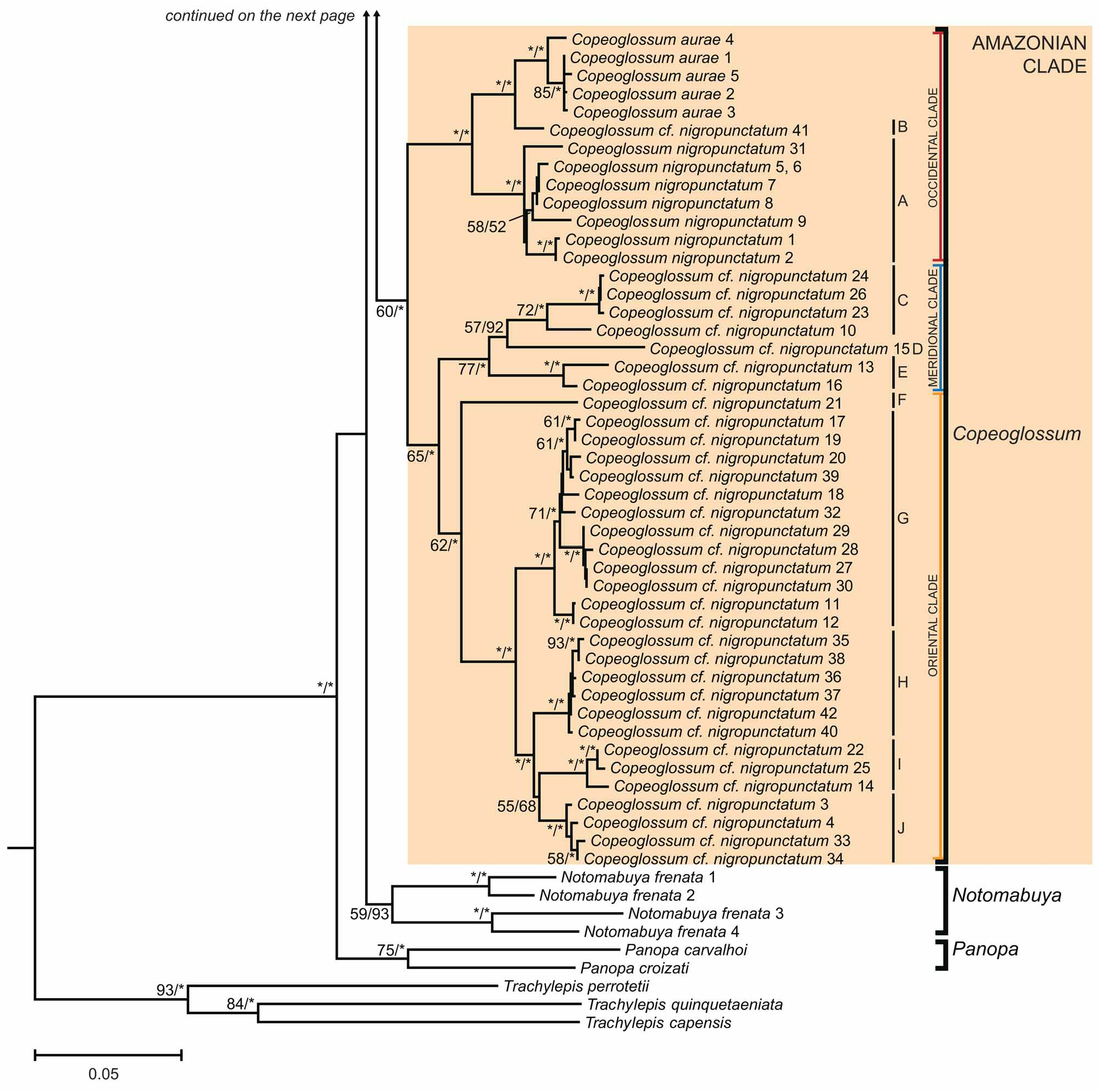
Marisora aurulae, Hedges & Conn, 2012
|
publication ID |
https://doi.org/10.11646/zootaxa.3288.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/39191A7F-0770-FF7D-2DA9-EDB17F01FDE4 |
|
treatment provided by |
Felipe |
|
scientific name |
Marisora aurulae |
| status |
sp. nov. |
Marisora aurulae sp. nov.
