Cancrion khanhensis, Oanh & Boyko, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4894.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:451A3DBD-A70C-426B-8455-8A554DE99144 |
|
DOI |
https://doi.org/10.5281/zenodo.4329024 |
|
persistent identifier |
https://treatment.plazi.org/id/5DACACB1-3B6D-4EB9-916A-27F4C6DC6490 |
|
taxon LSID |
lsid:zoobank.org:act:5DACACB1-3B6D-4EB9-916A-27F4C6DC6490 |
|
treatment provided by |
Plazi |
|
scientific name |
Cancrion khanhensis |
| status |
sp. nov. |
Cancrion khanhensis View in CoL sp. nov.
( Figs 1–4 View FIGURE View FIGURE 2 View FIGURE 3 View FIGURE 4 , 8 View FIGURE 8 A–C, 9C, D)
LSID urn:lsid:zoobank.org:act:5DACACB1-3B6D-4EB9-916A-27F4C6DC6490
Type material. Holotype. Female (24.4 mm TL, stage 12), Nha Trang bay , Khanh Hoa, Vietnam, from Ke Ga cape (12.30°N, 109.24°E) to Cu Hin cape (12.15°N, 109.23°E), ex male Monomia haanii (Stimpson, 1858) (82 mm CW), 25 Apr 2019, coll. L. T. K. Oanh ( NOMV-E.56943 ). GoogleMaps
Allotype. Male (4.9 mm), same locality and collector data as holotype, ex female M. haanii , 23 Feb 2019 ( NOMV-E.56945).
Paratypes. All same locality and collector data as holotype. January 2019. Mature female (26 mm TL, stage 12), ex female M. haanii (57 mm CW), 2 Jan 2019 ( CNVB 10002); Mature femae (24 mm TL, stage 12), ex male M. haanii (92 mm CW), 2 Jan 2019 ( CNVB 10003); Two mature females (26–35 mm TL, stage 12, larger infested with Stellatoniscus tentaculus gen. nov., sp. nov.), male (3.1 mm), ex female M. haanii (70 mm CW), 2 Jan 2019 ( CNVB 10004); Mature female (29 mm TL, stage 12), male (4.3 mm), ex male M. haanii (61 mm CW), 8 Jan 2019 ( CNVB 10005); Mature female (32 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), male (4.3 mm), ex male M. haanii (89 mm CW), 8 Jan 2019 ( CNVB 10006).
February 2019. Mature female (26 mm TL, stage 12, infested with Stellatoniscus tentaculus gen. nov., sp. nov.), ex female M. haanii (69 mm CW), 23 Feb 2019 ( CNVB 10007); Juvenile female (22 mm TL, stage 8), ex male M. haanii (65 mm CW), 23 Feb 2019 ( CNVB 10008); Mature female (31 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), male (4.5 mm), ex male M. haanii (65 mm CW), 28 Feb 2019 ( NOMV-E.56944); Two mature females (29–32 mm TL, stage 12) male (3.9 mm), ex female M. haanii (65 mm CW), 28 Feb 2019 ( CNVB 10009); Juvenile female (26 mm TL, stage 8), ex male M. haanii (64 mm CW), 28 Feb 2019 ( CNVB 10010); Two mature females (28–31 mm TL, stage 12, largest infested with S. tentaculus gen. nov., sp. nov.), two males (3.4–4.8 mm), ex immature male M. haanii (55 mm CW), 28 Feb 2019 ( CNVB 10011); Two mature females (24–34 mm TL, stage 12, both infested with S. tentaculus gen. nov., sp. nov.), male (4.2 mm), ex male M. haanii (61 mm CW), 28 Feb 2019 ( CNVB 10012).
