Anelosimus jucundus, sensu Levi, 1956
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2006.00213.x |
|
persistent identifier |
https://treatment.plazi.org/id/236D8D66-FFB1-FFBB-2455-2EC6FDA0643B |
|
treatment provided by |
Felipe |
|
scientific name |
Anelosimus jucundus |
| status |
|
THE JUCUNDUS View in CoL GROUP
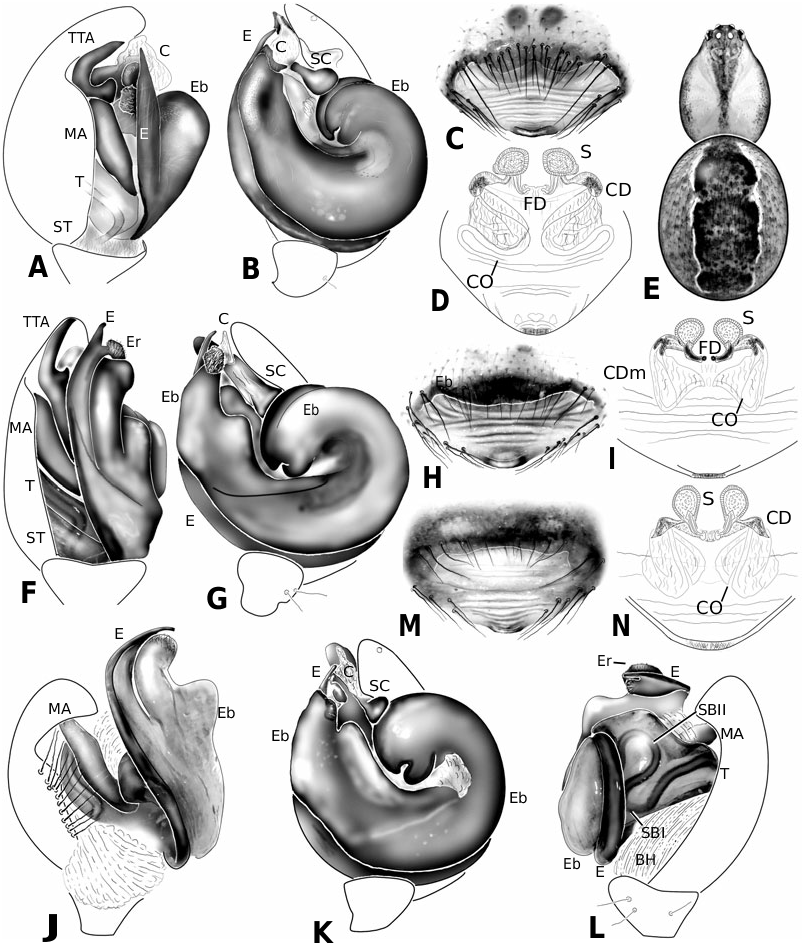
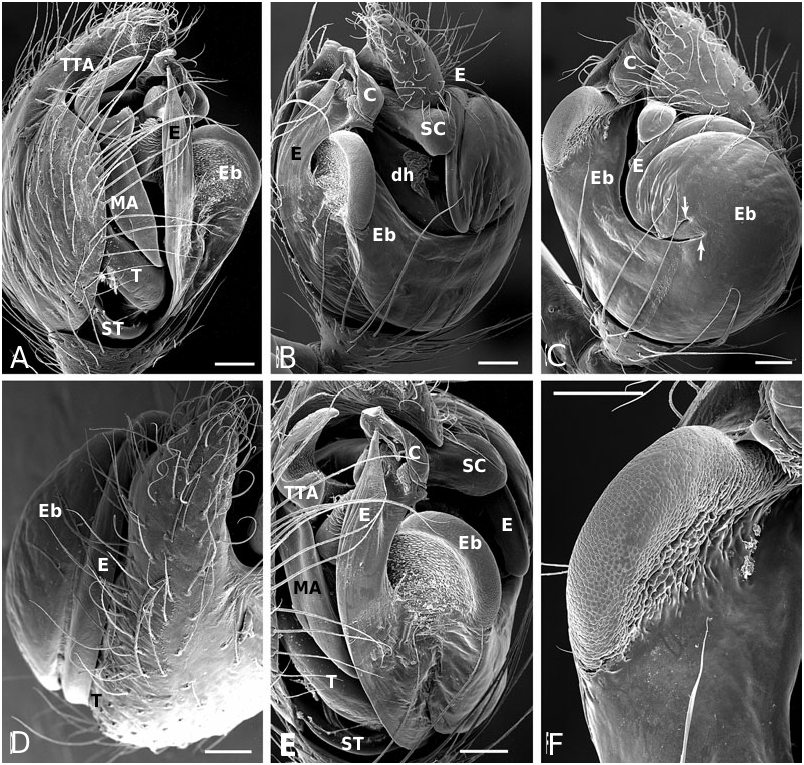
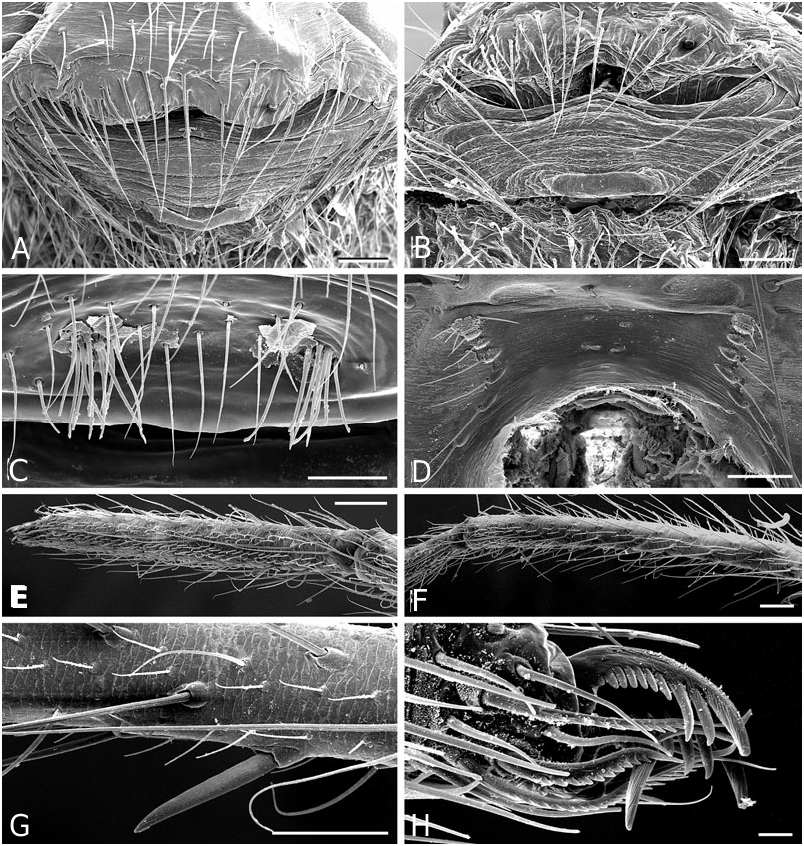
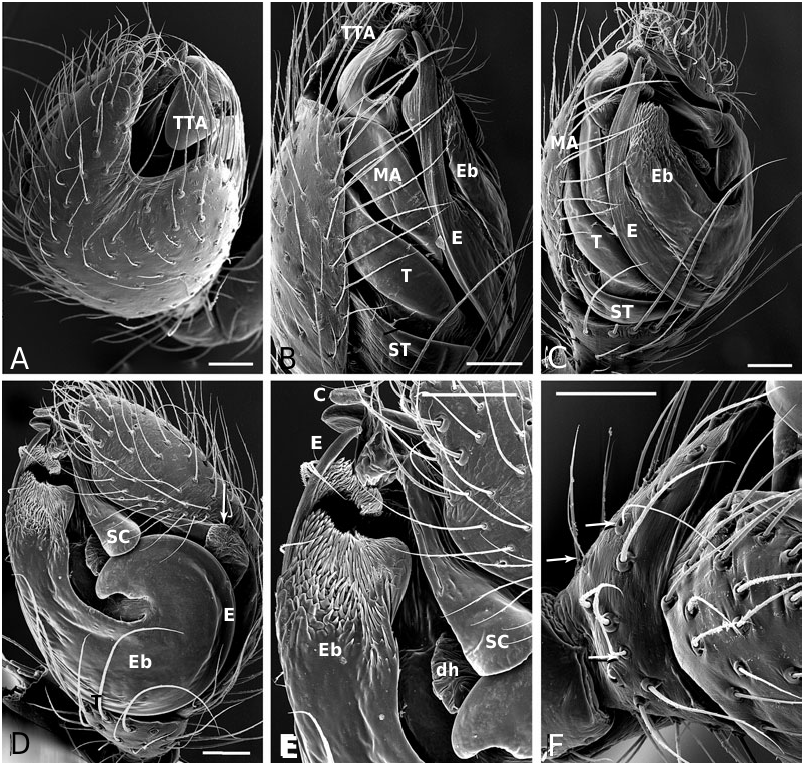
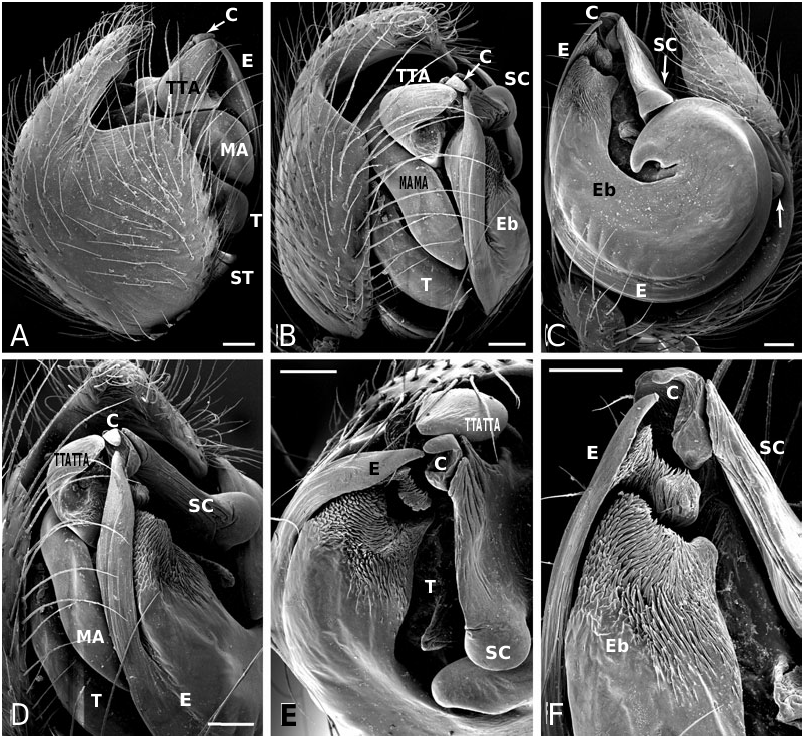
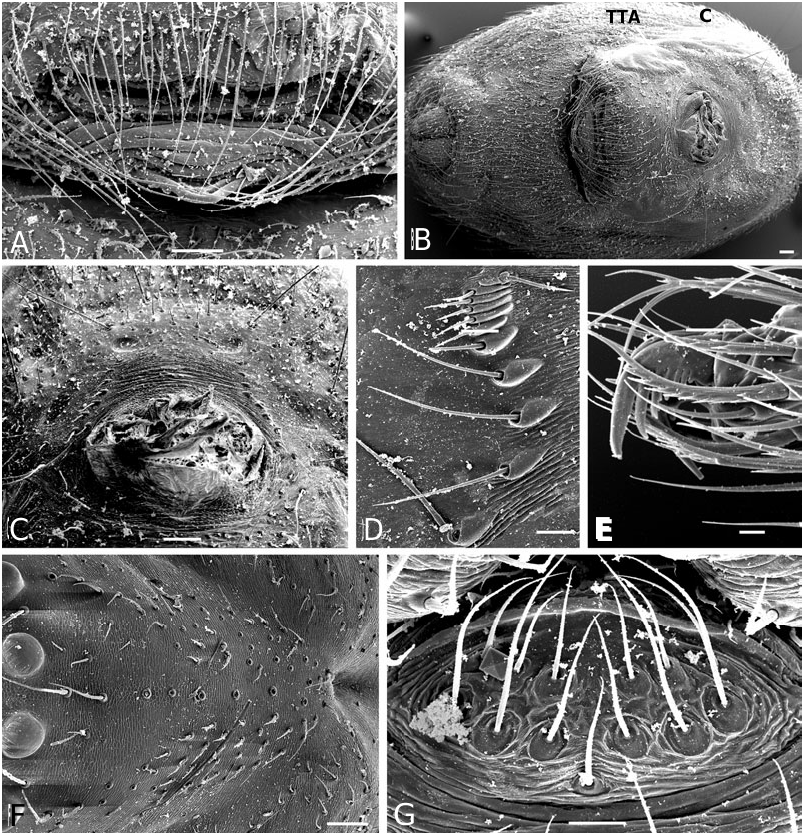
Diagnosis: Males of the jucundus group can be separated from the related studiosus group by a large, globose embolic division b (e.g. Fig. 19A, G, J View Figure 19 ) that is distally much broader than in species of the studiosus group. The basal lobe of the embolus always surpasses the hood of the subconductor, whereas it is hooked in it, or orientated towards it in the studiosus group. Epigyna are very similar among species, and to those of the studiosus group, but differ from the latter in the strongly sclerotized part of the copulatory ducts extending clearly beyond (ectal to) the ectalmost margin of the spermathecae ( Fig. 27F, G View Figure 27 ). The external epigyna in the jucundus group are always strongly ridged (e.g. Fig. 19C, H, M View Figure 19 ), whereas they range from weakly to strongly ridged in the studiosus group. Species of the jucundus group are generally larger than those of the studiosus group, although the overlap is considerable. Palpal organs and epigyna are similarly larger in the jucundus group than in the studiosus group.
Description: Males with a large embolus and embolic division b, together covering the entire ventral portion of the palp (e.g. Fig. 19A, G, K View Figure 19 ). The basal lobe of the embolus surpasses the hood of the subconductor. Females with strongly ridged epigynal plates, and internally the sclerotized portion of the copulatory duct extends beyond (ectal to) the ectalmost margin of the spermathecae ( Fig. 27F, G View Figure 27 ).
Phylogenetics: The jucundus group ( A. jucundus sensu Levi, 1956, 1963 ) monophyly is supported by two unambiguous synapomorphies with perfect fit to the cladogram: elongated theridiid tegular apophysis distal branch ( 53 -1, Fig.19A View Figure 19 ), and shallow embolus-distal hematodocha grooves ( 70 -1, Fig. 20C View Figure 20 ).