Lesser Windward Skink
( Figs. 46A View FIGURE 46 , 47A View FIGURE 47 , 48 View FIGURE 48 )
Mabuia agilis — Boulenger, 1887:191 (part).
Mabuia aenea — Garman, 1887:53 (part).
Mabuya aenea — Barbour, 1914:322 (part).
Mabuya aenea —Barbour, 1930:105 (part).
Mabuya mabouia — Barbour, 1935:129 (part).
Mabuya mabouya mabouya — Dunn, 1936:544 (part).
Mabuya mabouia — Barbour, 1937:147 (part).
Mabuya aenea — Underwood, 1963:83 (part).
Mabuya mabouya mabouya —Peters & Donoso-Barros, 1970:200 (part).
Mabuya mabouya mabouya — Schwartz & Thomas, 1975:141 (part).
Mabuya mabouya mabouya — MacLean et al., 1977:40–41 (part).
Mabuya mabouya mabouya — Schwartz & Henderson, 1988:150 (part).
Mabuya mabouya mabouya — Schwartz & Henderson, 1991:457 (part).
Mabuya bistriata — Powell et al., 1996:82 (part); Murphy, 1997:150 (part).
Mabuya sloanii — Mayer & Lazell, 2000:883 (part).
Mabuya mabouya —Miralles, 2005:49 (part?).
Mabuya falconensis —Miralles et al., 2009:609 (part).
Mabuya mabouya — Henderson & Powell, 2009:292 (part).
Holotype. MCZ R-38196, an adult female from Young's Island , St. Vincent, collected 11 November 1934 by J. B. Myers.
Paratypes (n = 12). Grenada. MCZ R-79743, James Lazell , Glover Island, 21 June 1964 ; USNM 72658–59 About USNM , Belmont , St. George (no collection date available) . Grenadines. KU 242049, Albert Schwartz , Saline Bay, Mayreau ( Mayero) Island, 13 December 1961 ; KU 242050, Albert Schwartz, Petit Bateau , Tobago Cays , 13 December 1961 ; MCZ R-79098, C. MacIntosh, Carriacou, 1963 . Tobago. KU 242012, Albert Schwartz , 1 mile E Canaan ( 13 May 1963); MCZ R-12079–80, W. E. Broadway (no specific locality or collection date available); MCZ R-55668, Garth Underwood, Scarborough, 5 September 1956 . Trinidad. MCZ R-100482–83, J. Boos, La Romain , 14 June 1967 .
Other material (n = 4). Grenada. MCZ R-4514, P. Sellinan, no specific locality, ca. 1882 (see Remarks).
Diagnosis. Marisora aurulae sp. nov. is characterized by (1) maximum SVL in males, 80.9 mm; (2) maximum SVL in females, 89.0 mm; (3) snout width, 2.47–3.08% SVL; (4) head length, 16.7–19.1% SVL; (5) head width, 13.0–15.0% SVL; (6) ear length, 1.00–2.13% SVL; (7) toe-IV length, 7.96–10.5% SVL; (8) prefrontals, two; (9) supraoculars, four; (10) supraciliaries, four (85%), five (15%); (11) frontoparietals, two; (12) supralabial below the eye, five (69%), six (31%); (13) nuchal rows, one; (14) dorsals, 57–63; (15) ventrals, 57–68; (16) dorsals + ventrals, 114–129; (17) midbody scale rows, 30–32; (18) finger-IV lamellae, 11–15; (19) toe-IV lamellae, 14–17; (20) finger-IV + toe-IV lamellae, 26–32; (21) supranasal contact, Y (46%), N (54%); (22) prefrontal contact, N; (23) supraocular-1/frontal contact, N; (24) parietal contact, Y; (25) pale middorsal stripe, N; (26) dark dorsolateral stripe, N; (27) dark lateral stripe, Y; (28) pale lateral stripe, Y; and (29) palms and soles, dark ( Tables 3–5).