March 2019. Juvenile female (21 mm TL, stage 8), ex immature female M. haanii (47 mm CW), 5 Mar 2019 ( CNVB 10013); Mature female (31 mm TL, stage 12), male (4.2 mm), ex female M. haanii (73 mm CW), 5 Mar 2019 ( CNVB 10014); Two mature females (25–34 mm TL, stage 12), two males (3.7–4.7 mm), ex female M. haanii (70 mm CW), 5 Mar 2019 ( CNVB 10015); Mature female (27 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), male (4.0 mm), ex female M. haanii (66 mm CW), 5 Mar 2019 ( CNVB 10016); Mature female (32 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), ex female M. haanii (58 mm CW), 21 Mar 2019 ( CNVB 10017); Two immature females (21–23 mm TL, stage 8), male (3.6 mm), ex male M. haanii (73 mm CW), 21 Mar 2019 ( CNVB 10018).
April 2019. Mature female (26 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), male (3.6 mm), ex male M. haanii (74 mm CW), 5 Apr 2019 ( CNVB 10019); Immature female (21 mm TL, stage 8, infested with S. tentaculus gen. nov., sp. nov.), ex male M. haanii (82 mm CW), 25 Apr 2019 ( CNVB 10020).
May 2019. Mature male (30 mm TL, stage 12), ex male M. haanii (75 mm CW), 5 May 2019 ( CNVB 10021); Mature female (29 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), male (3.9 mm), ex male M. haanii (65 mm CW), 5 May 2019 ( CNVB 10022); Immature female (5 mm TL, stage 2), mature female (24 mm, stage 12, infested with S. tentaculus gen. nov., sp. nov.), ex male M. haanii (59 mm CW), 5 May 2019 ( CNVB 10023); Two mature females (26–30 mm TL, stage 12, both infested with S. tentaculus gen. nov., sp. nov.), two males (3.2–4.7 mm), ex male M. haanii (71 mm CW), 5 May 2019 ( CNVB 10024); Mature male (32 mm TL, stage 12), ex female M. haanii (57 mm CW), 16 May 2019 ( CNVB 10025); Two mature females (26–29 mm TL, stage 12, smaller infested with S. tentaculus gen. nov., sp. nov.), male (4.1 mm), ex female M. haanii (57 mm CW), 16 May 2019 ( CNVB 10026).
June 2019. Mature female (28 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), male (4.1 mm), ex female M. haanii (66 mm CW), 9 Jun 2019 ( CNVB 10027); Mature female (33 mm TL, stage 12), male (4.4 mm), ex female M. haanii (65 mm CW), 30 Jun 2019 ( CNVB 10028); Immature female (18 mm TL, stage 5), two cryptoniscus larvae (partially damaged), ex female M. haanii (69 mm CW), 30 Jun 2019 ( CNVB 10029); Mature female (33 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), ex male M. haanii (82 mm CW), 30 Jun 2019 ( CNVB 10030).
July 2019. Immature female (3.5 mm TL, stage 1), mature female (26 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), ex ovigerous female M. haanii (73 mm CW), 6 Jul 2019 ( CNVB 10031); Mature female (29 mm TL, stage 12), male (4.1 mm), ex immature male M. haanii (57 mm CW), 6 Jul 2019 ( CNVB 10032); Two mature females (26–32 mm TL, stage 12, largest infested with S. tentaculus gen. nov., sp. nov.), two males (3.8–4.1 mm), ex female M. haanii (71 mm CW), 21 Jul 2019 ( CNVB 10033); Mature female (34 mm TL, stage 12), male (4.8 mm), ex male M. haanii (64 mm CW), 21 Jul 2019 ( CNVB 10034).
August 2019. Mature female (31 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), male (4.8 mm), ex female M. haanii (82 mm CW), 7 Aug 2019 ( CNVB 10035); Mature female (39 mm TL, stage 12, infested with S. tentaculus gen. nov., sp. nov.), ex male M. haanii (58 mm CW), 13 Aug 2019 ( CNVB 10036); Mature female (39 mm TL, stage 12), ex female M. haanii (76 mm CW), 24 Aug 2019 ( CNVB 10037).
