Mesobiotus anastasiae, Tumanov, Denis V., 2020
|
publication ID |
https://doi.org/ 10.5852/ejt.2020.726.1179 |
|
publication LSID |
lsid:zoobank.org:pub:CCB2BAEF-06D4-4FAD-A018-27A54553D3DA |
|
DOI |
https://doi.org/10.5281/zenodo.5634053 |
|
persistent identifier |
https://treatment.plazi.org/id/8651D164-4CBC-41DD-A5C4-FF9E584F8088 |
|
taxon LSID |
lsid:zoobank.org:act:8651D164-4CBC-41DD-A5C4-FF9E584F8088 |
|
treatment provided by |
Plazi |
|
scientific name |
Mesobiotus anastasiae |
| status |
sp. nov. |
Mesobiotus anastasiae View in CoL sp. nov.
urn:lsid:zoobank.org:act:8651D164-4CBC-41DD-A5C4-FF9E584F8088
Figs 1–6 View Fig View Fig View Fig View Fig View Fig View Fig , Tables 2–3
Etymology
I dedicate this new species to my daughter, Anastasia.
Material examined
Holotype REPUBLIC OF SOUTH AFRICA • ♀; Cape Town, top of Table Mountain; approx. 33°57′43.9″ S, 18°24′38.0″ E; = 1000 m a.s.l.; 10 Jan. 2008; I. Nikolaeva leg.; moss on soil, wet depression with a small temporary pond; SPbU 256(8) . GoogleMaps
Paratypes REPUBLIC OF SOUTH AFRICA • 5 ♀♀, 3 ♂♂, 22 eggs; same collection data as for holotype; SPbU 256(1) to 256(6) and 256(9) to 256(18) GoogleMaps • 2 adults, 7 eggs; same collection data as for holotype; SEM stub; SPbU_Tar16 GoogleMaps .
Comparative material
PEOPLE’S REPUBLIC OF CHINA • 7 eggs, paratypes of Mesobiotus mauccii ( Pilato, 1974) ; Canton; moss on tree bark; UNICT 2132 .