Composition: As with A. studiosus , F. O. P.- Cambridge’s (1902) and Levi’s (1956) concept of A. jucundus included several synonymies and bountiful geographical variation. Likewise, recent work discloses behavioural differences and breeding barriers (under laboratory conditions) between some geographically separate populations ( Tapia & De Vries, 1980; Nentwig & Christenson, 1986; Avilés & Gelsey, 1998; Bukowski & Avilés, 2002; L. Avilés, pers. comm.). I recognize five species of the jucundus species complex here: A. arizona , A. baeza , A. jucundus , A. octavius and A. puravida .
Distribution: From south-western USA bordering Mexico, to Brazil ( Figs 63C View Figure 63 , 64B, D View Figure 64 ). Most speciose in Ecuador and Mexico particularly at altitudes of 1000 m or above.
Natural history: Most species of the jucundus group are predominantly subsocial ( A. arizona , A. baeza , and probably A. octavius and A. jucundus ) while A. puravida appears to be social. Level of sociality varies within species in A. baeza , with some populations forming multiple female nests while others form single female nests.
ANELOSIMUS ARIZONA SP. NOV.
( FIGS 19A–E View Figure 19 , 20–22 View Figure 20 View Figure 21 View Figure 22 , 64B View Figure 64 )
Types: Male holotype from Huachuca mountains , Arizona, USA, iv.1989, Avilés & Maddison, deposited in NMNH [ IA40621 View Materials ]. Female and male paratypes from Garden Canyon, Huachuca mountains, Arizona, c. 31°33′N, 110°17′W, c. 1600 m, 28.vi.2003, T GoogleMaps . Bukowski, deposited in NMNH [ IA40622 View Materials ] .
Synonymies:
Anelosimus jucundus: ( Avilés & Gelsey, 1998: 2138) View in CoL . The authors discuss biology, but specimens are not illustrated, not A. jucundus View in CoL O. P.-Cambridge (vouchers examined).
Anelosimus cf. jucundus View in CoL : ( Bukowski & Avilés, 2002: 193; Powers & Avilés, 2003: 727). The authors discuss biology, but specimens are not illustrated, not A. jucundus View in CoL O. P.-Cambridge (vouchers examined).
Etymology: The species epithet is a noun in apposition referring to the type localitie’s state.
Diagnosis: Males of A. arizona differ from other Anelosimus in the jucundus group by having an enlarged distal Eb-ridge, being more prominent than in any other Anelosimus species ( Fig. 19A View Figure 19 ). Geography apart, I have not found a reliable way of separating females from others of the ‘ jucundus group’.