Comparative material examined. Cancrion australiensis Shields & Earley, 1993 : mature holotype female, 15.1 mm, Moreton bay GoogleMaps , 27°00’S, 153°00’E, between the mouth of Cabbage Tree Creek and the mouth of the Brisbane River, Queensland, Australia, 5–7 m depth, ex Thalamita sima H. Milne Edwards, 1834 , 20 Nov 1990 [in publication; 23 Nov 1990 on label in vial], coll. J. D. Shields ( QM W17079); Mature paratype male, 2.3 mm, same data as holotype except 20 Oct 1990 collection date; 1 mature female, Sta. 36A-25, Moreton bay, Mud island, Queensland, Australia, 27°15’S, 153°15’E, ex T. sima , 13 Feb 1991, coll. J. D. Shields ( USNM 284172 View Materials ) GoogleMaps ; 1 mature female, Sta. 31-A, Moreton bay, Queensland, Australia, 27°15’S, 153°15’E, ex T. sima , 4 Dec 1990, coll. J. D. Shields ( USNM 284173 View Materials ) GoogleMaps ; 1 mature female, Sta. 29-2, Moreton bay, Queensland, Australia, 27°15’S, 153°15’E, ex T. sima , 20 Dec 1990, coll. J. D. Shields ( USNM 284174 View Materials ) GoogleMaps .
Etymology. The name refers to the province of Vietnam, Khanh Hoa, in which the species was collected.
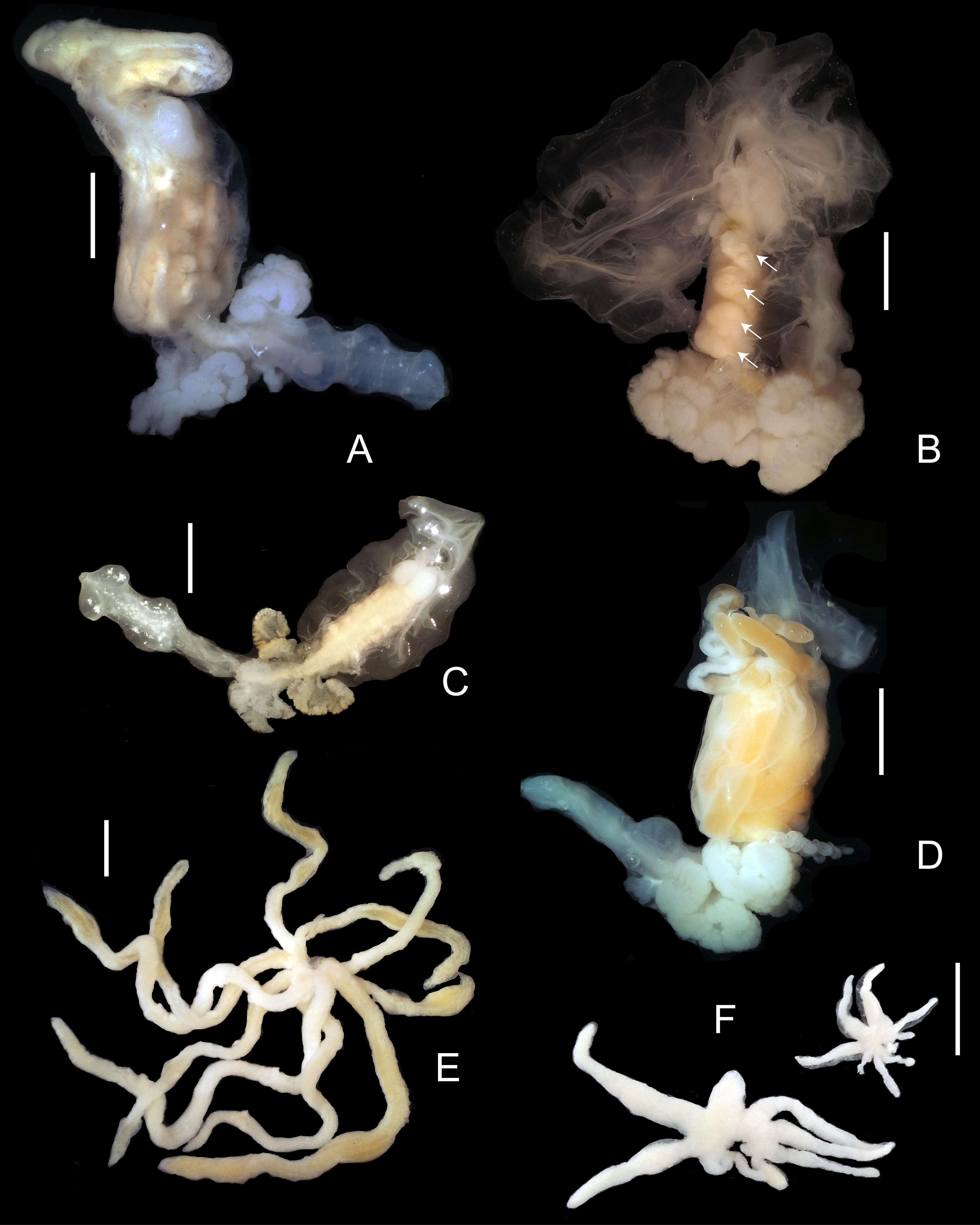
Description. Mature female (stage 12, see Remarks) ( Figs 1 View FIGURE A–D) body occupying most of host hemocoel; total length of cephalon, thorax, and abdomen 24–35 mm. Sheath formed by host surrounds entire parasite except for perforation at posterior end (exit pore not observed). Body dorsally recurved, V-shaped from cephalon to posterior end of pleon. Cephalon rounded, 3.0– 4.5 mm, dorsally distinctly divided into 2 bulbous lobes ( Fig. 1B View FIGURE ), with 2 pairs of antennae and pair of maxillipeds. Both pairs of antennae as slender lobes, subequal in length, inner pair slightly broader ( Fig. 1B View FIGURE ). Maxilliped with subquadrate coxopodite, folded concave, lamellar exopodite ( Fig. 1B View FIGURE ); endopodite absent or vestigial (not observed).