Morphological description
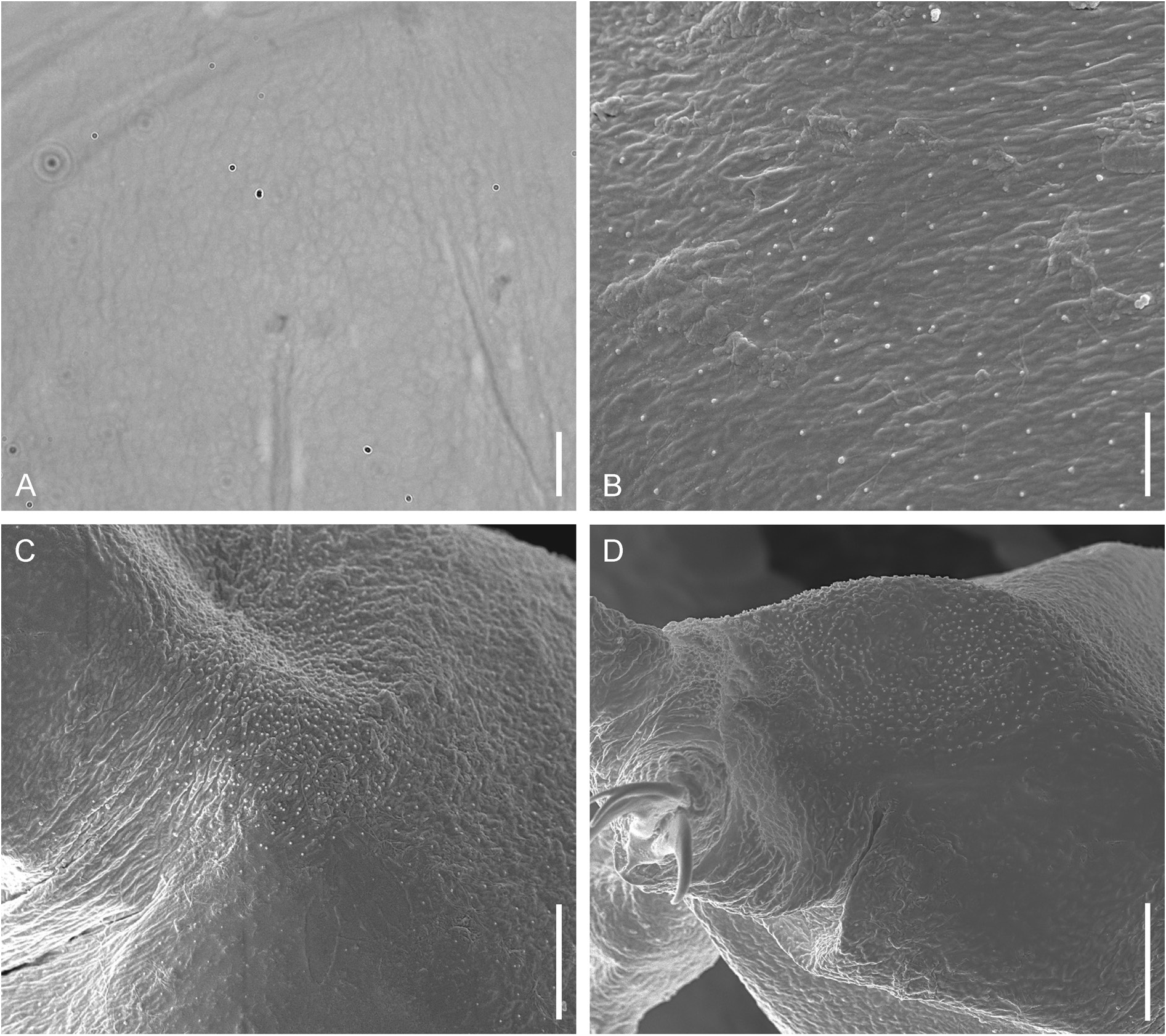
Body elongated ( Fig. 1 View Fig A–B) (morphometrics in Table 2, raw morphometric data are provided in Supplementary file 2). Fresh specimens uncolored or whitish with slightly greenish gut content, transparent after fixation in Hoyer’s medium. Four specimens with eyes ( Fig. 1A View Fig ), usually welldiscernible after slide mounting, three specimens without eyes; in two specimens processed for DNA extraction the presence or absence of eyes was not registered. Dorsal cuticle in LM with poorly visible network-like pattern ( Fig. 2A View Fig ). SEM investigation revealed presence of flat tubercles all over the body surface ( Fig. 2 View Fig B–C). Additionally, the body surface is covered with regularly distributed small granules (invisible in LM; Fig. 2B View Fig ). In the dorso-lateral position, above the bases of the hind legs, zones of concentrated granules are present ( Fig. 2C View Fig ). All legs with granulated areas. Legs I–III with small granulated areas on the external surfaces, near the claw bases ( Fig. 4A View Fig ), invisible or extremely poorly visible in LM ( Fig. 4D View Fig , black arrowhead), the internal leg surfaces without granulation, with indistinct pulvinus ( Fig. 4B View Fig , white asterisk). Legs IV with better-developed granulation dorsally ( Fig. 2D View Fig ) and around the claw bases ( Fig. 4G View Fig ).
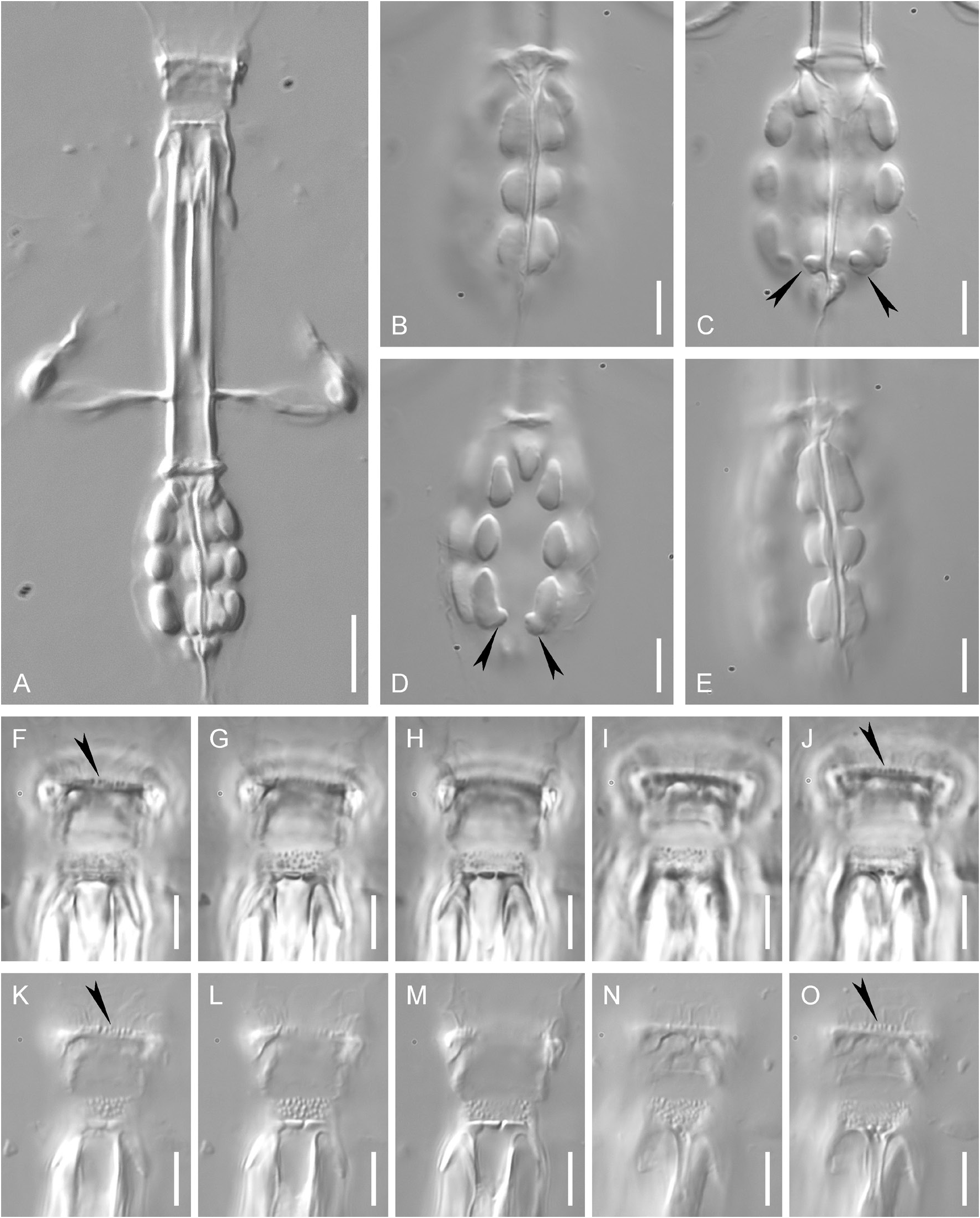
Buccal-pharyngeal apparatus of Macrobiotus type ( Fig. 3A View Fig ) with ventral lamina and ten peribuccal lamellae. Oral cavity armature (OCA) of modified krynauwi type (according to Kaczmarek et al. 2020) with three bands of teeth visible in LM. Evident first (anterior) band consists of a single uneven line of relatively large and slightly longitudinally elongated teeth ( Fig. 3F View Fig , J–K, O, black arrowheads). Second band consists of a wide zone of small dot-like teeth ( Fig. 3G, I, L, N View Fig ), teeth of the anterior-most and (rarely) posterior-most rows of the second band are slightly larger than others, sometimes teeth of the anterior-most row are very slightly longitudinally elongated. Third band comprises three dorsal and three ventral transverse ridges ( Fig. 3H, J, M, O View Fig ). Medio-ventral ridge is usually more or less clearly divided into two separate parts ( Fig. 3J, O View Fig ). Pharyngeal bulb with apophyses, three macroplacoids and a large microplacoid ( Fig. 3A View Fig ). Macroplacoid length sequence is 2 <3=1. First macroplacoid is anteriorly
narrowed, third macroplacoid without distinct subterminal constriction, but with strong terminal protrusion, directed towards pharynx lumen ( Fig. 3 View Fig B–E, black arrowheads).
Claws of Mesobiotus type with minute stalk, distinct distal part of the basal portion, short common tract and developed internal septum, defining a distal part. Primary and secondary branches diverge at a point near half the claw height, main branches with long accessory points, which at a large distance from the main claw ( Fig. 4C View Fig , E–F, H). Claws of fourth pair of legs slightly longer than claws of first three pairs of legs ( Fig. 4H View Fig ). All claws with smooth lunules ( Fig. 4C View Fig , E–F, H). Anterior (internal) and posterior (external) claws of legs IV are similar in shape, with equally sized lunules. Poorly developed bar-like cuticular thickenings are present below claw bases of the first three pairs of legs ( Fig. 4C View Fig , black arrowhead). Claws of legs IV are connected with a wide horseshoe-like structure ( Fig. 4F View Fig , black arrowhead).
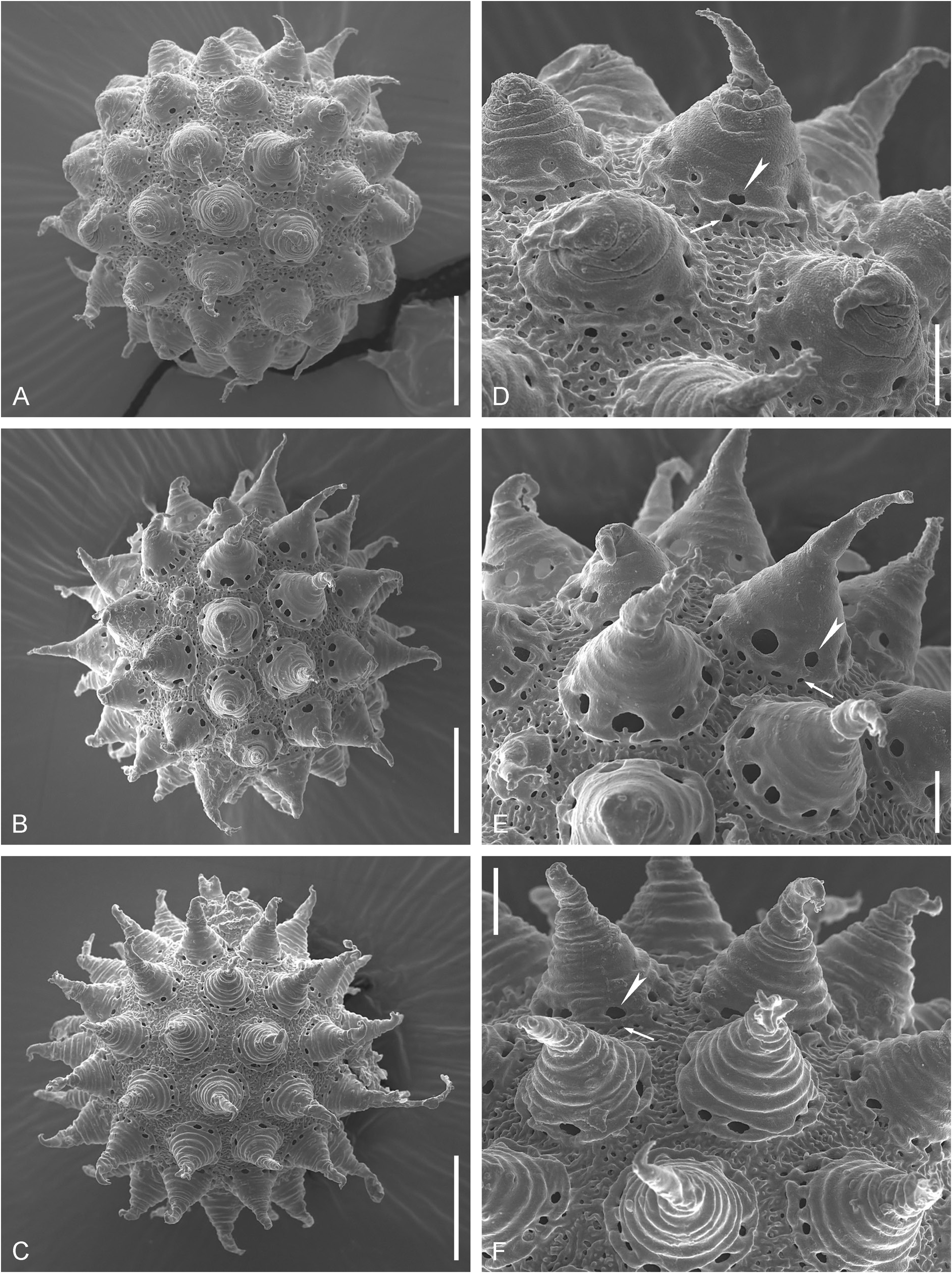
Eggs spherical, white, ornamented and laid freely ( Figs 5 View Fig A–B, 6A–C; morphometrics in Table 3). Chorion with conical processes that can be attributed to the “ sharp wide cones with collars ” and “ reticular design with “bubbles” ” morphotypes (according to Kaczmarek et al. 2020). Egg processes with wide bases and thinned and flexible apices ( Figs 5 View Fig C–I, 6D–F). Basal parts of the processes with bilayered walls, with a net of trabecular structures between the internal and external layers, forming irregular rounded meshes of different size, so the processes seem to be reticulated in LM ( Fig. 6 View Fig C–I). Apical parts of the processes with bubble-like internal structure ( Fig. 5 View Fig E–I), often branching ( Figs 5 View Fig E–F, 6E–F). The SEM observations revealed circular wrinkles on the outer surface of the processes ( Fig. 6 View Fig D–F). Basal parts of the processes with well-developed collar elevated above the egg surface ( Figs 5 View Fig G–I, black arrowheads; 6D–F). Large pores (1–3 µm in diameter), visible in LM and in SEM, are present on the surface of all processes, near the collar, in two rows: one row of larger pores above the collar and second row of smaller pores below the collar ( Figs 5 View Fig C–E, black arrowhead; 6D–F). Process bases are smooth, without a crown of granules or teeth. Egg surface between the processes without areolation, with a system of Fig, 7. Mesobiotus mauccii ( Pilato, 1974) . Paratype egg (UNICT 2132). A–B. Egg processes, white arrowhead indicates polygonate mesh-like pattern, black arrowheads indicate the collar, black arrows indicate pores, DIC. Scale bars: 10 µm.
irregularly distributed ridges and small pores between them ( Fig. 6 View Fig D–F). In some eggs underdeveloped small processes are present among normal processes ( Fig. 6B, E View Fig ).
Reproduction
The new species is dioecious.Adult males were identified by having testis filled with spermatozoa, visible under PhC on mounted slides. Males of M. anastasiae sp. nov. exhibit no secondary sexual dimorphism.
DNA sequences
Sequences of good quality for the four aforementioned molecular markers were obtained from one specimen (voucher slide SpbU 256(11)):
COI sequence (GenBank: MT904513 View Materials ), 658 bp long;
18S rRNA sequence (GenBank: MT903468 View Materials ), 1030 bp long;
28S rRNA sequence (GenBank: MT903612 View Materials ), 740 bp long;
ITS-2 sequence (GenBank: MT903470 View Materials ), 419 bp long.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Macrobiotoidea |
|
Family |
|
|
Genus |