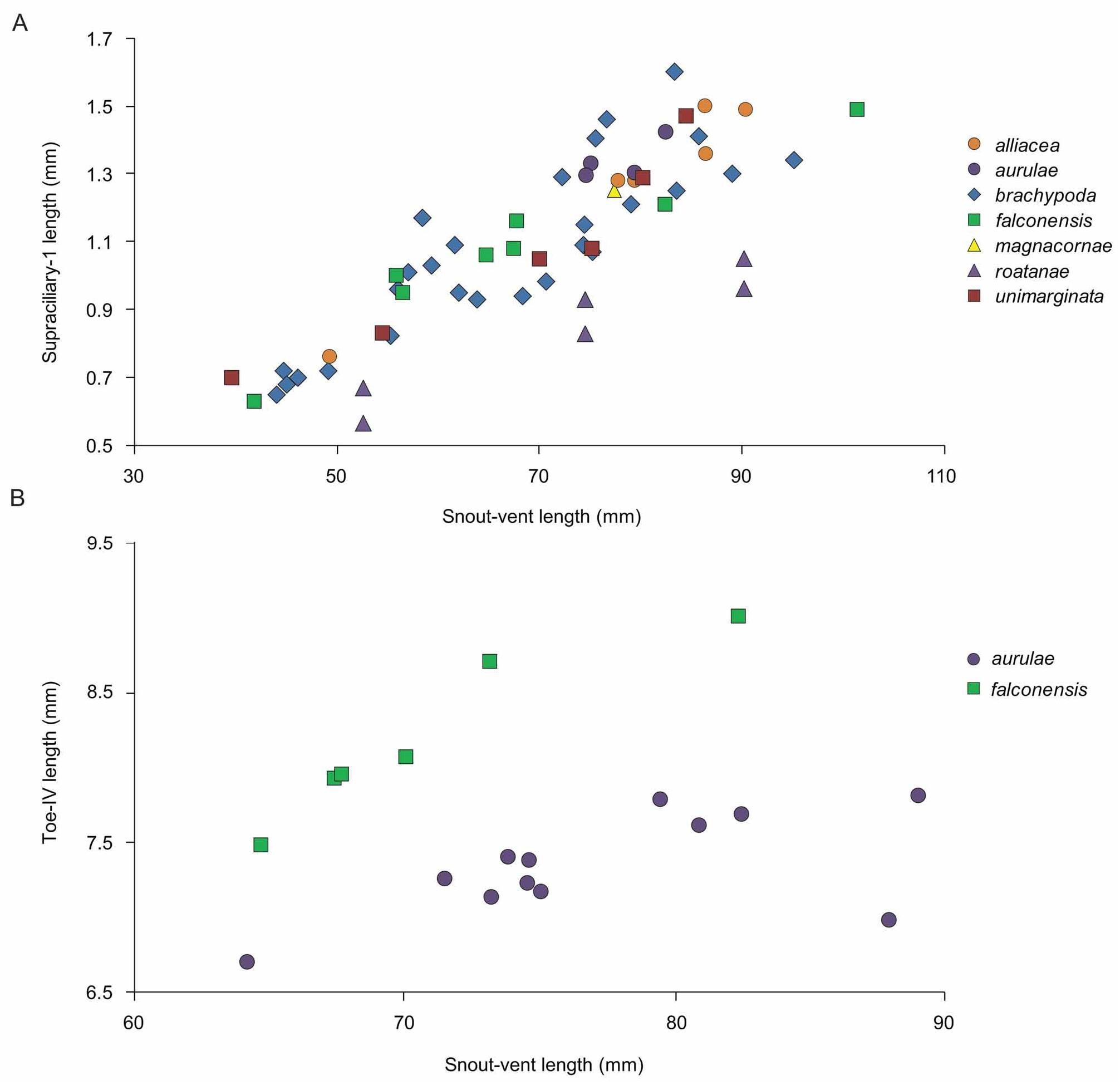
Marisora aurulae sp. nov. differs from M. alliacea , M. magnacornae sp. nov., and M. unimarginata in having shorter limbs (arm + leg length 53.7–55.9% SVL versus 55.7–69.1% SVL; Fig. 49 View FIGURE 49 ). From M. unimarginata and M. magnacornae sp. nov. it also differs in having 2–4 pairs of chin shields in contact with the infralabials versus one pair in M. magnacornae sp. nov. and usually one pair (88%) in M. unimarginata . From M. magnacornae sp. nov. it also differs in having shorter toes (toe-IV length 7.96–10.5% SVL versus 12.4% in M. magnacornae sp. nov.). Marisora aurulae sp. nov. is separated from M. roatanae sp. nov. in having a longer supraciliary-1 scale (1.65– 1.77% SVL versus 1.04–1.29% in M. roatanae sp. nov.; Fig. 50A View FIGURE 50 ). From M. falconensis , its closest relative ( Figs. 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7 ), M. aurulae sp. nov. differs in having shorter toes (toe-IV length 7.96–10.5% SVL versus 10.8–11.9% in M. falconensis ; Fig. 50B View FIGURE 50 ). Also, most M. aurulae sp. nov. that we examined (82%) have dark palms and soles and we score that as the fixed state in the species, assuming that the coloration has faded in the remaining 18%. However, M. falconensis is considered to have pale palms and soles ( Miralles et al. 2005a), and thus this may be another diagnostic difference. In body pattern, M. aurulae sp. nov. differs from all other species in the genus, including M. falconensis , in being paler and in having the standard stripe pattern weakly defined or nearly absent ( Figs. 47A View FIGURE 47 , and 48 View FIGURE 48 ). From M. alliacea it further differs in lacking dark dorsolateral stripes (present in M. alliacea ).