Pereon compact, 8.0–10.0 mm, ovaries cream to orange, four pairs of large dorsolateral ovarian processes, first pair slightly smaller than others, covering most or all of dorsal, lateral, and ventral margins of pereon ( Fig. 8B View FIGURE 8 ), pereopod 1 absent, pereopods 2–7 rudimentary; pereopods 2–5 present as small projections arising from dorsal side at median of respective oostegites, pereopods 6, 7 located on angle between pereon and pleon ( Fig. 1H View FIGURE ). Five pairs of oostegites forming brood pouch, margins entire. Oostegite 1 with ascendant lobe projecting anteriorly and dorsally, 2 simple transverse lobes, one recurrent lobe extending entire length of pereon with complex folding flap on lateral and flower-shaped fold on dorsal surface ( Fig. 1C View FIGURE ). Oostegites 2–5 delicate, scarcely overlapping, fused in mature females; oostegite 2 covering base of oostegite 1 and ventral surface of cephalon ( Fig. 1A View FIGURE ); oostegites 3–5 arising from lateral margins of dorsolateral ovarian processes.
Pleon white, 12.0–17.0 mm, composed of 5 segments. Pleomeres 1–5 each with distinct uniramous flattened pair of pleopods that overlay one another ( Fig. 1D View FIGURE ). Two pairs of complex pleural lamellae positioned medially on pleomeres 1 and 2 ( Fig. 1A, D View FIGURE ). Oval, bulbous heart in pleomere 3 ( Fig. 1A, H View FIGURE ). Distal end of pleon with two minute finger-like projections (possibly uropods) ( Fig. 1H View FIGURE ).
Immature females ( Figs 1 View FIGURE E–H, 8C) immature newly molted from cryptoniscus females found lying along the median shaft of host gill; vermiform, cylindrical, without appendages ( Fig. 1E View FIGURE ), immature females found in hemocoel ranging from vermiform, recurved, without appendages (stage 2, Fig. 1F View FIGURE ) to V-shaped with rudimentary pleural lamellae (stage 5, Fig. 1G View FIGURE ) to V-shaped with developing oostegites plus rudimentary pleural lamellae (stage 8, Fig. 1H View FIGURE ); latter stage with ovaries as two cream-colored wavy ridges lying along lateral margins of pereon ( Figs 1H View FIGURE , 8C View FIGURE 8 ); mature ovigerous females (stage 12, Fig. 8A, B View FIGURE 8 ) with orange gonadal tissue.
