Bryothinusa hauseri, Ashe, Desig. J. S. Ashe, 2003
|
publication ID |
https://doi.org/10.1649/691 |
|
persistent identifier |
https://treatment.plazi.org/id/2473C163-4D60-FFF6-73E6-FD4AFCA7FC21 |
|
treatment provided by |
Tatiana |
|
scientific name |
Bryothinusa hauseri |
| status |
sp. nov. |
Bryothinusa hauseri View in CoL , new species
( Figs. 1–15 View Fig View Figs View Figs View Figs )
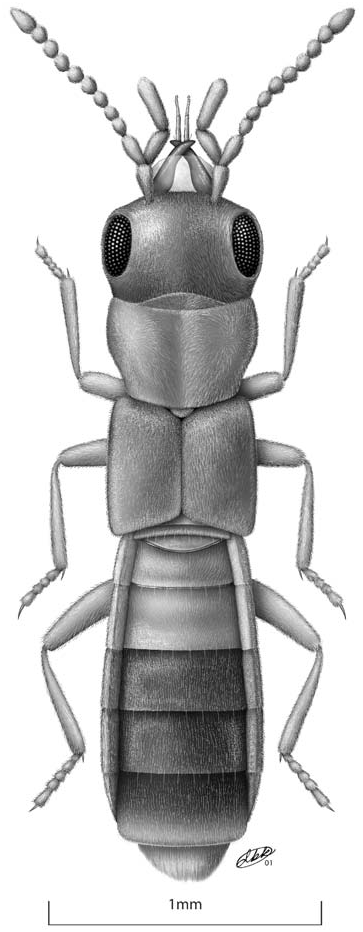
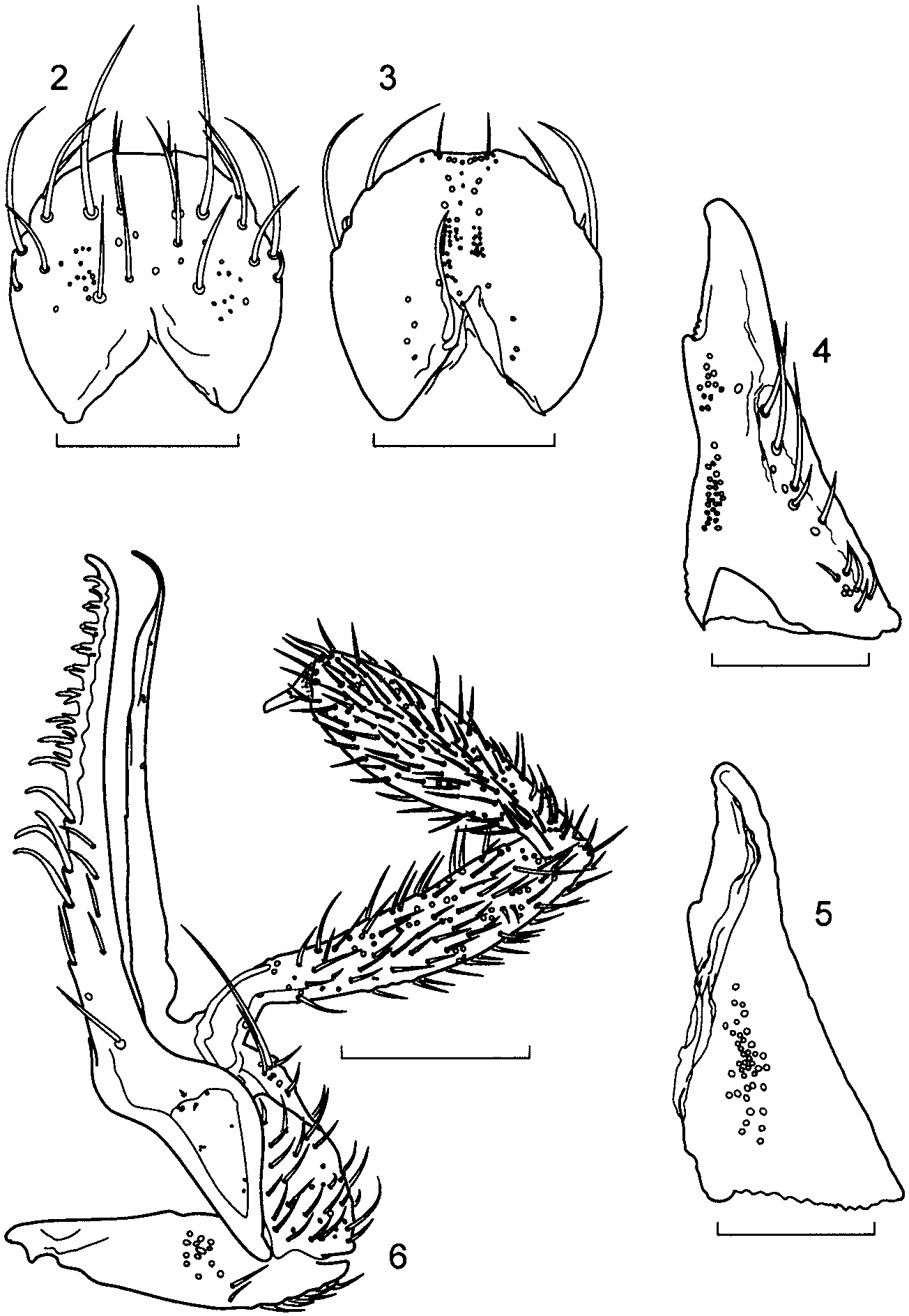
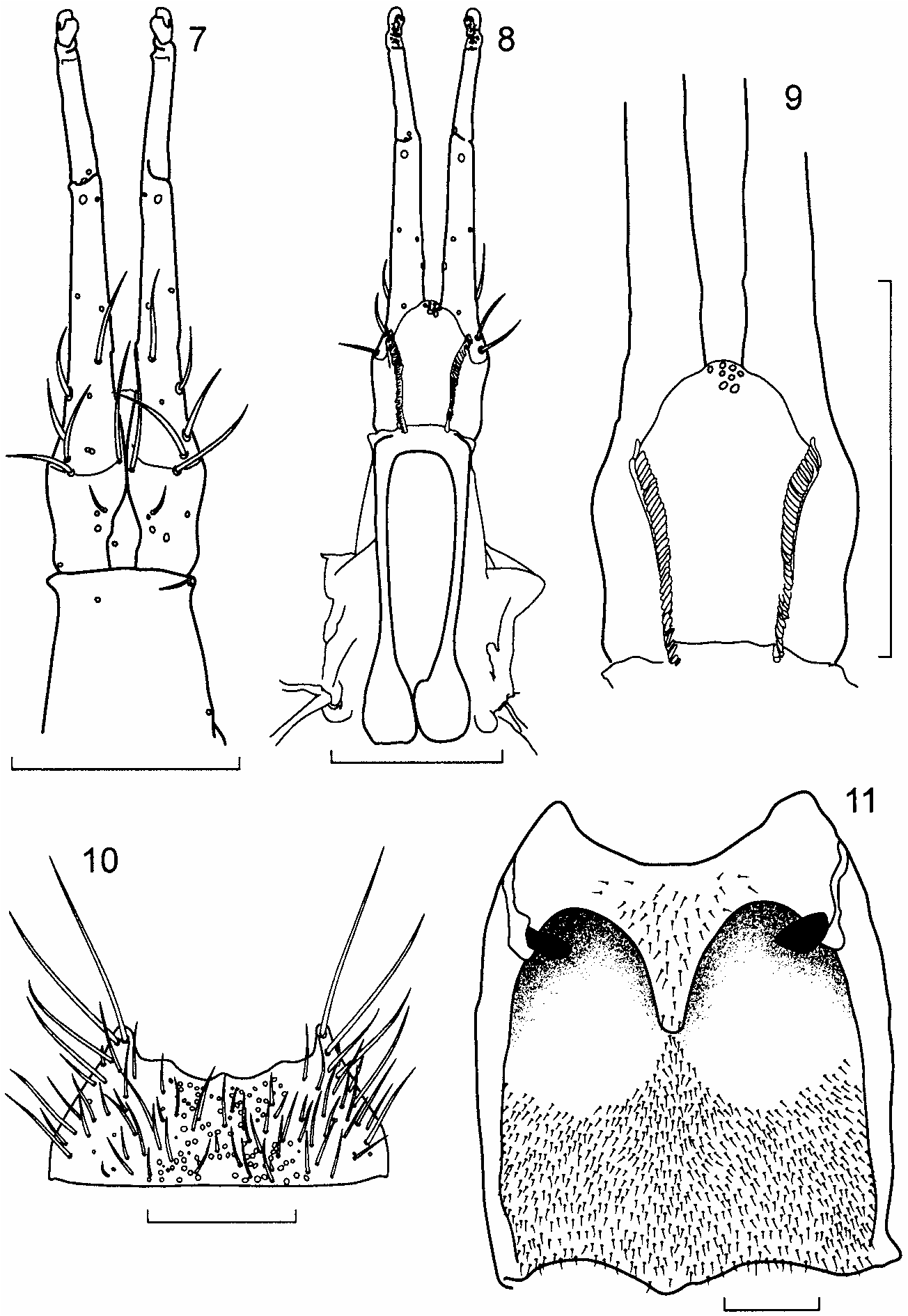
Description. Length 2.4–2.8 mm. Body parallel-sided, flattened ( Fig. 1 View Fig ); body, antennae, mouthparts and legs light brown, most specimens with head, pronotum and a diffuse area at the sutural base of the elytra medium brown, abdominal segments V, VI and the anterior 1/2–4/5 of VII medium to dark brown. Head strongly prognathous, without differentiated neck, subequal in size to pronotum in females, distinctly larger than