trade ( Nilson et al. 1999, http://www.iucnredlist.org), causing local extinctions. Because this species occurs only in Italy, we strongly suggest that a specific legal protection for the species should be implemented very quickly.
Longer term prospects and climatic change
Of course, it can be argued that. walser, as a restricted-range relic species, is likely heading down an evolutionary dead-end path ( Allendorf and Luikart 2007), in the sense of Darwin’s ‘wreck of ancient life’ ( Darwin 1859) or Jeannel’s ‘fossiles vivants’ ( Jeannel 1943). Its eventual natural extinction may take many millennia, but its ability to survive the next 100 years may hang on two important aspects of its biology. First, there is a real lack of genetic variability within the population as compared to that in other vipers (e.g. Ursenbacher et al. 2006a, b; Ferchaud et al. 2011). The population is already fragmented into two main subpopulations, and, presumably, the complex topography of ridges and valleys may work to further isolate populations, as in. berus ( Ursenbacher et al. 2009). A high priority for future study is an examination of habitat suitability at the landscape scale coupled with research on dispersal mechanisms and ability in the species.
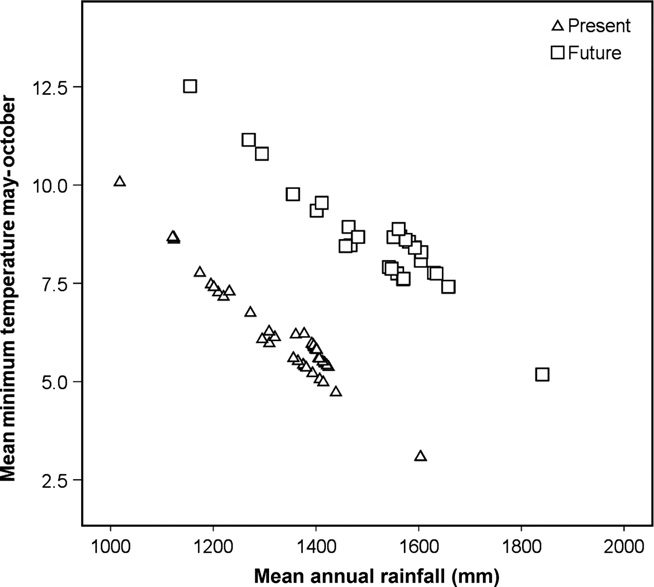
Second, and related to the above, its ability to withstand or adapt to climatic change expected to take place within its range will be crucial. The current habitat of. walser is restricted to an area of around 800 km 2 within a few valleys, which experience some of the highest rainfall in the Alps ( Mercalli et al. 2008). Point estimates of annual rainfall from presence locations within its area of occupancy range from 1018 to 1604 mm (mean = 1348 mm ± 111) and mean minimum temperature between May and October from 3.1 to 10.0°C (mean = 6.1 ± 1.2°C SD). Climate models (CMIP5 IPPC Fifth Assessment; www.worldclim.org) indicate that in the next 20 years, these valleys will become far wetter (mean = 1536 mm ± 129 SD) and warmer (mean = 8.5 ± 1.2°C SD; Fig. 10 View Fig. 10 ). Consequently, species distribution modelling, and how this distribution might change under realistic climate change scenarios, especially including the influence of habitat and habitat change and dispersal ability (e.g. Pearson and Dawson 2003), is clearly a priority.
Conclusion
The present study described and named a new viper species,. walser, which shows strong genetic divergence and clear morphological differentiation from all other known European viper species. The new taxon occurs in a restricted area of the southwestern Italian Alps and shows close affinities with the Caucasian species. dinniki,. darevskii and. kaznakovi, opening unexpected and interesting biogeographic scenarios. The very small extent of occurrence of the new species implies a particularly high threat level, and thus conservation managements should be developed. The protection of its habitat, the limitation of the forest regrowth, but also the evaluation of its likely future distribution given climatic changes (for the long term) or struggle against culling (short term) are key elements to investigate. Involvement of local authorities, foundations and other stakeholders will be crucial in realizing effective protection of this species.