Male ( Fig. 2 View FIGURE 2 ) body elongated, white, markedly curved ventrally, 3.1–4.9 mm long, 0.7–0.85 mm wide, all pereomeres clearly separated dorsally, pereomere 1 fused with rounded cephalon ( Fig. 2 View FIGURE 2 A–C). Cephalon with pair of minute dorsolateral eyes ( Fig. 2C View FIGURE 2 ). Antennulae as large ovate lobes fused medially, with group of short setae anteroventrally ( Fig. 2A, B View FIGURE 2 ); antennae absent. Mandibles inside oral cone; no vestiges of maxillipeds ( Fig. 2B View FIGURE 2 ).
Pereon maximal width at pereomeres 4–6, gradually tapering anteriorly and posteriorly; lateral margins of pleomeres 1–6 expanded and directed ventrally, overlying bases of pereopods; lateral margins of pleomere 7 directed posteriorly; pereomeres 2–7 each with small setose medioventral tubercle ( Fig. 2A View FIGURE 2 ). Six pairs of pereopods (absent on pereomere 7) ( Fig. 2A View FIGURE 2 ), pereopods subequal in size, each with minute dactylus; propodus, carpus fused into single cylindrical segment distally covered with small spinules, merus small, subcircular, variably distinct from propodus/carpus; ischium, basis distinctly separated ( Fig. 2A, B, D View FIGURE 2 ).
Pleon with 5 cylindrical segments plus pleotelson ( Fig. 2A View FIGURE 2 ). Pleomeres distinctly narrower posteriorly, all separated, with horizontal fold in posterior portion of each segment, when folded, each anterior pleomere overlies base of following segment, pleopods absent. Pleotelson with 2 short, distally rounded, spinulose posterolateral lobes ( Fig. 2A, E View FIGURE 2 ); anal cone and uropods absent.
Epicaridium larva ( Fig. 3 View FIGURE 3 ) approximately 250–265 µm long (anterior margin of cephalon to end of telson), 96–102 µm wide (excluding pereopods). Body tear-drop shaped; anterior margin of head rounded; black-pigmented posterolateral eyespots. Antennula of 2 rounded articles, article 1 wider but scarcely longer than terminal article; terminal article with 2 or 3 long setae and several short setae ( Fig. 3A View FIGURE 3 ). Antenna elongated, nearly as long as body ( Fig. 3A View FIGURE 3 ); composed of 6 articles, articles 1 and 2 subequal in size; articles 3 and 4 longer, flagellar article 1 approximately 1.5 times as long as terminal article, with 2 distolateral setae; terminal article bearing long medial seta nearly half as long as entire antenna ( Fig. 3A View FIGURE 3 ). Mandibles within oral cone.
Pereon with 5 anterior pairs of gnathopodal pereopods, subequal in size, each with slender, slightly curved dactylus extending approximately half length of propodus, tip of dactylus hooked; propodus, carpus fused with ventral area of blunt spinules; merus small, subrectangular, ischium and basis cylindrical ( Fig. 3A View FIGURE 3 ). Pereopod 6 greatly elongated, dactylus reduced, not adpressed against carpopropodus; propodus, carpus fused, larger than carpopropodus of other pereopods, ventrally with rounded expansion and distoventral finger-like extension; elongate, flattened, distally expanded and distomedially indented process articulated with propodus/carpus dorsal to dactylus, slightly longer than carpopropodus; 2 lateral setae on stalk and 2 long, simple setae at distolateral margins of process; merus triangular, approximately 1/3 length of ischium; ischium cylindrical, approximately subequal in size to basis; basis cylindrical; merus approximately 3 times longer than on other pereopods; ischium approximately 3 times than on other pereopods; basis approximately 2 times longer than on other pereopods ( Fig. 3B View FIGURE 3 ).
Pleon with 5 pairs of uniramous pleopods ( Fig. 3A View FIGURE 3 ); triangular sympod bearing 2 long setae at inner distal point, cylindrical exopod articulated with outer distal point of sympod bearing 3 long setae. Uropods biramous, cylindrical peduncle flared slightly at distal end with 1 stout seta on outer angle, distally with 2 slender rami, exopod ending in 2 thin, short setae and 1 long, robust seta, endopod subequal in length to exopod, with 1 long, robust seta ( Fig. 3A View FIGURE 3 ).