Marisora aurulae sp. nov. also differs in many ways from a sympatric species, Copeoglossum aurae sp. nov., described above, in the genus Copeoglossum . Two scale characters that may be used to separate them readily are parietal scales (not in contact, or rarely just touching, in C. aurae sp. nov.; in contact in M. aurulae sp. nov.) and paired chin scales (usually completely separated from infralabials by a row of scales in C. aurae sp. nov.; 2–4 pairs in contact with infralabials in M. aurulae sp. nov.).
Description of holotype ( Fig. 48A–B View FIGURE 48 ). An adult female in good state of preservation, with minor damage to snout tip and with an abdominal slit. SVL 74.6 mm; tail length 32.8 mm (broken and regenerated); HL 13.9 mm; HW 10.5 mm; SW 2.30 mm; EL 1.11 mm; and toe-IV length 7.40 mm; ear-opening average in size and round; toe length in the following order: I <V <II <III <IV.
Head scalation. Rostral wider than high, contacting first supralabials, nasals and supranasals. Paired supranasals in median contact, contacting anteriormost loreal. Frontonasal heptagonal (damaged), wider than long, laterally in contact with anterior loreal scale. A pair of quadrilateral prefrontals, separated medially, and in contact with frontonasal, both anterior and posterior loreals, first supraciliary, first supraoculars, and frontal. Frontal roughly octagonal, in contact with the second supraoculars and paired frontoparietals. Frontoparietals also in contact with parietals and interparietal. Interparietal tetragonal and lanceolate, separated from nuchals by parietals; parietal eye distinct. Parietals in contact with upper secondary and tertiary temporal scales. Four supraoculars, the second one being the largest. Four supraciliaries, the second the longest. Nostril in posterior part of the nasal. A small postnasal, bordered by supranasal, anterior loreal and first supralabial. Anterior loreal rectangular and posterior loreal squarish with posterodorsal projection on latter. Two upper preoculars and two lower preoculars. Seven supralabials, the fifth being the widest and forming the lower border of the eyelid. Three moderately delimited from the scales on the nape and the sides of the neck. Seven infralabials (eight on the left). Mental scale wider than long, posterior margin straight. Postmental scale and two pairs of adjoining chin shields (plus a third left chin shield scale) in contact with anterior infralabials. First pair of chin shields in contact medially; second and third pairs separated by a smaller cycloid scale.
Body and limb scalation. One row of nuchal scales. Other scales on nape similar to dorsals. On lateral sides of neck, scales slightly smaller. Dorsal scales cycloid, imbricate, smooth, 58 in a longitudinal row; ventrals similar to dorsals; 64 in a longitudinal row; 30 scales around midbody. No distinct boundaries between dorsals, laterals and ventrals. Scales on tail and limbs similar to dorsals, except smaller on limbs and paler on regenerated part of tail. Palmar and plantar regions with small tubercles, subequal in size and delimited by a surrounding region of flatter scales. Subdigital lamellae smooth, single, 13 under finger-IV and 16 under toe-IV. Preanal scales similar to ventrals. Enlarged median subcaudal scales on regenerated part of tail.
Pattern and coloration (faded slightly, apparently from age and preservation): Dorsal ground color medium grayish-brown with small dark brown spots, distributed on body, tail, and limbs. Forelimbs with larger spots or mottling. Dark dorsolateral stripes absent. Dark lateral stripes present, brown, irregular (series of close blotches), gradually fading from loreal region to hindlimbs. Pale middorsal stripe absent. Pale dorsolateral stripes absent. Pale lateral stripes present, whitish, extending from behind eye to midbody, bordered below by a series of brown spots. Ventral surface of body without pattern. Palmar and plantar surfaces pale brown or medium brown. No information is available on color in life of the holotype.
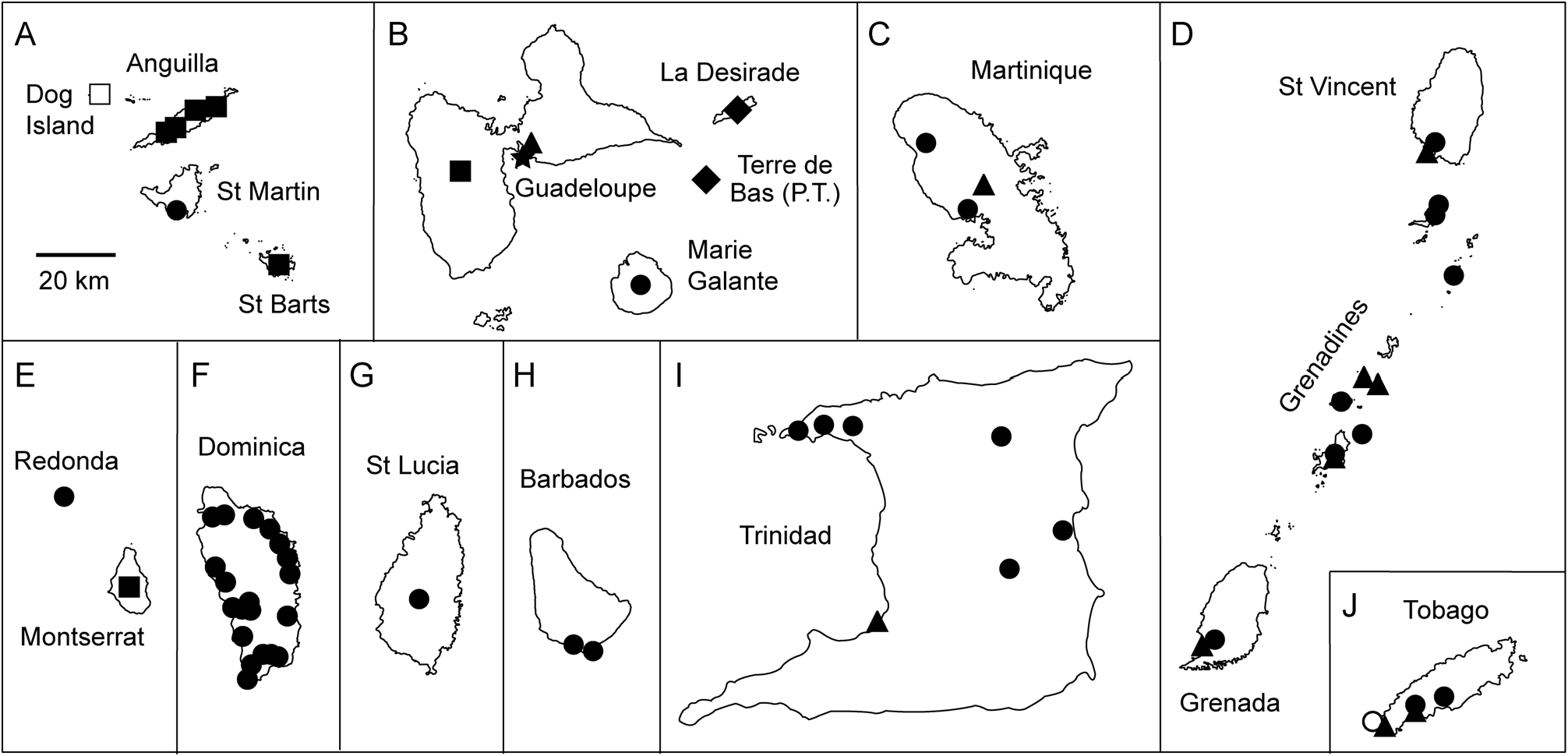
Distribution. The species is distributed in the southern Lesser Antilles and on Trinidad and Tobago. Specifically, it occurs on Young's Island (off St. Vincent), the Grenadines (Mayero Island, Carriacou, and Petit Bateau in the Tobago Cays), Grenada, Trinidad, and Tobago ( Fig. 11D, I–J View FIGURE 11 ).
Ecology and conservation. Because of confusion between this species and the sympatric species Copeoglossum aurae sp. nov., published ecological information on skinks from the region cannot be applied to either species with certainty. Past ecological information reported for skinks from Trinidad and Tobago, summarized in Murphy (1997), probably confounds C. aurae sp. nov. and Marisora aurulae sp. nov. In those reports, skinks were noted as occurring in a diversity of habitats, including rainforest, forest edge, coconut trash, and cultivated and disturbed areas. In the Grenadines, skinks have been found usually on the ground "in woody underbrush and between cacti" and climbing among cacti and on tree trunks ( Daudin & de Silva 2007). Apparently this species, and C. aurae sp. nov., have been extirpated from the large islands of St. Vincent and Grenada ( Barbour 1937), both of which have the introduced mongoose. The mongoose is present on Trinidad, although C. aurae sp. nov. has been collected there in recent years ( Murphy 1997); it may have adapted to continental mammalian predators on that island. Photographs of that species confirm its recent presence in the Grenadines (Fig. 25D). However, the last date of collection for M. aurulae sp. nov. on any island, from material we examined, was 1967 ( Trinidad), although two specimens from Tobago (ZFMK 62602–03), not examined here, were collected more recently. Black rats ( Rattus rattus ) are also likely predators, and these are on many islands. We identified more than twice as many specimens in museums of C. aurae sp. nov. than of M. aurulae sp. nov., suggesting that M. aurulae sp. nov., over the years, has been less frequently collected (for whatever reasons) than C. aurae sp. nov.
Based on IUCN Redlist criteria ( IUCN 2011), and considering that this species has not been seen on any island within its range (except Tobago, which is mongoose-free) in nearly a half-century, we assess the conservation status of Marisora aurulae sp. nov. as Critically Endangered (CR A2ace). It faces a primary threat from the introduced mongoose, which has apparently led to its extirpation from Grenada and Trinidad, and near-extinction. A secondary threat is predation from other introduced mammals, including black rats. Studies are needed to determine if the species still exists, the health of any remaining populations, and threats to the survival of the species. Captive breeding programs should be considered, if the species still exists, because eradication of introduced mammalian predators is not possible on large islands.
Reproduction. No data on reproduction are available for this species.
Etymology. The species name ( aurulae ) is a feminine genitive singular noun, from the Latin noun aurula (small wind, breeze) alluding to both its smaller size (compared with sympatric Copeoglossum aurae sp. nov.) and its distribution on the Windward Islands: the southern Lesser Antilles, sometimes including Trinidad and Tobago (see Etymology of C. aurae sp. nov. for further comments on the term "windward"). The first part of the common name (Lesser Windward Skink) refers to the smaller body size of this species, compared with C. aurae sp. nov. (Greater Windward Skink), described above.
Remarks. There are no records of this species from mainland St. Vincent, but it likely occurred there, prior to the introduction of the mongoose, given its occurrence on the satellite islet, Young's Island. The USNM specimens from Belmont, Grenada, have no date or collector. However, they appear to be from the 19th century because the catalog number immediately preceding those numbers is from 1885, and the assigned name (" Mabuya aurata ") is one used at about that time ( Boulenger 1887). Therefore, there are no definite records of this species on the main island of Grenada subsequent to the introduction of the mongoose.
Marisora aurulae sp. nov., like Copeoglossum aurae sp. nov., violates a common pattern of Caribbean island skinks in its occurrence on multiple islands separated by deep water. Most other species are single island (or island bank) endemics. In fact, M. aurulae sp. nov. has a nearly identical distribution as that of C. aurae sp. nov., with both species being taken together at two localities (see discussion above, in Remarks for C. aurae sp. nov.). Literature reports of skinks on these islands (e.g., Murphy 1997) confuse the two species, and therefore the precise ecological habits of each species remain to be determined. The photo of a skink from near Arima, Trinidad ( Murphy 1997) is of C. aurae sp. nov. (as opposed to M. aurulae sp. nov.) because it shows separated parietal scales. The molecular phylogeny ( Fig. 5 View FIGURE 5 ) indicates that the closest relative of M. aurulae sp. nov. is the Venezuelan species M. falconensis , as was shown earlier ( Miralles et al. 2009b). This makes sense from a geographic standpoint, as M. falconensis is the closest species to M. aurulae sp. nov. (in our limited examination of M. Lesser Antilles on flotsam carried by ocean currents known to flow in a southeast to northwest direction ( Hedges 1996b). The systematic evidence indicates that neither of the two species ( C. aurae sp. nov. and M. aurulae sp. nov.) was introduced by humans to these islands (see discussion in Remarks for C. aurae sp. nov.).
Marisora aurulae sp. nov. exhibits some geographic variation. Three specimens from Tobago (MCZ R- 12079–80, 55668) differ from all other M. aurulae sp. nov. in having a higher number of midbody scale rows: 31– nov. A fifth specimen, the oldest museum specimen of the species (MCZ R-4514), deserves special comment. It was collected in Grenada (no specific locality indicated) and apparently donated to the MCZ in ca. 1882 by P. Sellinan, who deposited some other reptiles in the MCZ collection. One of those reptiles was later described as an endemic subspecies of snake from Grenada ( Greer 1965), and the others are consistent with an origin on Grenada, inferring that the skink (MCZ R-4514) from Sellinan is likely from Grenada. However, that specimen differs in several characters from other M. aurulae sp. nov.: it has the lowest dorsals + ventrals count (111), lacks parietal contact, has the longest toe-IV (11.4% SVL), and has a pattern more like C. aurae sp. nov. Nonetheless, its other scale characters, including chin scale configuration, are consistent with M. aurulae sp. nov. and not C. aurae sp. nov. It is also curious that the other specimen with a low dorsals + ventrals count (MCZ R-79743) was collected in Grenada (Glover Island) as well; however, that specimen agrees more with M. aurulae sp. nov. in other characters. Whether MCZ-R-4514 represents a geographic variant, a cryptic species, or a hybrid with sympatric C. aurae sp. nov. is unknown. The specimens from Tobago (ZFMK 62602–03) identified as M. falconensis by Miralles et al. (2009), although not examined here, are assumed to be M. aurulae sp. nov.
One specimen, ZMH R09305, labeled as " Mabuya agilis " from St. Thomas, was collected in 1877 by "Riise." It is a member of the genus Marisora (not known from the Greater Antilles) and agrees in all respects (scalation and pattern) with Marisora aurulae sp. nov. Because of the essentially pre-mongoose date of collection, the specific locality, and the collector—Albert Heinrich Riise, a Danish pharmacist and naturalist on St. Thomas—we were intrigued by this specimen and at first considered that it might represent an endemic species to St. Thomas. However, when we discovered that another ZMH specimen (ZMH R09298) with identical catalog information turned out to be the Jamaican species, Spondylurus fulgidus , we realized there was likely some confusion in collection data of these specimens. Because the mongoose has significantly altered the diversity of Mabuyinae on Caribbean islands, we could not completely dismiss the possibility that the collection data are correct and that St. Thomas was previously inhabited by fulgidus -like and aurulae -like species. However, we were unable to find any unique traits in these specimens that would suggest that they were endemic to St. Thomas. Because of this apparent locality confusion we did not treat this specimen as a paratype.
| MCZ |
Museum of Comparative Zoology |
| KU |
Biodiversity Institute, University of Kansas |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Marisora aurulae
| Hedges, S. Blair & Conn, Caitlin E. 2012 |
Mabuya mabouya
| Henderson, R. W. & Powell, R. 2009: 292 |
Mabuya sloanii
| Mayer, G. C. & Lazell, J. D., Jr. 2000: 883 |
Mabuya bistriata
| Murphy, J. C. 1997: 150 |
| Powell, R. & Henderson, R. W. & Adler, K. & Dundee, H. A. 1996: 82 |
Mabuya mabouya mabouya
| Schwartz, A. & Henderson, R. W. 1991: 457 |
Mabuya mabouya mabouya
| Schwartz, A. & Henderson, R. W. 1988: 150 |
Mabuya mabouya mabouya
| MacLean, W. P. & Kellner, R. & Dennis, H. 1977: 40 |
Mabuya mabouya mabouya
| Schwartz, A. & Thomas, R. 1975: 141 |
Mabuya aenea
| Underwood, G. 1963: 83 |
Mabuya mabouia
| Barbour, T. 1937: 147 |
Mabuya mabouya mabouya
| Dunn, E. R. 1936: 544 |
Mabuya mabouia
| Barbour, T. 1935: 129 |
Mabuya aenea
| Barbour, T. 1914: 322 |
Mabuia agilis
| Boulenger, G. A. 1887: 191 |
Mabuia aenea
| Garman, S. 1887: 53 |