pronotum in males, covered with very fine vestiture of microsetae; microsetae on dorsum of head oriented laterally at base of head and along inner margins of eyes, medially in middle of head and antero-medially on anterior margin of frons. Eyes very large, about as long as tempora in females and up to 1.5 times as long as tempora in some males. Infraorbital carina absent. Antenna with articles 4–7 subquadrate and articles 8–10 very slightly transverse. Labrum ( Fig. 2 View Figs ) about as long as wide, broadly rounded apically. Epipharynx ( Fig. 3 View Figs ), asymmetrical, with a distinct sclerotized bar on one side associated with an asymmetrical distribution of sensory pores. Mandibles ( Figs. 4, 5 View Figs ), long, nearly straight with only a slightly curved apex, with numerous setae on external face, velvety patch absent; prostheca reduced to membranous process, without fringe of fimbriate processes. Maxilla ( Fig. 6 View Figs ) very elongate, slender: lacinia with widely spaced spines interdigitating with spinose scales; galea subequal in length to lacinia, strongly hooked at apex, without internal setae subapically. Labium ( Figs. 7, 8 View Figs ) with palpi very elongate and stylate; palpi appearing 2-segmented with articles 1 and 2 fused; 2 discal setae present, bases close, slightly staggered one behind the other; medial pseudopore field extremely narrow, without pseudopores; lateral pseudopore fields each with 1 spinose pore and 2 real pores, 1 or 2 irregularly scattered pseudopores present in some specimens; ligula ( Fig. 9 View Figs ) short, very broad and broadly rounded apically. Hypopharyngeal lobes ( Fig. 9 View Figs ) with single row of closely-arranged, short processes. Mentum ( Fig. 10 View Figs ) with latero-apical angles prolonged into short, sub-spinose processes; anterior margin broadly concave medially with small and broad medial projection.
Pronotum ( Fig. 1 View Fig ) broadest near anterior 1/5, narrowed to base; anterior margin produced into broad lobe medially; hind angles distinct; uniformly and densely covered with very fine microsetae; microsetae directed anteriorly in broad medial band, directed laterally on each side of medial band, and directed medially along lateral margins. Hypomera broadly visible in lateral aspect. Meso-metasternum as in Figure 11 View Figs , mesosternal process extended about 1/2 length of mesocoxal cavities, narrowly rounded apically, mesocoxae narrowly separated; mesocoxal cavities not margined posteriorly.
Elytra length at suture subequal to length of prothorax; uniformly and densely covered with very fine microsetae, microsetae directed posteriorly; macrosetae absent; postero-lateral margins not sinuate. Hind wings present.
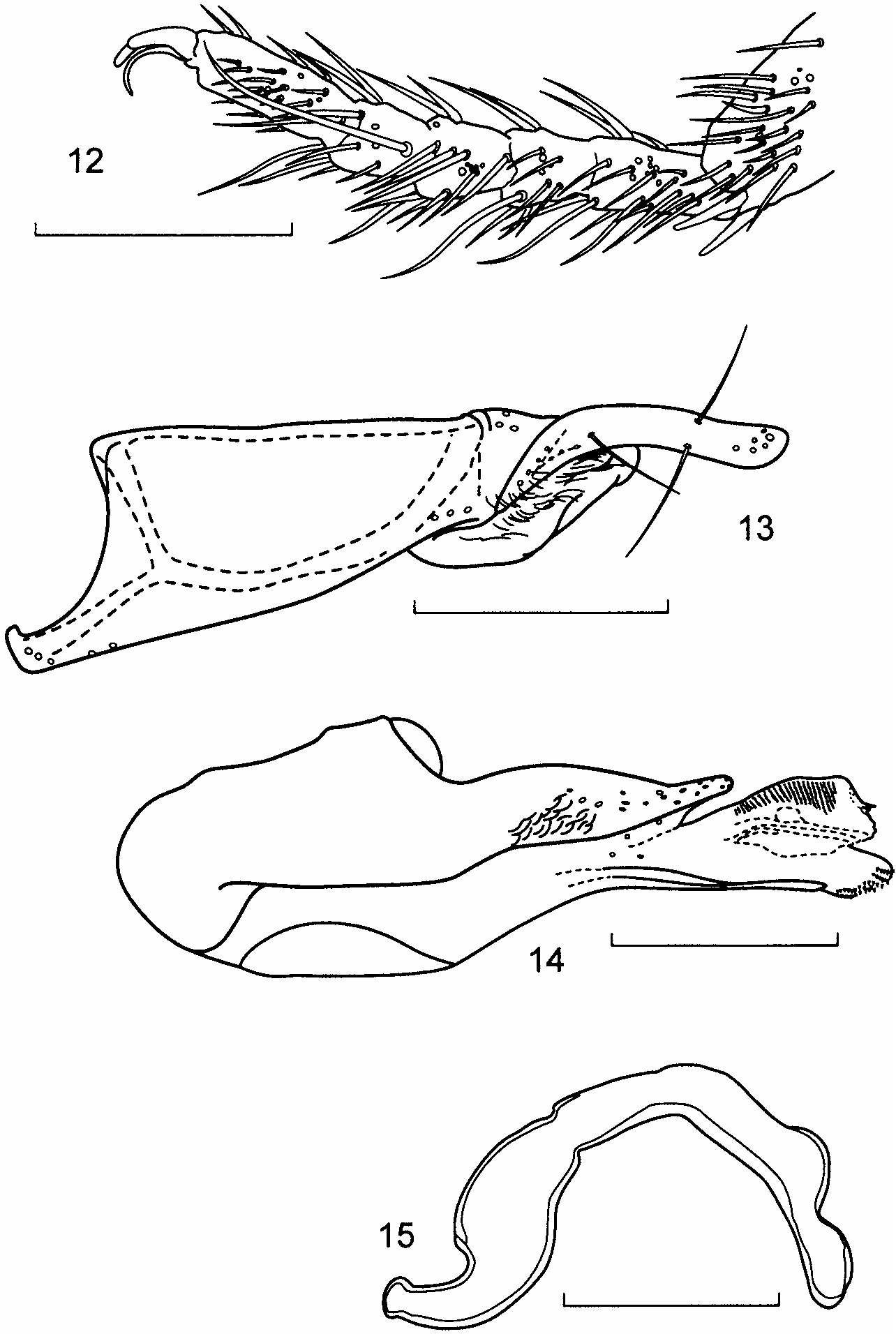
Tarsi 4-4-5, tarsomere 1 of hind tarsus subequal in length to articles 2 þ 3 together ( Fig. 12 View Figs ), all tarsal articles with numerous setae; tarsal claws short, 1 distinctly shorter than the other, claws closely confluent in most specimens so that only a single tarsal claw appears present in dried and pointed specimens; 1 empodial bristle present, subequal to, or slightly longer than, longest tarsal claw ( Fig. 12 View Figs ).