Cryptoniscus larva ( Fig. 4A View FIGURE 4 ) body fusiform, length 700 µm, maximum width at pereomeres 3 and 4. Cephalon anterior margin round (anterior portion of sole specimen damaged). Body pigmentation lacking. Antennula missing. Antenna with four peduncular and 1+ flagellar articles (damaged) ( Fig. 4A View FIGURE 4 ), distal 2 peduncular articles longer than proximal 2; flagellar article approximately half width of peduncular articles ( Fig. 4A View FIGURE 4 ). Oral cone triangular, anteriorly directed ( Fig. 4A View FIGURE 4 ). Pereomeres 1–7 with entire (not toothed) coxal plates ( Fig. 4A View FIGURE 4 ). Pereopods 1–5 subequal in shape with long, curved dactylus, propodus expanded proximally with distoventral ridge corresponding to dactylus position bearing small bulges; carpus slender, distally forking; merus triangular, approximately half length of carpus; ischium subrectangular, approximately 2.5 times as long as merus; basis cylindrical, approximately twice as long as ischium ( Fig. 4B View FIGURE 4 ). Pereopods 1 and 2 subequal in size, 3–5 subequal in size and slightly larger than 1 and 2 ( Fig. 4A View FIGURE 4 ). Pereopod 6 and 7 missing. Each pleomere with a median ventral acute projection ( Fig. 4A View FIGURE 4 ); 5 pairs of uniramous pleopods; sympod with distomesial short, subquadrate, extension, proximomesial and distomesial corner with few long setae; exopods recurved with few long terminal setae ( Fig. 4C View FIGURE 4 ). Pleotelson subquadrate. Uropods biramous, composed of wide subquadrate sympod with single long seta at distolateral corner, endopod slightly longer than exopod, pair of long distal setae on endopods and exopods, short seta at distolateral and distomesial margins of endopods and exopods ( Fig. 4D View FIGURE 4 ).
Distribution. Known only from Monomia haanii collected in Nha Trang bay, Khanh Hoa province, Vietnam.
Remarks. The new entoniscid isopod appears to belong to the genus Cancrion with some of whose species it shares the following characters: oostegite 1 of the female of 3 parts; female without ventral ovarian processes; female with two pairs of pleural lamellae; epicaridium larva with elongate pereopod 6 with a highly modified process arising from the distodorsal margin of the propodus. However, it needs to be noted that Cancrion is a potentially heterogeneous grouping in that not all the females have the same number of pleural lamellae (2 in C. australiensis Shields & Earley, 1993 , C. carolinus Pearse & Walker, 1939 , C. deltoides Shiino, 1942 , and C. needleri Pearse & Walker, 1939 ; 2 or 3 (unclear) in C. cancrorum Müller, 1864 ; more than 2 in C. miser Giard & Bonnier, 1887 ; unknown number in C. floridus Giard & Bonnier, 1887 . Additionally, the epicaridium larvae are known from only three other species: C. australiensis , C. deltoides , and C. carolinus , and only C. australiensis and C. deltoides show the long process arising from the propodus of pereopod 6 as seen in C. khanhensis sp. nov. It is possible that Cancrion is paraphyletic but a complete redescription of C. miser , the type species, is needed, including details of the epicaridium larval morphology, before any conclusions can be drawn.
In comparison to other species of Cancrion , C. khanhensis sp. nov. most resembles C. australiensis and C. deltoides in all known life history stages based on the characters noted above (cryptoniscus larvae for the latter two species are unknown). However, females, males and epicaridium larvae of C. khanhensis sp. nov. possess several characters that distinguish this species from the other two. The length of both females (24–35 mm) and males (3.1–4.9 mm) is considerably larger than for the other two species ( C. australiensis : females 16.4–21.2 mm, males 2.1–2.6; C. deltoides : female 8.7 mm, male 1.58 mm); for females this is probably a function of host size limitation but male size is not strongly correlated to host size due to their being the much smaller sex. The size of epicaridium larvae for C. khanhensis sp. nov. (250–265 µm) is intermediate between those of the other two species ( C. australiensis 228–239 µm; C. deltoides 345 µm).