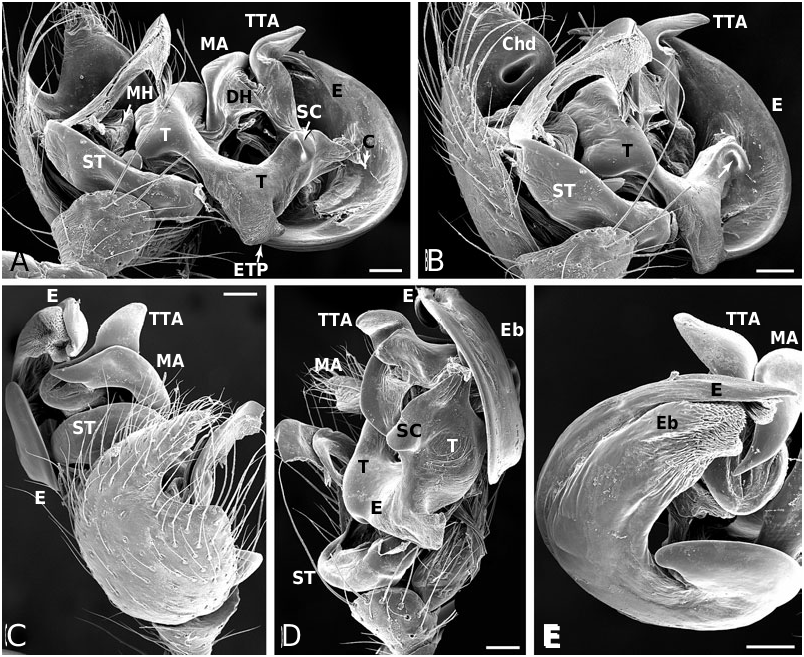
Male ( holotype): Total length 4.42. Prosoma 1.95 long, 1.65 wide, 1.42 high, pale yellow to brown, centre and rim darker. Sternum 1.16 long, 1.07 wide, extending between coxae IV, pale yellow, centre darker. Abdomen 2.80 long, 1.90 wide, 2.06 high. Pattern as in Figure 19E View Figure 19 . Eyes subequal, about 0.11 in diameter. Clypeus height about 4 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4–5 denticles retrolaterally. Leg I femur 3.41, patella 0.91, tibia 3.41, metatarsus 3.15, tarsus 1.04, tibia and metatarsus unusually long. Femur about 8 times longer than wide, metatarsus I about 19 times longer than wide, with a ventral row of thick setae. Leg formula 1243. Leg base colour as carapace, femur 1 slightly darker than other leg segments, distal tip of tibia slightly darkened. Tarsal organs slightly distal (0.50–0.55) on tarsus I, central (0.50) on II, proximal (0.40–45) on III–IV. Five to six trichobothria dorsally on all tibia, 5–6 on tibia I, 5 on tibia III. Trichobothria on metatarsi I–III proximal (about 0.35– 0.40), absent on metatarsus IV. Two prolateral and one retrolateral trichobothria on palpal tibia. Palp as in Figures 19A, B View Figure 19 , 20A–F View Figure 20 , 21A–G View Figure 21 .
Female ( paratype): Total length 4.62. Prosoma 2.08 long, 1.57 wide, 1.40 high, yellowish, with cephalic region and rim darker ( Fig. 19E View Figure 19 ). Sternum 1.16 long, 1.04 wide, extending between coxae IV, yellowish, covered with darker spots, rim dark brown. Abdomen 2.73 long, 2.23 wide, 2.31 high. Pattern as in Fig. 19E View Figure 19 . AME slightly the smallest, other eyes subequal, about 0.10 in diameter. Clypeus height about 3.0 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4–5 denticles retrolaterally. Leg I femur 2.60, patella 0.81, tibia 1.95, metatarsus 2.18, tarsus 0.91. Femur about 6 times longer than wide, metatarsus I about 12 times longer than wide. Leg formula 1423 with legs 4 and 2 subequal. Leg base colour as carapace, femora slightly darkened, especially at tip. Tips of patella, tibia and metatarsi darkened, tibia also with a darker central ring. Tarsal organs slightly distal on tarsi I–II (around 0.50–0.55), proximal (0.40–0.45) on III–IV. Five to seven small trichobothria dorsally on all tibia, 5–6 on tibia I, 6 on tibia III. Trichobothria on metatarsi I–III proximal (about 0.40–0.45), absent on metatarsus IV. Three trichobothria dorsally on palpal tibia. Epigynum as in Figures 19C, D View Figure 19 , 22A, B View Figure 22 .
Variation: Carapace and sternum (in preserved specimens) range from unicolorous yellowish or light brown, to having centre and rim darker than rest. Male holotype pale coloured, some males much darker, with distinctly darkened femur I. Male total length from 3.25–5.00, carapace 1.69–2.60, femur I 2.67– 4.10. Males from Mexico smaller than any from USA The palps of this species vary in subtle details (compare Figs 20A–F View Figure 20 and 21A–G View Figure 21 ), males from Mexico have a more strongly developed E fork. The size of the male palp also varies, and some specimens have up to about 20% larger palps than that of the holotype. Female total length 4.50–6.18, carapace 2.00–2.47, femur I 2.60–2.99. Size of Mexican females overlaps with those from USA. Apparently populations from the Huachuca mountains and Patagonia mountains (about 40 km apart) differ in behaviour ( T. Bukowski, pers. comm.). However, despite much variation, I did not find any consistent differences in their morphology and thus treat them as conspecific here .
Additional material examined: MEXICO. Districto Federal, Pedregal [ 19°18′0″N, 99°8′0″W], 8.viii.1947, c. 2200 m ( H. Wagner, AMNH), 2♂ [IA40525]; xixii.1943, 2400m ( AMNH), 3♀ [cf. IA40518]. Oaxaca [ 17°3′0″N, 96°43′0″W] ( AMNH), 1♂, [IA40520]. Chihuahua, Santa Bárbara [ 28°26′0″N, 107°23′0″W], 18.vii.1947 ( W. J. Gertsch, AMNH), 5♀ [cf. IA40514]. Veracruz, Jalapa City in residential park ( 19°31′1″N, 96°54′2″W), 17.xi.2003, on Ficus sp. , 1400 m ( T. J. Henry & E. Barrera, NMNH) 1♀, 15juv [cf. IA40528] GoogleMaps . USA. Arizona, Patagonia, Sonoita Creek Preserve ( 31°30′0″N, 110°50′0″W), 27.vi.2003, 1500 m ( K. Powers, NMNH), 1♂, 1♀ [IA40557] GoogleMaps .
Distribution: Only known from Arizona, USA, and Mexico ( Fig. 64B View Figure 64 ). All collections from 1500−2500 m altitude.
Natural history: Anelosimus arizona is a univoltine subsocial species, forming mother-offspring associations that persist for several months but break up prior to mating ( Avilés & Gelsey, 1998 [identified as A. jucundus ]; Bukowski & Avilés, 2002; Powers & Avilés, 2003 [identified as A. cf. jucundus ]). The nests are typical basket-shaped sheet webs with intercepting aerial threads extending upwards. Nests occur at the ends of branches, either singly or in clusters. New nests are established by individual subadults, or young adult males and females during the dispersal season (May–August in Arizona). Most individuals disperse at the fifth instar, but between fourth and seventh (adult) and males appear to disperse further. Mating takes place (during July–August in Arizona), typically in the female web, and she shortly thereafter lays a single egg sac containing 21– 53 eggs ( Avilés & Gelsey, 1998). Maternal care continues until the mother dies (sometimes eaten by her offspring), usually when the offspring are only a couple of moults away from adulthood. Siblings continue to collaborate in the natal nest until dispersal, including cooperative prey capture and prey sharing. However, tolerance and co-operation breaks down with age, and also broke down among juveniles in a laboratory experiment under conditions of crowding or low food supply, when the spiders readily cannibalized each other ( Avilés & Gelsey, 1998).
Nest reoccupation occurs by older instar females (subadult or adult) and sometimes two females may reoccupy an old nest ( Avilés & Gelsey, 1998). Remaining in natal nest seems to lower the probability of extinction, while dispersal may be a result of resource competition, mate competition and inbreeding avoidance ( Bukowski & Avilés, 2002; Powers & Avilés, 2003). Bukowski & Avilés (2002) found that maturation of same-generation female and male kin is asynchronous, resulting in limited inbreeding. They therefore concluded that inbreeding avoidance is not key in Anelosimus (see also Powers & Avilés, 2003), but rather that resource competition seems most important.
Avilés & Gelsey (1998) reported equal sex ratios prior to dispersal, but distinctly female-biased postdispersal sex ratios. They attributed this to male dispersal beyond the local area, resulting in greater cost of dispersal for males.
In Mexico, the mirid Ranzovius crinitus Distant (see Henry, 1984) has recently been collected (by T. J. Henry, pers. comm.) in the nests of A. arizona . Henry (1999) also reported R. crinitus in webs of Anelosimus sp. , quite likely also A. arizona .
ANELOSIMUS JUCUNDUS (O. P.- CAMBRIDGE, 1896)
( FIGS 19F–H View Figure 19 , 23–24 View Figure 23 View Figure 24 , 64B View Figure 64 )
Types: Male types, Mexico, Omilteme, col. Godman & Salvin BM1905.4.28.1811-30 (part), in BMNH, examined. O. P.- Cambridge (1896: 166–167) did not designate types, but later F. O. P.- Cambridge (1902: 394) indicated a male type. F. O. P.- Cambridge (1902: fig. 14a–d) illustrated two different specimens and the type vial contains two heterospecific males. One of them matches the original drawing of O. P.- Cambridge (1896, pl. 21, fig. 13) and is here designated as a LECTOTYPE ( Fig. 19F, G View Figure 19 ). The other specimen belongs to A. octavius sp. nov., which is here described (see taxonomic history below).
Synonymies:
Theridion jucundum O. P.- Cambridge, 1896: 166, pl. 21, fig. 13, ♀.
Theridion jucundum: Petrunkevitch, 1911 , 29: 198; 1925, 27: 67.