Abdomen parallel-sided, with base of terga III–V broadly V-shaped ( Fig. 1 View Fig ), abdominal terga without deep transverse depressions basally; uniformly and densely covered with microsetae.
14) median lobe of aedeagus; 15) spermatheca. Scale line ¼ 0.1 mm.
Aedeagus. Parameres as in Figure 13 View Figs ; median lobe as in Figure 14 View Figs .
Secondary Sexual Characteristics. Males with noticeably larger heads than females.
Spermatheca. As in Figure 15 View Figs .
Holotype. Male, with labels as follows: ‘‘ MALAYSIA: Malay Peninsula, 40 mi N of Kuala Dungan , 58049N, 1038179 E. M. Hauser’ ’; ‘‘ HOLOTYPE, Bryothinusa hauseri Ashe, Desig. J. S. Ashe, 2003 .’’ Deposited in the Snow Entomological Collection , Division of Entomology , KU Natural History Museum / Biodiversity Research Center , University of Kansas , Lawrence, Kansas ( KSEM) .
Paratypes. 10, same data as type ( KSEM) .
Habitat and Distribution. Known only from type locality; collected from among sand and seaweed on beach (M. Hauser, pers. comm.).
Discussion. This species is easily recognized, among known Bryothinusa , by the very broad ligula of the prementum ( Fig. 8 View Figs ), the distinctive galea of the maxillae (apically curved as a spinose process and without internal setose processes) ( Fig. 6 View Figs ), and the distinctive spermatheca ( Fig. 15 View Figs ) and aedeagus ( Fig. 14 View Figs ). There is a slight possibility that this species is the same as Bryothinusa testaceipennis (Cameron) from Singapore since both occur on the Malay Peninsula. However, B. hauseri does not fit most features in the description of B. testaceipennis given by Haghebaert (1991), and B. hauseri is significantly larger (up to 2.8 mm in length).
Bryothinusa hauseri shares many distinctive features with the type of B. catalinae , and fits most of the characterization of Bryothinusa given by Ahn (1998). The following features are particularly noteworthy and may be synapomorphies: the distinctively shaped labrum, with similar distribution of setae and sensory elements; the asymmetrical bar and the similar distribution of sensory elements in the epipharyngeal region; the number of setae on the external surface of the mandibles; similar distribution of spines and setae on the lacinia of the maxilla; similar shape of the pronotum, with a broad band of micriosetae in the midline directed anteriorly; the contiguous mesocoxal cavities not margined behind; and the intertidal habitat.
Bryothinusa hauseri is similar in overall form, and in many important characteristics, to the type species of Bryothinusa ( B. catalinae Casey ), and to the characterization of the genus given by Ahn (1998). However, this species differs in several significant features that are often used to distinguish among genera in the Aleocharinae. The most striking difference is the very broad, distally rounded ligula, without apical sensory spinules, of B. hauseri (shorter, distinctly slender and elongate, with a pair of apical sensory spinules in B. catalinae ). The mandibles of B. hauseri are more elongate, and less curved at the tips than those of B. catalinae . The tarsal claws of B. hauseri are distinctly different in size with only a single empodial seta between them; the difference in sizes between the tarsal claws is much less in B. catalinae , and two empodial setae are present. The apico-lateral margins of the mentum of B. hauseri are not as elongate and spinose as that of B. catalinae . The maxilla of B. hauseri is similar in overall shape to that of B. catalinae , but that of B. hauseri is much more elongate, including a much longer cardo. However, the galea of the maxilla of B. hauseri is characterized by dramatic reduction of the apical setae to a single curved apical spinose process; B. catalinae , as well as all other species that I have examined and those for which the maxillae have been illustrated, have a few long hair-like processes internally near the apex of the galea. The pronotum of B. hauseri and B. catalinae are similar in overall shape and in microsetation; however, the pronotum of B. catalinae lacks the mediallydirected setae along the lateral margins found on the pronotum of B. hauseri (all lateral setae directed laterally in B. catalinae ). Finally, the metasternal process is longer and more apically rounded than that of B. catalinae (mesosternal process short and acutely pointed).
These differences do not seem sufficient to assign B. hauseri to a new genus, but they do suggest that a complete review of the structural variation among species in the genus Bryothinusa would be useful, and could either result in a redefinition of Bryothinusa or in splitting it into two or more genera. In this regard, illustrations of the labium, maxillary palpus, and paramere of the aedeagus given for B. parvula Haghebaert appear to be quite outside of the known range for these features in other Bryothinusa . Such a full review is beyond the scope of this paper.
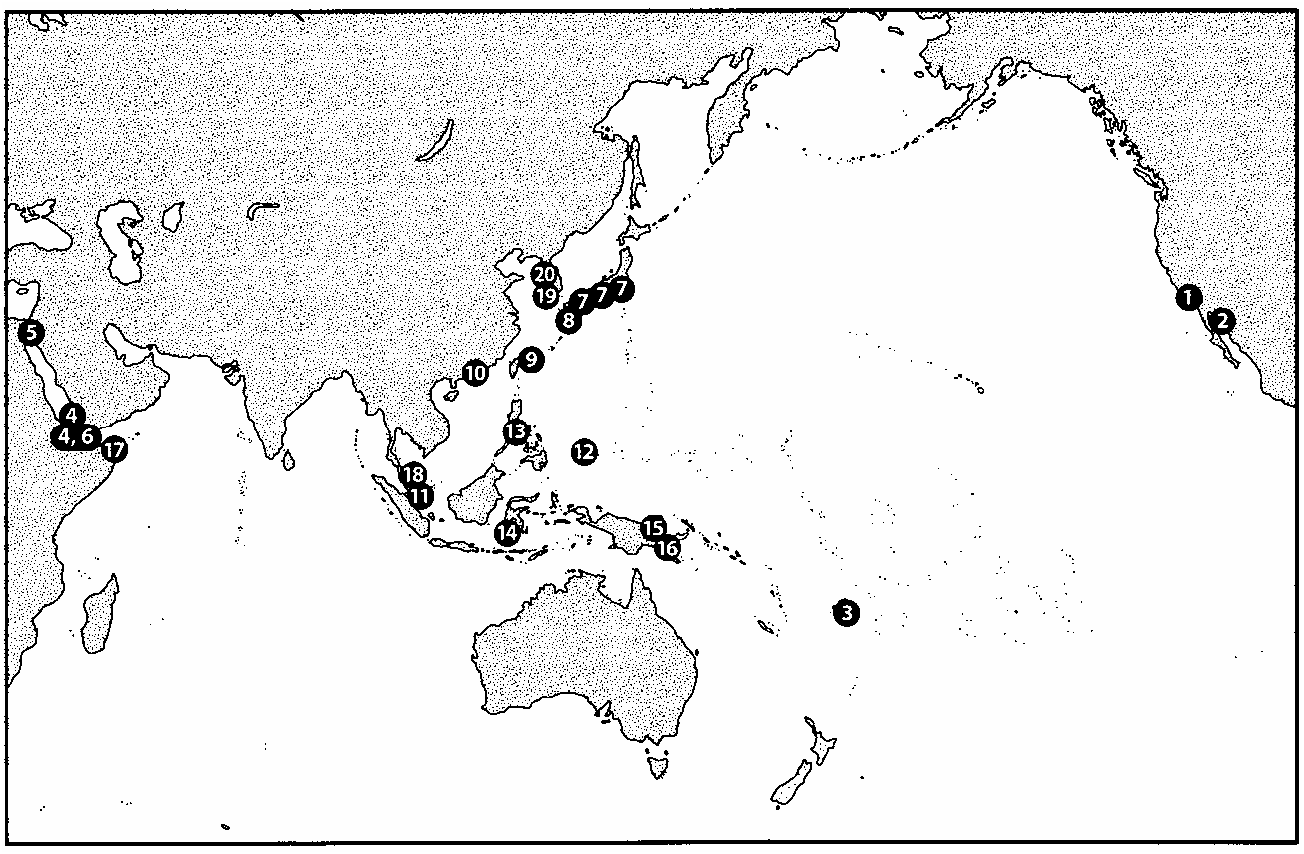
Distribution of Bryothinusa . The known distribution of Bryothinusa is quite remarkable ( Fig. 16 View Fig ) with species occurring on beaches in the Red Sea (Gulf of Aqaba, Perim and Kamaran Islands), and throughout the Pacific Basin (Gulf of California and southern California on the western coast of North America, Japan, South Korea, Hong Kong, Singapore, the Philippines, Sulawesi, Papua New Guinea, the Caroline Islands, Samoa, and the Malay Peninsula). The distribution of known species is largely limited to tropical and subtropical beaches, though B. algarum from Japan is known from as far north as Tokyo, a clearly temperate region of Japan, and Ahn and Jeong (2004) recently reported 4 species, including 2 new ones, from South Korea. Species of Bryothinusa are not known from the north-temperate beaches of Japan and the northern Pacific Coast of North America, which otherwise have a large fauna of intertidal aleocharines (primarily Liparocephalini), and no Bryothinusa species are known from shores of the Atlantic Basin. The fact that most species are only known from the type series ( Haghebaert 1995) suggests that the distribution within the Pacific basin is likely to be much broader, and includes many more species than currently known.
It is difficult to imagine how the broad distribution indicated in Figure 16 View Fig could have been achieved. Some currently separate areas occupied by various species of Bryothinusa were probably contiguous landforms at various times in the past, particularly during the Pleistocene when sea levels were lower ( e.g., Malaysia, most of Indonesia and the Philippines, depending on full extent of sea level depression; see Pirazzoli 1991) or are close to source areas for colonization. However, other areas are clearly historically separate ( e.g., localities in the Red Sea, Japan, Caroline Islands, Samoa, New Guinea and the Pacific Coast of North America) and appear to require substantial over-water dispersal for colonization. Nothing is known about the dispersal abilities of species of Bryothinusa ; though species are fully winged, I am not aware of any records of specimens observed or captured in flight, and while adults and larvae survive emersion in sea water for many hours (Wong and Chan 1977), there is no information about their ability to survive long journeys on the open ocean.
This broad distribution suggests a very ancient origin for these beetles in the Pacific basin. However, understanding the origins of this remarkable distribution will require an analysis of the phylogenetic relationships among the species of Bryothinusa , a much more through knowledge of their distribution in the Pacific Basin, and better knowledge of the biology and dispersal characteristics of Bryothinusa . Current data do not provide the basis for these studies, and they are outside the goals of this paper.
| KU |
Biodiversity Institute, University of Kansas |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.