The females of C. khanhensis sp. nov. each possess 4 pairs of dorsolateral ovarian processes, while those of C. australiensis and C. deltoides bear 3 and 2 pairs, respectively. In addition, the recurrent lamella of C. khanhensis sp. nov. is developed into an elaborate flap with dorsal flower-shaped folds not seen in the other two species. The males of the new species have horizontal ridges with small median spines on pereomeres 2–7 and horizontal folds on pleomeres 1–5 that are lacking in the other two species. The epicaridium larva has a distally expanded and medially indented process on pereopod 6 whereas this process is spoon-shaped in C. australiensis and rounded in C. deltoides .
This is the first description, albeit a partial one, of any cryptoniscus larva of a species of Cancrion . Only two cryptoniscus larvae were found on hosts’ gills, one on each of two crabs. Unfortunately, the first cryptoniscus was damaged during removal and the latter was covered by a dark membrane, possibly caused by host melanization (see Kuris et al. 1980).
Kuris et al. (1980) divided the growth of female entoniscids into 12 stages based on development of morphological features and reproductive condition. We have only observed female entoniscids corresponding to four of these stages (2, 5, 8, 12) as well as a stage not reported by Kuris et al. (1980): a cylindrical vermiform stage which appears to be one immediately following molting of the cryptoniscus exuvia as it is the most reduced in structure and simplest shape.
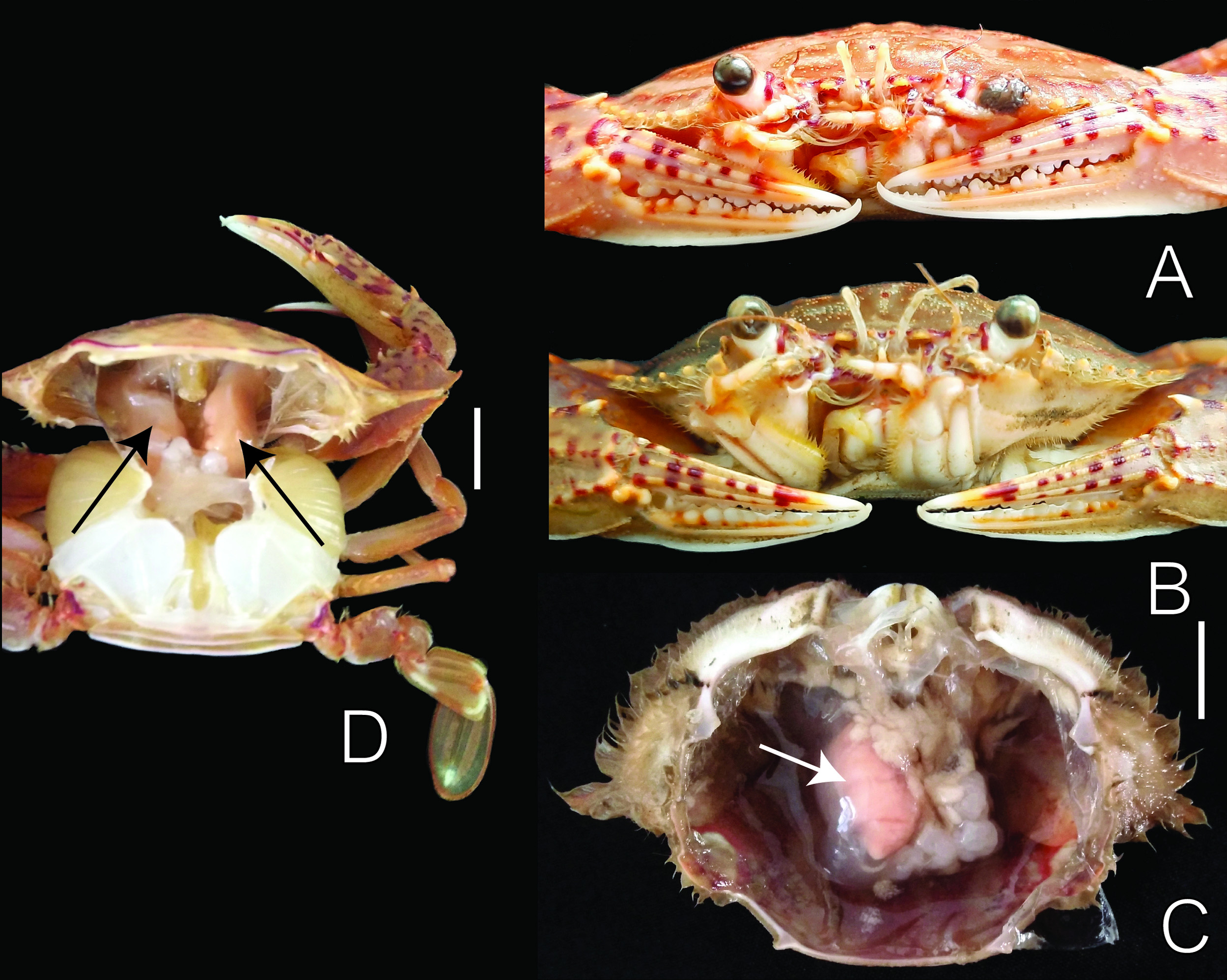
Discussion. Parasitic position. As with all other Cancrion species, C. khanhensis sp. nov. lies in the hemocoel of the host and is enclosed by the host response sheath ( Fig. 9C, D View FIGURE 9 ; see also Shiino 1942; Shields & Earley 1993). The female is curved dorsally with the whole marsupium, including the cephalon and pereon, laying in one side of the host’s hemocoel cavity with the “hood” (distal extension of the oostegites) extending to the anterior border of the host carapace while the abdomen is curved under the host’s stomach, reaching to the other side of the host ( Fig 9C, D View FIGURE 9 ). The exit pore, via which the parasite isopod communicates with the external environment, was not observed but was likely entering into the branchial chamber (see McDermott et al. 2019). The male is of the type which has a very strongly curved body ( Shiino 1942) and is usually found in the brood-chamber of the female. The marsupia of most females that were not hyperparasitized were filled with epicaridium larvae.