Anelosimus jucundus View in CoL : F. O. P.- Cambridge, 1902: 394 (in part), pl. 37, figs 14a, 15, ♂ ♀ Levi, 1956: 417 (most of Levi’s text and figures do not refer to A. jucundus O. P. Cambridge View in CoL , see A. octavius View in CoL and A. baeza View in CoL ); Levi, 1963: 43; Stejskal, 1976: 344, figs 4.4, 5.4, 6.3, ♂ ♀ (synonymy uncertain, from the figures it is not possible to tell what species Stejskal worked with, but based on the locality ( Venezuela) it was most likely A. baeza View in CoL . [Note, however, that some specimens labelled A. jucundus View in CoL by Stejskal are in fact A. eximius View in CoL (pers. obs.).] Platnick, 2006.
Anelosimus studiosus: Bryant, 1940 View in CoL , 86: 311, not A. studiosus (Hentz) View in CoL according to Levi (1956); however, I have not been able to locate the Cuban specimens discussed by Bryant (1940) and thus cannot confirm their identity.
Etymology: O. P.-Cambridge did not explain the etymology, but jucundus is Latin for agreeable or merry, possibly referring to the social behaviour of this species.
Diagnosis: Males of A. jucundus differ from other Anelosimus , except A. octavius , by having a distinct ridge ectally on the Eb distal portion facing the embolus base ( Fig. 19G View Figure 19 ). Males differ from A. octavius in a more robust embolus fork. I have not found a reliable way of separating females from others of the ‘ jucundus group’.
Male (IA40623): Total length 3.25. Prosoma 1.50 long, 1.19 wide, 0.92 high, yellowish-brown, with centre and rim darker. Sternum 0.92 long, 0.76 wide, extending between coxae IV, yellowish-brown with a darker rim. Abdomen 1.89 long, 1.47 wide, 1.58 high. Pattern as in A. arizona . Eyes subequal, about 0.11 in diameter. Clypeus height about 2.9 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4–5 denticles retrolaterally. Leg I femur 2.28, patella 0.65, tibia 2.11, metatarsus 2.02, tarsus 0.78. Femur about 8 times longer than wide, metatarsus I about 20 times longer than wide. Leg formula 1423. Leg base colour yellowish, with distal tip of all segments darkened, a darker central bands on tibia, and femur I darker than other segments. Tarsal organs distal on tarsi I (0.55–60) and II (0.50–55), proximal III (0.45– 50) and IV (0.40). Five to six small trichobothria dorsally on all tibia, 6 on tibia I and III. Trichobothria on metatarsi I–III proximal (about 0.35–0.45), absent on metatarsus IV. Two prolateral and one retrolateral trichobothria on palpal tibia. Palp as in Figures 19F, G View Figure 19 , 23A–F View Figure 23 .
Female (IA40623): Total length 4.36. Prosoma 1.76 long, 1.45 wide, 1.22 high, yellowish-brown, with centre and rim darker. Sternum 1.17 long, 0.97 wide, extending between coxae IV, brown with a darker rim. Abdomen 2.86 long, 2.15 wide, 2.15 high. Pattern as in male. Eyes subequal, about 0.09 in diameter. Clypeus height about 4.0 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4–5 denticles retrolaterally. Leg I femur 2.28, patella 0.81, tibia 1.95, metatarsus 1.79, tarsus 0.85. Femur about 6 times longer than wide, metatarsus I about 14 times longer than wide. Leg formula 1423. Leg base colour yellowish, with distal tip of all segments darkened, a darker central bands on tibia, and femur I darker than other segments. Tarsal organs distal on tarsi I (0.60–65) and II (0.55–60), proximal on III and IV (0.4–45). Five to six small trichobothria dorsally on all tibia, 5–6 on tibia I, 5 on tibia III. Trichobothria on metatarsi I–III proximal (about 0.35–0.40), absent on metatarsus IV. Two prolateral and one retrolateral trichobothria on palpal tibia. Epigynum as in Figures 19H, I View Figure 19 , 24B View Figure 24 .
Variation: Male total length 3.15–3.35, prosoma 1.45– 1.55, femur I 2.20–2.28, female total length 4.20–4.55, prosoma 1.70–1.80, femur I 2.20–2.30. A female specimen in very poor condition had a prosoma reaching 2.6 mm, but the identity of the specimen, collected with a male A. studiosus , is doubtful.
Additional material examined: COLOMBIA. Antioquia, San Vicente [ 6°17′0″N, 75°20′0″W], 30.xii.1986, c. 2000 m ( M. A. Serna, MCZ), 1♀ [IA030301] GoogleMaps . COSTA RICA. Puntarenas, Monteverde Cloud Forest Reserve [ 10°2′0″N, 83°27′0″W], 28.vii.1979, 1500 m ( J. Coddington, NMNH), 1♀ [cf. IA40408] GoogleMaps . HONDURAS. [approx. country centre 14°50′0″N, 86°43′0″W] ( Dyer, AMNH), 1♂ [cf. IA40212] GoogleMaps . MEXICO. Chiapas, 5 km W of San Cristobal de Las Casas on HWY 190 ( 16°44′0″N, 92°41′0″W), 27–28.vii.1983, 2134 m (W. Maddison et. al, MCZ), 1♂ [IA0220]; Lagos de Montebello [ 16°6′0″N, 91°43′0″W] ( CAS), 1♂ [IA40782] GoogleMaps . Morelos, Cuernavaca [ 18°55′0″N, 99°13′0″W], c. 1500 m ( N. Banks, MCZ), 1♂ [IA010401] GoogleMaps . Oaxaca, S of Oaxaca at Monte Alban [ 17°2′0″N, 96°47′0″W], 22.ix.1989 ( T. J. Henry, NMNH), 1♂, 5♀ [IA40623] GoogleMaps . Veracruz, Fortín de las Flores [ 18°54′0″N, 96°59′0″W], vii–viii.1986, c. 1000 m ( NMNH), 1♀ [cf. IA40636]. PANAM A. Chiriqui, Road between Volcan-Concepcion [ 8°38′0″N, 82°38′0″W], 28.x.1983, 1021 m ( MCZ), 1♀ [IA031001] GoogleMaps .
Distribution: Mexico – Colombia ( Fig. 64B View Figure 64 ), at altitudes of c. 900–2500 m.
Natural history: Anelosimus jucundus is reportedly a subsocial species, building single-mother/offspring nests. However, it is not certain that any behavioural studies have actually dealt with ‘true’ A. jucundus . The vial label of description series states: ‘Webs abundant on large Ficus sp. , heavily infested with laurel thrips; two species of the mirid genus Ranzovius present.’ The webs were approximately 20 × 20 cm, numerous individual webs tightly grouped (T. J. Henry, pers. comm.). This is consistent with the species being predominantly subsocial.
Nentwig & Christenson (1986) studied ‘ A. jucundus ’ in Panama (likely either A. jucundus or A. baeza ). Occasional nests had more than one adult female, but these appeared not to be co-operating. The spiders seemed to feed mostly on flying ants, cicadina and coleoptera. As in other Anelosimus species nests, kleptoparasites were common: Faiditus caudatus , Argyrodes elevatus and A. spinosus have been documented in the webs of ‘ jucundus -like’ species. Nentwig & Christenson (1986) suggest that their study species is more social than A. studiosus which, in Brach’s (1977) study, did not tolerate the presence of other females. Also their species sometimes had more than one generation of spiders in the nest. However, the populations of A. studiosus studied by Furey (1998) showed much higher degree of social behaviour.
The species studied by Avilés & Gelsey (1998), Bukowski & Avilés (2002) and Powers & Avilés (2003), in their papers identified as A. jucundus , and A. cf. jucundus , respectively, is Anelosimus arizona . Tapia & de Vries (1980) studied ‘ A. jucundus ’ in Ecuador, but given their findings (they discuss a predominantly social species, from a lowland rainforest) the species was almost certainly A. eximius (see also Vollrath & Windsor, 1983).
Two kleptoparasitic mirid species live in the webs of A. jucundus , Ranzovius crinitus and R. bicolor Henry. The latter has only been found in nests of A. jucundus (T. J. Henry, pers. comm.).
Taxonomic history: Reverend Octavius Pickard- Cambridge (1896: 166–167) described this species presumably based on two males from Mexico. O. P.- Cambridge (1896) did not designate types, but later his nephew Frederick O. P.- Cambridge (1902: 394) indicated a male type. However, he illustrated two different specimens (F. O. P.- Cambridge, 1902, fig. 14a– d); both are in the type vial and are here found to be heterospecific, differing considerably in palpal morphology. I here designate one of them as a LECTO- TYPE ( Fig. 19F, G View Figure 19 ), chosen as it matches the original drawing of O. P.- Cambridge (1896, pl. 21, fig 13). The other specimen belongs to A. octavius sp. nov. (see F. O. P.- Cambridge, 1902, fig. 14b–d).
Simon (1897) suggested that A. jucundus and A. studiosus are synonymous due to difficulty of telling the females apart (this would mean the jucundus and studiosus groups as treated here represent only a single species). F. O. P.- Cambridge (1902: 394) conceded that ‘I am unable as yet to satisfactorily distinguish between the females of A. jucundus and A. studiosus ’. He (F. O. P.- Cambridge, 1902: 395) agreed with Simon that ‘It is possible, however, that both T. studiosum, Hentz , and T. jucundum , O. P.- Cambr. (as Simon thinks probable), the varieties here figured, and also those from Bogota, are all one and the same species, the larger and more highly developed examples being T. jucundum , the smaller and more slender being T. studiosum ...’. However, another possibility was suggested by F. O. P.- Cambridge (1902: 395) ‘ [i]t is also possible, on the other hand, that there are several species of these social spiders, and that the varieties above noted may prove to be really good species. I cannot at present reconcile myself to either view, but must be content with giving drawings and descriptions of them’. Levi (1956: 418) closely followed F. O. P.-Cambridge, and remarked on the variation of what he called A. jucundus : ‘There is considerable variation in the structure of the palpi. The epigynum of a number of specimens (from Michoacan: Pátzacuaro [ Mexico]; Guatemala: Yepocapa, Antigua; Costa Rica: San José) lacked the deep groove... which usually distinguishes A. jucundus . These specimens, however, otherwise resemble this species. It is very likely that this is a distinct but similar species.’ It seems clear now that the differences F. O. P.- Cambridge (1902) and Levi (1963) noted between specimens from different localities represented different species rather than intraspecific variation. This conservative and broad formulation of A. jucundus by F. O. P.-Cambridge and Levi is understandable given the difficulty of identifying these species. However, it has muddled subsequent work on Anelosimus , and as a result of a lack of voucher specimens in particular, it is now unclear what species some literature is referring to.
ANELOSIMUS OCTAVIUS SP. NOV.
( FIGS 19J–N View Figure 19 , 25–26 View Figure 25 View Figure 26 , 64B View Figure 64 )
Types: Male holotype and female paratype from Costa Rica, San Jose Province, San Antonio de Escazu , 9°56′N, 84°08′W, J. Coddington, in NMNH GoogleMaps .
Synonymies:
Anelosimus jucundus View in CoL : F. O. P.- Cambridge, 1902: 394, pl. 37, figs 14b–d, 15a,b, ♂ ♀, not A. jucundus View in CoL O. P.- Cambridge; Levi, 1956: 417, figs 27, 34–35, not A. jucundus View in CoL O. P.-Cambridge (note that it is not certain that Levi’s illustrations are of A. octavius View in CoL ); Stejskal, 1976: 344, figs 4.4, 5.4, 6.3, ♂ ♀ (note that Stejskal’s photographs are not recognizable and it is therefore not clear what species he discusses).
Etymology: Octavius Pickard-Cambridge (1896) described A. jucundus , but the type vial contains two heterospecific males, one of which belongs to this new species. The species epithet is a noun in apposition in honour of O. P.-Cambridge.
Diagnosis: Males of A. octavius differ from other Anelosimus , except A. jucundus , by having a distinct ridge ectally on the Eb distal portion (facing the embolus base) ( Fig. 19J, K View Figure 19 ). Males differ from A. jucundus in a less robust embolus fork, and in the entire embolus being more roundish. I have not found a reliable way of separating females from others of the ‘ jucundus group’.
Male (from Mexico, Omilteme, Godman & Salvin, BM1905.4.28.1811−30 (part), in BMNH): Total length 3.77. Prosoma 1.82 long, 1.65 wide, 1.20 high, brown, slightly darkest centrally. Sternum 1.07 long, 0.91 wide, extending between coxae IV, brown. Abdomen 2.02 long, 1.62 wide, 1.73 high. Pattern as in A. baeza . Eyes subequal, about 0.08 in diameter. Clypeus height about 3.8 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4–5 denticles retrolaterally. Leg I femur 2.70, patella 0.72, tibia 2.34, metatarsus 2.08, tarsus 2.08. Femur about 8 times longer than wide, metatarsus I about 18 times longer than wide. Leg formula 1243. Leg base colour yellowish to brown, with distal tip of all segments darkened, and femur I dark. Tarsal organs distal (0.65–0.70) on tarsi I–II, proximal (0.40–0.45) on III–IV, most proximal on tarsus III. Four to five small trichobothria dorsally on all tibia, 4–5 on tibia I, 5 on tibia III. Trichobothria on metatarsi I–III slightly proximal (about 0.45–0.50), absent on metatarsus IV. Two prolateral and one retrolateral trichobothria on palpal tibia. Palp as in Figures 19J–L View Figure 19 , 25A–F View Figure 25 .
Female ( paratype): Total length 5.01. Prosoma 1.95 long, 1.60 wide, 1.40 high, light brown. Sternum 1.17 long, 1.07 wide, extending between coxae IV, light brown. Abdomen 2.93 long, 2.84 wide, 2.64 high, pattern as in A. baeza . Eyes subequal, about 0.12 in diameter. Clypeus height about 3.5 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4–5 denticles retrolaterally. Leg I femur 2.60, patella 0.81, tibia 2.21, metatarsus 2.21, tarsus 0.88. Femur about 5 times longer than wide, metatarsus I about 13 times longer than wide. Leg formula 1423. Leg base colour yellowish with distal tip of all segments darkened, and a central band on tibia and femora, most distinct on femur I. Distal part of femur IV darker than others. Tarsal distal tarsi I (0.65–70) and II (0.60–0.65) proximal on III (0.35–4-) and IV (0.40– 0.45). Five to eight small trichobothria dorsally on all tibia, 7 on tibia I, 6 on tibia III, tibia IV, unusually, with 8. Trichobothria on metatarsi I–III proximal (0.35–0.45), absent on metatarsus IV, distal (0.85) on palpal tarsus. Three dorsal trichobothria on palpal tibia. Epigynum as in Figures 19M, N View Figure 19 , 26A View Figure 26 .
Variation: Male total length 3.64–3.77, prosoma 1.8– 1.85, femur I 2.70–2.86, female total length 3.77–5.01, prosoma 1.89–1.95, femur I 2.47–2.60.
Additional material examined: COSTA RICA. San José, San Antonio de Escazú ( 9°56′0″N, 84°8′0″W], 28–31.iii.1989, c. 1200 m (J. Coddington, NMNH), 1♂ [IA40532]; 30.iii.1989, holotype and paratype, and additional 1♂, 1♀ [IA40617]; 17.iii.1997 (L. Avilés, NMNH), 1♂, 1♀ [IA40535] GoogleMaps . GUATEMALA. Alta Verapaz, Cobán [ 15°27′0″N, 90°22′0″W], vii.1947, 1300 m (C. & P. Vaurie, AMNH), 1♂ [cf. IA40738] GoogleMaps . Chimaltenango, Yepocapa [ 19°30′0″N, 90°56′0″W], 27.vii.1949, 1400 m ( T. H. Farr, AMNH), 1♂ [cf. IA40737]; iii– vi.1935 (E. Elishewitz, AMNH), 1♀ [cf. IA40744] GoogleMaps . Quiché, Nebaj [ 15°24′0″N, 91°9′0″W], 9–10.viii.1947, 2000 m (C. & P. Vaurie, AMNH), 1♀ [cf. IA40739] GoogleMaps . MEXICO. Chiapas, San Cristóbal de las Casas [ 16°44′0″N, 92°38′0″W], 22.vii.1947, 2200 m (C. & M. Goodnight, AMNH), 2♂, 1♀ [cf. IA40733]; 2.ix.1972, 2164 m (C. Mullinex, CAS), 1♂ [IA40784] GoogleMaps . Hidalgo, 4 km NE. of Tlanchinol on Highway 105 ( 21°2′0″N, 98°39′0″W), 14.vi.1983, cloud forest and edge, 1500 m (W. Maddison, MCZ), 1♀ [IA40505] GoogleMaps . Guerrero, Omiltemi [ 17°30′0″N, 99°40′0″W], c. 2800 m ( Godman & Salvin, BMNH, in vial with A. jucundus holotype), 1♂ GoogleMaps .
Distribution: Mexico, Guatemala, Costa Rica ( Fig. 64B View Figure 64 ), from altitudes of 1000−2800 m.
Natural history: The only information on the natural history of this species is from field label and notes from J. Coddington (pers. comm.). He found adult males and females in individual webs or wandering (males), and thus the species is probably a typical subsocial species with a solitary life phase after dispersal from the natal nest (during the time males are adult).
ANELOSIMUS BAEZA SP. NOV.
( FIGS 27A–M View Figure 27 , 28–32 View Figure 28 View Figure 29 View Figure 30 View Figure 31 View Figure 32 , 64D View Figure 64 )
Types: Male holotype and female paratype from Tena Road, 17 km S of Baeza, Napo administrative division, Amazon river basin, Ecuador, 0°37′S, 77°53′W, 3.viii.1999, L. Avilés, deposited in NMNH [ IALA0601 ] GoogleMaps .
Synonymies:
Anelosimus jucundus: Levi, 1956: 417–418 View in CoL (in part), fig. 26 (possibly also 27, 31–33); Levi, 1963: 35–36 (in part).
Anelosimus cf. jucundus: Agnarsson, 2004 View in CoL : figs 20A–F, 21A–G.
Etymology: The species epithet is a noun in apposition based on the name of the type locality.
Diagnosis: Males differ from all other Anelosimus , except A. puravida , in having an ectal tegular outgrowth ( Fig. 27B, C View Figure 27 ) and in the shape of the Eb, with a distinct basal lobe pointing caudally, and an evenly broad, terminally ridged distal portion ( Fig. 27B, C View Figure 27 ). Morphologically this species is extremely similar to A. puravida . Subtle differences in the male palp separate the two species, the ectal tegular outgrowth of A. baeza (Tr, Fig. 27B, C View Figure 27 ) is much broader than that of A. puravida ( Fig. 27O View Figure 27 ), the connection of the distal hematodocha with the embolus is smaller. I have not found a reliable way of separating females from others in the jucundus group. Anelosimus baeza appears to be less social than A. puravida , and sex ratio is unbiased, but most likely female biased in A. puravida .
Male ( holotype): Total length 2.86. Prosoma 1.43 long, 1.12 wide, 0.92 high, brown. Sternum 0.79 long, 0.73 wide, extending between coxae IV, brown. Abdomen 1.56 long, 1.07 wide, 1.04 high. Pattern as in Figure 27H, I, L, M View Figure 27 . Eyes subequal, about 0.09 in diameter. Clypeus height about 3.1 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4–5 denticles retrolaterally. Leg I femur 1.92, patella 0.55, tibia 1.76, metatarsus 1.50, tarsus 0.68. Femur about 7 times longer than wide, tibia I about 18 times longer than wide Leg formula 1243 with legs two and four subequal. Leg base colour yellowish, distal tip of tibia I very slightly darker, femur I darker than other femora, their distal tip not noticeably darkened. Tarsal organs slightly distal (around 0.55) on tarsi I and II, proximal (0.40–0.45) on III and IV. Four to eight small trichobothria dorsally on all tibia, 7–8 on tibia I, 5 on tibia III. Trichobothria on metatarsi I–III proximal (about 0.40–0.45), absent on metatarsus IV. Two prolateral and one retrolateral trichobothria on palpal tibia. Palp as in Figures 27A–C View Figure 27 , 28A–F View Figure 28 , 29A–F View Figure 29 , 30A–E View Figure 30 .
Female ( paratype): Total length 4.00. Prosoma 1.82 long, 1.45 wide, 0.99 high, brown. Sternum 1.19 long, 0.86 wide, extending between coxae IV, brown. Abdomen 2.47 long, 1.62 wide, 1.47 high. Pattern as in Figure 27J, K View Figure 27 . Eyes subequal, about 0.09 in diameter. Clypeus height about 3.5 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4– 5 denticles retrolaterally. Leg I femur 2.37, patella 0.72, tibia1 0.95, metatarsus 1.76, tarsus 0.91. Femur about 6 times longer than wide, tibia I about 16 times longer than wide. Leg formula 1423. Leg base colour light brown, tibia I with an indistinct ventral central band. Tarsal organs distal (0.60–0.65) on tarsi I and II, central (0.5) on III, slightly proximal (0.40) on IV, distal (0.85) on female palp, positions vary slightly between specimens. Five to eight small trichobothria dorsally on all tibia, 8 on tibia I, 5–6 on tibia III (variable between sides of the animal). Trichobothria on metatarsi I–III proximal (about 0.40–0.45, absent on metatarsus IV. Three dorsal trichobothria on female palpal tibia. Epigynum as in Figures 27D–G View Figure 27 , 31A View Figure 31 , but very variable, sometimes more distinctly ridged.
Additional material examined: BRAZIL. Rio de Janeiro, Parque Nacional Tijuca, Paineiras [ 22°57′0″S, 43°16′0″W], 1.iv.1987 (H. Levi, MCZ), 1♀ [IA0208]; 1♀ [IA0219]. São Paulo, São Paulo botanical garden [ 23°34′0″S, 46°37′0″W], 10.iii.1985, c. 600 m (H. & L. Levi, NMNH), 1♀ [cf. IA0205]; 1♀ [cf.IA0206]; 1♀ [cf. IA40666]; 9.iii.1985, 1♀ [cf. IA081101]; Serra do Japi, Jundiaí [ 23°15′0″S, 47°0′0″W], 4.ii.1998, c. 1100 m (M. O. Gonzaga, NMNH), 1♂, 1♀ [IA40540]. COLOMBIA. Antioquia, La Estrella [ 7°15′0″N, 75°57′0″W], 24.i.1974, 1720 m (P. Schneble, MCZ), 1♂ [IA0201]; v-vi.1973, 1720 m (P. A. Schneble, MCZ), 1♀ [cf. IA030101]; San Vicente [ 6°17′0″N, 75°20′0″W], 30.xii.1986, c. 2000 m (M. A. Serna, MCZ), 1♂ [IA0213]; 26.xii.1986, picked from leaves (M. A. Serna, MCZ), 1♀ [cf. IA030501]. Huila, 12 km E. of Santa Leticia ( 2°20′0″N, 76°6′0″W), hand collected (NMNH), 5♂, 1♀ [IA020701]. Valle, Cali-Bitura road ( 25 km) [ 3°25′0″N, 76°33′0″W], ix.1975, 1700 m (MCZ), 1♀ [IA023101]; 1♀ [IA022401]; Cali [ 3°25′0″N, 76°33′0″W], v.1976, c. 750 m (W. Eberhard, MCZ), 1♂, 2♀, 1juv [IA031401]; (around house) [ 3°25′0″N, 76°33′0″W], 28.ii.1973, 1000 m (H. Levi, MCZ), 1♀ [cf. IA022501]; Lago Colima, Río Colima [ 3°42′0″N, 76°33′0″W], vi.1976, looking up, 1400 m (W. Eberhard, MCZ), 1♀ [IA010201]; Río Calima [ 3°42′0″N, 76°33′0″W], v.1976, picked from colony (W. Eberhard, MCZ), 1♂ [IA023201]; iv.1976, 1400 m (W. Eberhard, MCZ), 1♀, 4juv [cf. IA031301]; vi.1976, 1400 m (W. Eberhard, NMNH), 1♀ [IA40509]. Mala Valley, 23.iv.1964 (M. Guerovich, CAS), 1♂, 1♀ [IA40780]. COSTA RICA. San José, San Antonio de Escazú ( 9°56′0″N, 84°8′0″W], 28–31.iii.1989, c. 1200 m (J. Coddington, NMNH), 1♂, 2♀ [cf. IA40511]; San José, [ 9°55′0″N, 84°4′0″W], 1150 m (E. Schmidt, AMNH), 1♀ [cf. IA40734]. ECUADOR. Manabí, Salaite [1°23’29′S, 80°45’29′W], 6.v.1994, hand collected (W. Maddison, MCP), 1♂ [IALA14]; Puerto Lopez ( 1°32′56″S, 80°48′36″W), 1–5.viii.2004, 5 m (W. Maddison, NMNH), 1♂ [IAV07]. Morona Santiago, km 4 from Limón to Gualaceo, Napo, Amazon river basin, 1.3 km S of Baeza ( 0°28′0″S, 77°53′0″W), 17.xii.2002 (L. Avilés, NMNH), 1♂ [IA40561]; 10.15 km S of Baeza (0°30′76″S, 77°52′73″W), 17.xii.2002, hand collected (L. Avilés, NMNH), 1♀ [IALA26]; 11.3 km S of Baeza ( 0°30′0″S, 77°52′0″W), 17.xii.2002, hand collected (L. Avilés, NMNH), 1♂ [IALA25]; 5.6 km S of Baeza ( 0°28′59″S, 77°52′17″W), 17.xii.2002, hand collected, c. 1500 m (L. Avilés, NMNH), 1♂ [IALA21]; 6 km S of Baeza ( 0°31′0″S, 77°53′0″W), 22.iv.1994, hand collected (V. Roth, MCP), 1♀, 1♀ [IALA0801]; Baeza [ 0°27′0″S, 77°53′0″W], 13.viii.1999 (L. Avilés, NMNH, paratype), 1♀ [IA40625]; Las Caucheras, 16.6 km rd. to Sierra Azul, near Cosanga river ( 0°37′0″S, 77°55′0″W), 20.viii.1999 (L. Avilés, NMNH), 2♂, 2♀ [IA40560]; 5.3 km on road to Sierra Azul ( 0°37′0″S, 77°55′0″W), 20.viii.1999, hand collected, 2200 m (L. Avilés, NMNH), 2♂ [IALA28]; Las Caucheras, between Aliso & Cosanga rivers ( 0°33′5″S, 78°46′0″W), 6.i.2002, hand collected (L. Avilés, NMNH), 1♂ [IALA27]; Las Caucheras ( 0°37′0″S, 77°55′0″W), 20.viii.1999, hand collected, 2200 m (L. Avilés, NMNH), 2♂, 1♀, 3juv [IALA0401]; 3♂, 1♀, 1juv [IALA0501]; Oritoyacu, 8.4 km S of Baeza (0°29′98″S, 77°72′48″W), 17.xii.2002, hand collected, c. 2000 m (L. Avilés, NMNH), 2♂ [IALA24]; Road to Bermejo 0.52, 10 km S. of Baeza (0°30′97″S, 77°53′2″W), 17.xii.2002, hand collected, c. 1501 m (L. Avilés, NMNH), 1♂ [IALA23]; 10 km S. of Baeza ( 0°31′21″S, 77°53′85″W), 17.xii.2002, hand collected, c. 1500 m (L. Avilés, NMNH), 1♂, 1♀ [IALA22]; Tena Road, 17 km S of Baeza ( 0°37′0″S, 77°53′0″W), 3.viii.1997, hand collected, 2200 m (L. Avilés, NMNH, with holotype), 3♂, 2♀ [IALA0601]; 13.viii.1999, hand collected, 2200 m (L. Avilés, NMNH), 1♂, 1♀ [IALA0701]. Pichincha, near El Cisne, N of Pedro Vicente Maldonado ( 0°8′57.48″N, 79°1′54.12″W), 26.vii. 2004, 600 m (I. Agnarsson et al., NMNH), ♂♂ ♀♀ [IAV08]. Tungurahua, Baños [ 1°23′0″S, 78°25′0″W], iv.1939,1850− 2000 m (W.C. Macintyre, MCZ), 1♂, 2♀ [IA050501]; 15–21.vi.1943 (MCZ), 1♀ [cf. IA051326]; 1–15.iii.1939, 1800 m (F. M. Brown, AMNH) [cf. IA40740]. EL SAL- VADOR. San Salvador, San Salvador [ 13°42′0″N, 89°12′0″W], i-iii.1954 (J. B. Boursot, AMNH), 1♀ [cf. IA40736]. GUATEMALA. Sacatepéquez, Antigua [ 14°33′0″N, 90°44′0″W], 16–17.viii.1947,1600 m (C. & P. Vaurie, AMNH), 1♀ [cf. IA40743]. HONDURAS. Atlántida, Lancetilla [ 15°41′0″N, 87°28′0″W], 1.vii. 1929, 400 m (A. M. Chickering, MCZ), 1♀ [cf. IA011101]; vii.1929, 1♀ [cf. IA011301]. MEXICO. Hidalgo, 4 km NE of Tlanchinol on Highway 105 ( 21°2′0″N, 98°39′0″W), 14.vi.1983, 1500 m (W. Maddison, MCZ), 1♂ [IA010601]. Chiapas, Las Cruces Arriaga [ 16°17′0″N, 93°48′0″W], 15.ix.1947 (H. Wagner, AMNH), 3♀ [IA40516]; Rincón [ 16°28′0″N, 93°34′0″W], 6.iv.1953, c. 900 m (L. I. Davis, AMNH), 1♀ [cf. IA40522]; San Cristobal [ 16°44′0″N, 92°38′0″W], 21.vii.1950, c. 2200 m (C. & M. Goodnight, AMNH), 1♂ [cf. IA40513]. Guanajuato, Guanajuato [ 21°0′0″N, 101°16′0″W], 22.vii.1975, c. 2100 m (J. W. Burgess, AMNH), 1♀ [cf. IA40519]. Michoacán, Cerro Tancitare [ 19°25′0″N, 102°18′0″W], vii-viii.1941, Sweaping, 2377 m (H. Hoogstraal, MCZ), 1♀ [cf. IA010801]. Oaxaca, 7 miles S of Nochixtlan [ 17°21′0″N, 97°17′0″W], 27.vi.1947 (L. I. & A. M. Davis, AMNH), 1♀ [cf. IA40521]. San Luis Potosí, Tamazunchale [ 21°15′0″N, 98°47′0″W], 20.v.1944, c. 200 m (C. Bolivar, AMNH), 1♂ [IA40515]. Veracruz, Fortín [ 18°54′0″N, 96°59′0″W], 22.vii.1955 (P. Vaurie, AMNH), 1♂ [IA40523]. PANAMA. Panamá, Canal Zone, Barro Colorado Island [9°9’17′N, 79°50’53′W], vi.1950 (A. M. Chickering, MCZ), 1♀ [IA0124]; 5.vii.1936, 1♀ [cf. IA022901]; 1–3.viii.1939, 1♀ [cf. IA40748]; 23–30.vi.1939, 1♀ [cf. IA023001]; viii.1950, 1♀ [IA010501]; 3.vii.1954, 1♀ [cf. IA032001]; 5.ii- 4.iii.1958, 1♀ [IA011001]; 17–20.iii.1967 (Patterson expedition, MCZ), 1♂ [IA40569]; 16.v-15.vii.1934 (A. M. Chickering, NMNH), 1♀ [cf. IA0221]; 29.vii.1936 (MCZ), 1♀ [cf. IA40749]; Canal Zone, Ft. Sherman [ 9°22′0″N, 79°57′0″W], 15.viii.1939 (A. M. Chickering, MCZ), 1♀ [IA010701]; Perlas Islands, Isla San José (816′0″N, 79°6′0″W), 5.ii.1973, mangroves (MCZ), 1♂ [IA0210]; El llano (330) ( 8°24′0″N, 80°9′0″W), 28.v.1975 (F. Vollrath, MCZ), 1♂, 1juv [cf. IA033401]. Chiriqui, Boquete [ 8°46′0″N, 82°25′0″W], vii.1939 (A. M. Chickering, MCZ), 1♀ [cf. IA022701]; El Volcán [ 8°46′0″N, 82°38′0″W], 9–14.viii.1950 (A. M. Chickering, MCZ), 2♀ [IA0121]; La Fortuna [ 8°44′0″N, 82°15′0″W], 5.iv.1984, 1100−1200m (W. Eberhard, MCZ), 1♀ [cf. IA0202]; Road between Volcan- Concepción [ 8°38′0″N, 82°34′0″W], 28.x.1983, 1100 m (NMNH), 1♀ [cf. IA40504]. Bocas del Toro, Pipeline road [913′0″N, 82°30′0″W] 6.iv.1984 (NMNH), 1♀ [IA0216]. PERU. [? Piura], Chira road, Mallares [ 4°50′0″S, 80°26′0″W], 4.i.1942 (D. & H. Frizzel, CAS), 1♂, 1♀ [IA40781]. Cajamarca, San Andres de Cutervo [ 6°12′0″S, 78°40′0″W], 16.iii.1989, 2000 m (D. Silva, MHNSM), 2♀ [cf. IA40601]; 15.iii.1989, 1♀ [cf. IA40604]. Lima, Lima [ 12°2′0″S, 77°2′0″W], 31.v.1989 (D. Silva, MHNSM), 1♀ [cf. IA40548]. Urubamba, Machu Picchu, Pueblo Guzvo [ 13°9′0″S, 72°31′0″W], 20–22.iii.1947, 2400 m (J. C. Pallister, AMNH), 1♀ [cf. IA40741]. Pasco, Oxapampa [ 10°34′0″S, 75°23′0″W], 22.vi.1986 (D. Silva, MHNSM), 1♂, 3♀ [IA40590]; 5 km SE. of Oxapampa [ 10°40′0″S, 75°18′0″W], 20.vi.1986, 2000 m (D. Silva, MHNSM), 2♂, 1♀ [IA40552]. San Martín, Vilcapasa [ 7°6′0″S, 76°42′0″W], 7.i.1985, 2080 m ( A. Delgado et al., MCZ), 1♂, 7juv [IA030701]. SURINAM. Commewijne, Matapica Reserve [5°80′0″N, 54°50′0″W], 20.v.1986 (D. Smith Trail, NMNH), 3♂, 1♀ [IA40640]. VENEZU- ELA. Monagas, Café, Caripe [ 10°10′0″N, 63°30′0″W], 15.ix.1975 (W. Stejskal, NMNH), 1♀ [cf. IA0203]. [No locality data], 11.viii.1983 (H. & L. Levi, MCZ), 1♀ [cf. IA010101].? HONDURAS. Label only states ‘Dyer Tenne- ix.13 1917 ’. Unlikely from Dyer in Tennessee, USA as the species has never been found in USA. Possibly the collector is Dyer, whose other collections of Anelosimus all came from Honduras. 13.ix.1917 (AMNH), 1♂ [IA40742].
Variation: Male total length 2.65–4.10, prosoma 1.35–1.85, first femur 1.80 – 2.90. Female total length 3.80–6.10, prosoma 1.75–2.25, first femur 2.20–2.50. Coloration in general variable, some populations have very dark individuals, others lightly coloured individuals. Prosoma coloration varies from nearly uniformly brown to yellowish with darker areas in centre and around rim. Leg coloration varies from fairly unicolorous brown to yellowish-brown with distal tip of femora slightly darkened, tips and centre of tibia, and tip of metatarsus dark. Abdomen pattern and coloration also variable ( Fig. 27H–M View Figure 27 ). The number of trichobothria on tibia 1 varies from 6 to 9 in male, and from 6 to 8 in female. Male palpal organ quite variable ( Figs 27A–C View Figure 27 , 28A–F View Figure 28 , 29A–F View Figure 29 , 30A–E View Figure 30 ), especially the shape of the distal portion of the Eb, the orientation of the E lobe and the size and shape of the weakly sclerotized area of the embolus where the distal hematodocha connects with it. Palpal coloration ranges from nearly white appearing lightly sclerotized to dark reddish-brown. A male (identified as A. cf. baeza ) from San Antonio de Escazu, Costa Rica, has an abnormal left palp, appearing functional, but entirely different from any Anelosimus species (I. Agnarsson, unpubl. data). The right palp is similar to A. baeza , although differing in detail. Females collected with this male also differ from ‘normal’ A. baeza . Behaviour is also variable (see Natural history). Based on current evidence I consider this variation to be intraspecific, but further studies are necessary to understand this variation better, and A. baeza as here circumscribed may well represent two or more distinct species. Epigyna vary in the number and prominence of the ridges on the epigynal plate; no variation was observed in the internal female genitalia.
Distribution: From Panama to Peru ( Fig. 64D View Figure 64 ), at a range of altitudes from c. 200 to 2500 m.
Natural history: At the type locality, Anelosimus baeza is subsocial, typically with single-mother and offspring association ( Avilés et al., 2001; L. Avilés, pers. comm.). The webs are a typical basket, the largest one encountered by Avilés et al. (2001) measured 20 × 15 × 10 cm. Of 13 webs documented by Avilés et al. (2001), four contained solitary adults, two contained an adult female and one or two males, two a mother with egg sac, three contained a group of juveniles or subadults, and two a mother and her offspring, up to 89 individuals of three cohorts. Sex ratios were not biased. Interestingly, in the nearby Las Caucheras A. baeza sometimes forms larger colonies containing offspring of several females (L. Avilés, pers. comm.). However, as at Baeza, sex ratios are not biased, which is unusual for colonies containing more than one adult female. The lack of sex ratio bias indicates outbreeding; males, females or both must exit their natal colony to seek mates. Given this behavioural difference, these possibly represent different species. However, no morphological characters have been found to separate the two, and further studies on behavioural variation and inbreeding are necessary to solve this issue adequately.
ANELOSIMUS PURAVIDA SP. NOV.
( FIGS 27N–Q View Figure 27 , 33 View Figure 33 , 34 View Figure 34 , 63C View Figure 63 )
Types: Male holotype and female paratype from Costa Rica, San José Province, San Antonio de Escazú , 1300 m, viii.1988, W. Eberhard, deposited in NMNH [ IA40620 View Materials ] .
Synonymy:
Anelosimus jucundus: Levi, 1956: 417–418 View in CoL (in part); Levi, 1963: 35–36 (in part).
Etymology: The species epithet comes from the Costa Rican phrase ‘pura vida’ (literally ‘pure life’). It translates to ‘everything is fine’, a cheerful salut characteristic of the ‘Ticos’.
Diagnosis: Males differ from all other Anelosimus , except A. baeza , in having an ectal tegular outgrowth Tr ( Fig. 27O View Figure 27 ) and in the shape of the Eb, with a distinct basal lobe pointing caudally, and an evenly broad, terminally ridged distal portion ( Fig. 27O View Figure 27 ). Morphologically this species is extremely similar to A. baeza , but both sexes tend to be slightly larger. Subtle differences in the male palp separate the two species, the non-sclerotized region of the embolic base is typically larger in A. puravida , and the tegular outgrowth (Tr) is much narrower at its tip. The male of A. puravida also has a more strongly developed SPR. Behaviourally the two seem to differ: A. puravida seems to be prominently a social species with multiple adult spiders and a biased sex ratios (J. Coddington, pers. comm.), whereas A. baeza has equal sex ratios, but ranges from single-mother nests to nests containing several females. Apart from natural history I have not found any reliable way of distinguishing between females of the two.
Male ( holotype): Total length 4.03. Prosoma 1.89 long, 1.65 wide, 1.16 high, brown, darker in centre and around rim. Sternum 1.20 long, 1.02 wide, extending between coxae IV, dark brown, darkest in centre and around rim. Abdomen 2.41 long, 1.65 wide, 1.65 high. Pattern as in A. baeza . Eyes subequal, about 0.11 in diameter. Clypeus height about 3.8 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4–5 denticles retrolaterally. Leg I femur 2.60, patella 0.75, tibia 2.37, metatarsus 2.11, tarsus 0.85. Femur about 6 times longer than wide, metatarsus I about 14 times longer than wide. Leg formula 1243. Leg base colour yellowish, distal tip of all segments slightly darkened, femur I darker than other femora, their distal tip not noticeably darkened. Tarsal organs slightly distal on tarsi I (0.60) and II (0.50–55), proximal on III (0.4–0.45) and IV (0.45–50). Six to seven small trichobothria dorsally on all tibia, 6 on tibia I, 6–7 on tibia III. Trichobothria on metatarsi I–III proximal (about 0.35–0.45), absent on metatarsus IV. Two prolateral and one retrolateral trichobothria on palpal tibia. Palp as in Figures 27N, O View Figure 27 , 33A–F View Figure 33 .
Female ( paratype): Total length 5.07. Prosoma 2.02 long, 1.73 wide, 1.25 high, brown, darker in centre and around rim. Sternum 1.29 long, 1.07 wide, extending between coxae IV, dark brown, darkest in centre and around rim. Abdomen 3.38 long, 2.48 wide, 2.72 high. Pattern as in A. baeza . Eyes subequal, about 0.10 in diameter. Clypeus height about 3.5 times AME diameter. Chelicerae with one large and two small prolateral teeth, 4–5 denticles retrolaterally. Leg I femur 2.37, patella 0.81, tibia 1.98, metatarsus 2.24, tarsus 0.88. Femur about 5 times longer than wide, metatarsus I about 12 times longer than wide. Leg formula 1423 Leg base colour yellowish, distal tip of all segments slightly darkened, femur I darker than other femora, their distal tip not noticeably darkened. Tarsal organs slightly distal on tarsus I (0.55), proximal on tarsi II (0.45–0.50), III (0.40–0.45) and IV (0.35– 40). Four to seven small trichobothria dorsally on all tibia, 4–5 on tibia I, 6 on tibia III. Trichobothria on metatarsi I–III proximal (about 0.40–0.45), absent on metatarsus IV. Three dorsal trichobothria on female palpal tibia. Epigynum as in Figures 27P, Q View Figure 27 , 34A View Figure 34 .
Additional material examined: COSTA RICA. Cartago, Cartago [ 9°51′0″N, 83°55′0″W], xi.1953, c. 1400 m ( N. L. Krauss, MCZ), 1♀ [cf. IA022601] GoogleMaps . Puntarenas, 6 km S of San Vito (642′0″N, 83°0′0″W), 13–18.iii.1967 ( OTS course, NMNH), 1♀, 4juv [IA0223]; Monteverde Cloud Forest Reserve [ 10°2′0″N, 83°27′0″W], 28.vii.1979, c. 1350 m ( J. Coddington, NMNH), 1♀ [cf. IA40533]. San José, Pico Blanco ( 9°56′0″N, 84°8′0″W), iii.1988, 1500 m ( W. Eberhard, MCZ), 1♀ [IA0212]; San Antonio de Escazú [ 9°56′0″N, 84°8′0″W], vi.1988, 1300 m ( W. Eberhard, NMNH), 6♀ [cf. IA40634]; 28–31.iii.1989, c. 2300 m ( J. Coddington, NMNH), 1♀ [IA40506]; viii.1988, 1300 m ( W. Eberhard, NMNH), 1♂, 2♀ [IA40507]; 7♂, 43♀, 7juv [IA40512]. Chíral Paraíso ( N. Banks, MCZ), 6♀ [cf. IA0209]. GUATE- MALA. El Quiché, Chichicastenango [ 14°56′0″N, 91°6′0″W], 6–7.viii.1947, c. 2200 m ( C. & P. Vaurie, AMNH), 1♀ [cf. IA40735] GoogleMaps . PANAMA. Chiriqui, Boquete [ 8°46′0″N, 82°25′0″W], 10–25.vii.1939, c. 1100 m ( A. M. Chickering, MCZ), 1♂, 7♀ [IA011201] GoogleMaps . Panamá, El Valle [ 8°36′0″N, 80°8′0″W], 27.viii.1983 ( T. Christenson, MCZ), 1♀ [cf. IA0214]; ( T. Christenson, NMNH), 1♀ [cf. IA40508] GoogleMaps .
Variation: Male total length 3.90–4.50, prosoma 1.80 – 2.10, first femur 2.50 – 3.00. Female total length 4.70–6.20, prosoma 1.90–2.30, first femur 2.30–2.60.
Distribution: Central America from Guatemala to Panama ( Fig. 63C View Figure 63 ), at altitudes of c. 1000−2200 m.
Natural history: Anelosimus puravida is apparently social. The types came from a nest containing in total 43 adult females, 7 adult males, 8 juveniles (several instars) and 9 egg sacs (collection label states that all were from the same nest, coll. W. Eberhard). The observed sex ratio (approximately 6 females per male) may not represent primary sex ratio bias, but would not be atypical for a social species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Anelosimus jucundus
| Agnarsson, Ingi 2006 |
Anelosimus cf. jucundus
| Powers KS & Aviles L 2003: 727 |
| Bukowski TC & Aviles L 2002: 193 |
Anelosimus jucundus : ( Avilés & Gelsey, 1998: 2138 )
| Aviles L & Gelsey G 1998: 2138 |
Anelosimus jucundus : Levi, 1956: 417–418
| Levi HW 1963: 35 |
| Levi HW 1956: 418 |
Anelosimus jucundus : Levi, 1956: 417–418
| Levi HW 1963: 35 |
| Levi HW 1956: 418 |
Theridion jucundum
| Cambridge OP 1896: 166 |