Table 2. Mean sizes, general and head scalation of Vipera walser sp. nov. and other related species, with standard deviations and minimal / maximal values, when provided. Origin of the data: (1) this study; (2) Ursenbacher et al. 2005; (3) Scali and Gentilli 1998; (4) Joger and S ẗumpel 2005; (5) Nilson et al. 1995; (6) Orlov and Tuniyev 1990; (7) Geniez and Teyníe, 2005; (8) Göcʔmen et al. 2014; (9) Avćy et al. 20 - 10. The number of males and females is indicated except for the last two species, where data have been gathered from studies and the information was not available.
| Vipera walser sp. | nov.(1) | Vipera berus (Italian | clade)(1) | Vipera berus (Italian clade)( 2) | Vipera berus (Po Plain) (3) | Vipera berus (Northern clade)( | 2) | Vipera berus bosniensis (4) | Vipera kaznakovi (4, 5, 6) | Vipera darevskii recalculated from (6, 7, 8 and 9) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (31) | Male (17) | Female (70) | Male (65) | Female (35) | Male (28) | Female (13) | Male (11) | Female (54) | Male (47) | Female | Male | Female | Male | Female | Male | |
| Ventral scales | 148.50 ± 3.45 141–156 | 143.33 ± 3.28 138–149 | 145.13 ± 4.96 131–165 | 142.68 ± 4.49 131–158 | 145.77 ± 3.23 137–153 | 142.43 ± 3.73 134–149 | 145.9 ± 1.3 140–154 | 142.1 ± 1.1 136–148 | 147.15 ± 3.55 139–155 | 144.11 ± 3.54 135–152 | 144.90 ± 2.81 139–159 | 141.75 ± 3.19 136–149 | 136.2 ± 2.60 130–139 | 135.0 ± 1.81 133–139 | 136.5 ± 4.4 132–144 | 134.6 ± 3.5 129–136 |
| Subcaudal scales | 27.96 ± 2.41 23–32 | 35.06 ± 2.41 30–38 | 28.39 ± 3.28 22–41 35.05 ± 3.27 27–43 | 35.05 ± 3.27 27–43 | 27.63 ± 2.13 23–34 | 34.36 ± 2.39 29–39 | 33.8 ± 1.1 29–42 | 41.5 ± 1.1 35–47 | 30.50 ± 3.59 36.82 ± 2.71 21–39 30–42 | 36.82 ± 2.71 21–39 30–42 | 36.87 ± 2.71 32–42 | 28.60 ± 2.18 24–32 | 28.4 ± 1.69 26–32 | 33.6 ± 2.80 23–41 | 28.9 ± 2.76 25–33 33.4 ± 3.68 29–38 | 33.4 ± 3.68 29–38 |
| Loreals (both side) | 9.45 ± 2.16 | 9.19 ± 1.72 | 7.29 ± 2.29 | 6.12 ± 2.18 | 6.71 ± 2.47 | 5.89 ± 2.60 | 5.54 ± 2.40 | 4.47 ± 1.99 | 11.06 ± 3.13 | |||||||
| Perioculars (both side) | 20.00 ± 1.51 | 19.50 ± 1.83 | 18.20 ± 1.69 | 18.55 ± 1.94 | 18–22 | 20.0 ± 1.98 | 18.04 ± 1.55 | |||||||||
| Apicals | 2.29 ± 0.74 | 1.94 ± 0.43 | 2.00 ± 0.24 | 1.95 ± 0.21 | 1.50 ± 0.54 | 1.57 ± 0.5 | ||||||||||
| Crown scales | 18.07 ± 4.41 | 16.00 ± 2.30 | 13.70 ± 3.51 | 12.26 ± 3.27 | 14.94 ± 3.79 | 7.5 ± 2.4 | ||||||||||
| Subocular | 1.55 ± 0.30 | 1.50 ± 0.16 | 1.14 ± 0.31 | 1.09 ± 0.23 | ||||||||||||
| ranks | ||||||||||||||||
| Total length (mm) | 455.56 ± 167.1 | 386.00 ± 50.83 | 491.83 ± 71.79 | 451.88 ± 71.25 | 420.0 ± 37.1 | 437 ± 21.3 | 479.4 ± 45.8 | 466.4 ± 40.4 | 382.1 ± 46.7 | 376.8 ± 32.0 | ||||||
| Tail length (mm) | 43.42 ± 17.69 | 50.83 ± 14.16 | 52.27 ± 8.75 | 60.02 ± 10.19 | 56.6 ± 5.7 | 76.3 ± 5.0 | 52.0 ± 6.9 | 64.0 ± 5.7 | 45.1 ± 6.3 | 55.1 ± 5.3 | ||||||
| % Tail | 9.90% ± 0.70% | 12.8% ± 1.09% | 10.7% ± 1.25% | 13.3% ± 1.54% | 11.9% | 14.9% | 10.8% | 13.7% | 11.8% 1.10% | 14.7% 1.71% |
Table 3. Details of the morphological measurements of the investigated individuals of V. walser sp. nov.
| Total | Tail | Perioculars | Parietals | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| length | length | Crown | Loreals | (sum left+ | Suboculars | (sum left+ | |||||||||
| ID | ID locality | Age | (in mm) | (in mm) | scales | Rostral | (mean) | right) | (mean) | Apicals | Frontal | right) | Subcaudals | Ventrals | MSR |
| M1 | v.stronam 1 | ad | 14 | 1 | 4 | 20 | 1,5 | 2 | 1 | 3 | 36 | ||||
| M2 | v.stronam 2 | ad | 18 | 1 | 4,5 | 21 | 1,5 | 2 | 2 | 3 | 36 | ||||
| M3 | v.elvom 1 | ad | 10 | 1 | 4,5 | 16 | 1,5 | 2 | 1 | 9 | 34 | ||||
| M4 | v.olocchiam 1 | ad | 14 | 1 | 5,5 | 22 | 1,25 | 2 | 1 | 2 | 38 | ||||
| M5 | v.mastallonem 1 | ad | 17 | 2 | 4 | 20 | 1,25 | 2 | 1 | 6 | 31 | ||||
| M6 | v.dolcam 1 | juv | 20 | 1 | 5,5 | 21 | 2 | 1 | 1 | 3 | 38 | ||||
| M7 | a.meggianam1 | ad | 410 | 61 | 17 | 2 | 5,5 | 21 | 1,5 | 2 | 1 | 4 | 38 | 142 | 22 |
| M8 | oropam1 | ad | 5 | 3 | 7 | ||||||||||
| M9 | v.elvom 2 (MRSN)(MZUT R 2069) | ad | 16 | 1 | 1,5 | 2 | 1 | 7 | 36 | ||||||
| M10 | m.rossodelcrosom1 (MSNG33638M) | ad | 481 | 61 | 15 | 1 | 2,5 | 18 | 1,5 | 1 | 1 | 2 | 33 | 149 | 21 |
| M11 | cimarascàm1 (MSNG32286) | ad | 480 | 60 | 19 | 1,5 | 2 | 1 | 2 | 35 | 143 | 21 | |||
| M12 | a.finestrem1 (MSNG30818C) | subad. | 306 | 40 | 15 | 1 | 3,5 | 16 | 1,5 | 2 | 1 | 2 | 37 | 143 | 21 |
| M13 | a.finestrem2 (MSNG33637B) | juv | 224 | 27 | 16 | 1 | 4,5 | 19 | 1,5 | 2 | 1 | 2 | 36 | 147 | 21 |
| M14 | sesseram1 (MISN N° cat.2) | ad | 17 | 1 | 5,5 | 19 | 1,5 | 2 | 1 | 6 | 36 | 138 | 21 | ||
| M15 | v.stronam 3 | ad | 505 | 16 | 1 | 4,5 | 18 | 1,5 | 2 | 1 | 2 | 33 | 141 | 19 | |
| M16 | v.stronam 4 | juv | 210 | 17 | 1 | 5 | 22 | 1,5 | 2 | 1 | 4 | 34 | 142 | 21 | |
| M17 | v.riobachm 1 | ad | 472 | 56 | 16 | 1 | 5,5 | 20 | 1,5 | 2 | 1 | 4 | 30 (28–32) | 145 | 21 |
| F1 | v.chiobbiaf 1 | juv | 21 | 1 | 6 | 21 | 1,5 | 2 | 1 | 5 | 31 | ||||
| F2 | v.stronaf 1 | ad | 17 | 1 | 4,5 | 21 | 1,5 | 2 | 1 | 9 | 26 | ||||
| F3 | v.stronaf 2 | ad | 20 | 1 | 5,5 | 21 | 2 | 3 | 1 | 3 | 27 | ||||
| F4 | v.masttallonef 1 | juv | 19 | 1 | 5,5 | 19 | 1,625 | 2 | 1 | 4 | 30 | ||||
| F5 | v.stronaf 3 | subad. | 26 | 1 | 5 | 23 | 1,5 | 2 | 1 | 10 | 27 | ||||
| F6 | v.stronaf 4 | ad | 25 | 1 | 7,5 | 22 | 2 | 4 | 1 | 13 | 27 | ||||
| F7 | v.dolcaf 1 | ad | 16 | 1 | 6 | 19 | 1,5 | 1 | 1 | 12 | 32 | ||||
| F8 | v.mastallonef 2 | juv | 16 | 1 | 4 | 20 | 1,5 | 3 | 1 | 8 | 27 | ||||
| F9 | v.mastallonef 3 | ad | 18 | 1 | 4 | 20 | 1,5 | 2 | 1 | 6 | 29 | ||||
| F10 | v.elvof 1 | ad | 610 | 65 | 18 | 1 | 5 | 18 | 1,5 | 3 | 2 | 14 | 32 | 148 | 21 |
| F11 | v.vognaf 1 | ad | 527 | 55 | 13 | 1 | 4,5 | 18 | 1 | 1 | 1 | 2 | 25 | 149 | 21 |
| F12 | v.vognaf 2 | ad | 548 | 59 | 13 | 1 | 4,5 | 21 | 1,5 | 2 | 1 | 4 | 28 | 150 | 21 |
| F13 | a.lepianef1 (MSNG33637A) | ad | 588 | 59 | 14 | 1 | 2 | 19 | 1,5 | 2 | 1 | 2 | 29 | 147 | 21 |
| F14 | s.giovannidandornof1 (MSNG34485) | ad | 570 | 55 | 16 | 1 | 5,5 | 21 | 2 | 2 | 1 | 5 | 31 | 147 | 21 |
| F15 | a.finestref1 (MSNG30818B) | subad. | 263 | 27,5 | 17 | 1 | 5 | 20 | 2 | 2 | 1 | 4 | 29 | 148 | 21 |
| F16 | m.rossodelcrosof1 (MSNG30286) | juv | 232 | 24,5 | 17 | 1 | 4,5 | 22 | 1,5 | 2 | 1 | 6 | 27 | 147 | 21 |
| F17 | a.finestref2 (MSNG33637C) | juv | 213 | 21 | 20 | 1 | 4,5 | 19 | 1,5 | 2 | 1 | 2 | 28 | 154 | 19 |
| F18 | a.finestref3 (MSNG33637D) | juv | 191 | 17,5 | 16 | 1 | 2,5 | 20 | 1 | 1 | 1 | 6 | 23 | 149 | 21 |
| F19 | v.sesiaf 1 (MSNG2171A) | ad | 593 | 55 | 7 | 17 | 1 | 1 | 1 | 3 | 30 | 156 | 21 | ||
| F20 | r.valdobbiaf1 (MSNG2171B) | juv | 219 | 22 | 20 | 1,5 | 2 | 1 | 29 | 151 | 23 | ||||
| F21 | a.finestref4 (MSNG30818A) | ad | 19 | 1 | 5 | 18 | 1,5 | 3 | 1 | 6 | |||||
| F22 | oropaf1 | ad | 17 | 1 | 5,5 | 19 | 2 | 3 | 1 | 3 | |||||
| F23 | oropaf2 | juv | 19 | 1 | 5 | 23 | 2 | 3 | 1 | 6 | |||||
| F24 | sesseraf1 | ad | 15 | 1 | 3 | 20 | 1 | 2 | 1 | 3 | |||||
| F25 | v.stronaf 5 | ad | 535 | 50 | 17 | 1 | 4 | 18 | 1,5 | 3 | 1 | 9 | 30 | 148 | 21 |
| F26 | v.stronaf 6 | ad | 520 | 20 | 1 | 5,5 | 21 | 2 | 3 | 1 | 3 | 27 | 145 | ||
| F27 | v.stronaf 7 | ad | 460 | 22 | 1 | 4 | 20 | 1,5 | 3 | 1 | 7 | 24 | 141 | ||
| F28 | v.stronaf 8 | ad | 16 | 1 | 5,5 | 20 | 1,5 | 3 | 1 | 7 | 27 | 151 | |||
| F29 | v.stronaf 9 | ad | 580 | 21 | 1 | 4,5 | 22 | 1,5 | 3 | 2 | 4 | 26 | 145 | 21 | |
| F30 | sesseraf2 | ad | 640 | 54 | 14 | 1 | 4,5 | 19 | 1,5 | 2 | 1 | 2 | 24 | 146 | |
| F31 | piedicavallof1 (MSNG41663) | ad | 16 | 1 | 4,5 | 19 | 1,5 | 2 | 1 | 10 | 30 | 151 |
| MSNG |
Museo Civico di Storia Naturale di Genova 'Giacomo Doria' |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.