Prevalence and intensity. Out of a total of 366 Monomia haani examined, there were 37 crabs infested with C. khanhensis sp. nov.; a prevalence of 10.1%. The prevalence of isopod infestation in female crab hosts was 13.2% (18/136) and was significantly higher than the 0.83% (19/230) found in male hosts (Chi-square test, χ2 = 127.75, df = 1, p <0.01). Each host was parasitized by one ( Fig. 9C View FIGURE 9 ) or two ( Fig. 9D View FIGURE 9 ) female entoniscids with double infestations observed in 12 of the hosts. Of these 12 double infestations, there were eight crabs infested by one larger female entoniscid with a marsupium full of epicaridium larvae and one smaller female with eggs; three crabs bore one mature and one immature female entoniscid; and one crab was infested with two juvenile entoniscid females. Females and males of C. khanhensis sp. nov. were not always present together, with the number of males being equal to or often fewer than the number of females in the same host. Specifically, 12 of the 25 single female infestations and two of the 12 double female infestations had no males present, whereas one male accompanying a pair of females occurred in six hosts, and two males present with two females occurred in four hosts.
Infested host crabs can often be recognized by a vertical swelling of the carapace when the female entoniscids are large ( Fig. 9B View FIGURE 9 ) but modification of the host is not apparent when the isopod parasite is small and immature and the carapace appears the same as that of unifested hosts ( Fig. 9A View FIGURE 9 ). The sizes (carapace width) of infested crabs are in the range of 47 to 82 mm for females and 55 to 92 mm for males. Castration of hosts infested by C. khanhensis sp. nov. was not observed in male or female hosts, as 14 of 18 infested female (1 being ovigerous) and 17 of 19 infested male hosts were sexually mature.
| QM |
Queensland Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |