Brachymeria tibialis ( Walker, 1834 )
|
publication ID |
https://doi.org/10.11646/zootaxa.5178.5.1 |
|
publication LSID |
lsid:zoobank.org:pub:922527AF-2DC1-49DF-9D09-9EA6E3F52ACB |
|
DOI |
https://doi.org/10.5281/zenodo.7039811 |
|
persistent identifier |
https://treatment.plazi.org/id/21176831-FFAD-FFE3-FF6D-F975FBB5FA7D |
|
treatment provided by |
Plazi |
|
scientific name |
Brachymeria tibialis ( Walker, 1834 ) |
| status |
|
Brachymeria tibialis ( Walker, 1834) View in CoL
Chalcis tibialis Walker, 1834: 29 View in CoL . Original description. France. Synonymy with C. intermedia View in CoL by Ruschka, 1922: 225. Bouček, 1992: 92, senior synonymy, lectotype designation.
= Chalcis distinguenda Walker, 1834: 29 View in CoL . Original description. France. Synonymy with C. intermedia View in CoL by Ruschka, 1922: 225. Bouček, 1992: 92, synonymy confirmed.
= Chalcis cingulata Walker, 1834: 30 View in CoL . Original description. France. Synonymy with C. intermedia View in CoL by Ruschka, 1922: 225. Bouček, 1992: 92‒93, synonymy confirmed, lectotype designation.
= Chalcis intermedia Nees, 1834: 29 View in CoL . Original description. Germany. Brachymeria intermedia (Nees) View in CoL : Masi, 1916: 78. Synonymy with B. tibialis (Walker) View in CoL by Bouček, 1992: 93. Type lost.
= Chalcis intermedia var. rufofemorata Rosenhauer, 1856: 175 View in CoL . Original description. Spain. Synonymy with C. intermedia View in CoL by Masi, 1951: 45. Bouček, 1992: 92, synonymy confirmed.
= Chalcis scirropoda Förster, 1859: 97 View in CoL . Original description. Hungary. Synonymy with C. intermedia View in CoL by Ruschka, 1922: 225. Bouček, 1992: 92‒93, synonymy confirmed.
= Chalcis boops Thomson, 1876: 19 View in CoL . Original description. Germany. Ruschka, 1922: 225. Synonymy with C. intermedia View in CoL by Bouček, 1992: 92.
= Oncochalcis quettaensis Cameron, 1906: 94 View in CoL . Original description. Pakistan. Narendran, 1989: 251. Synonymy by Bouček, 1992: 92; lectotype designation.
= Brachymeria intermedia var. turkestanica Masi View in CoL : 1951: 45. Original description. Uzbekistan. Host: Genesis sp. Synonymy by Bouček, 1992: 92.
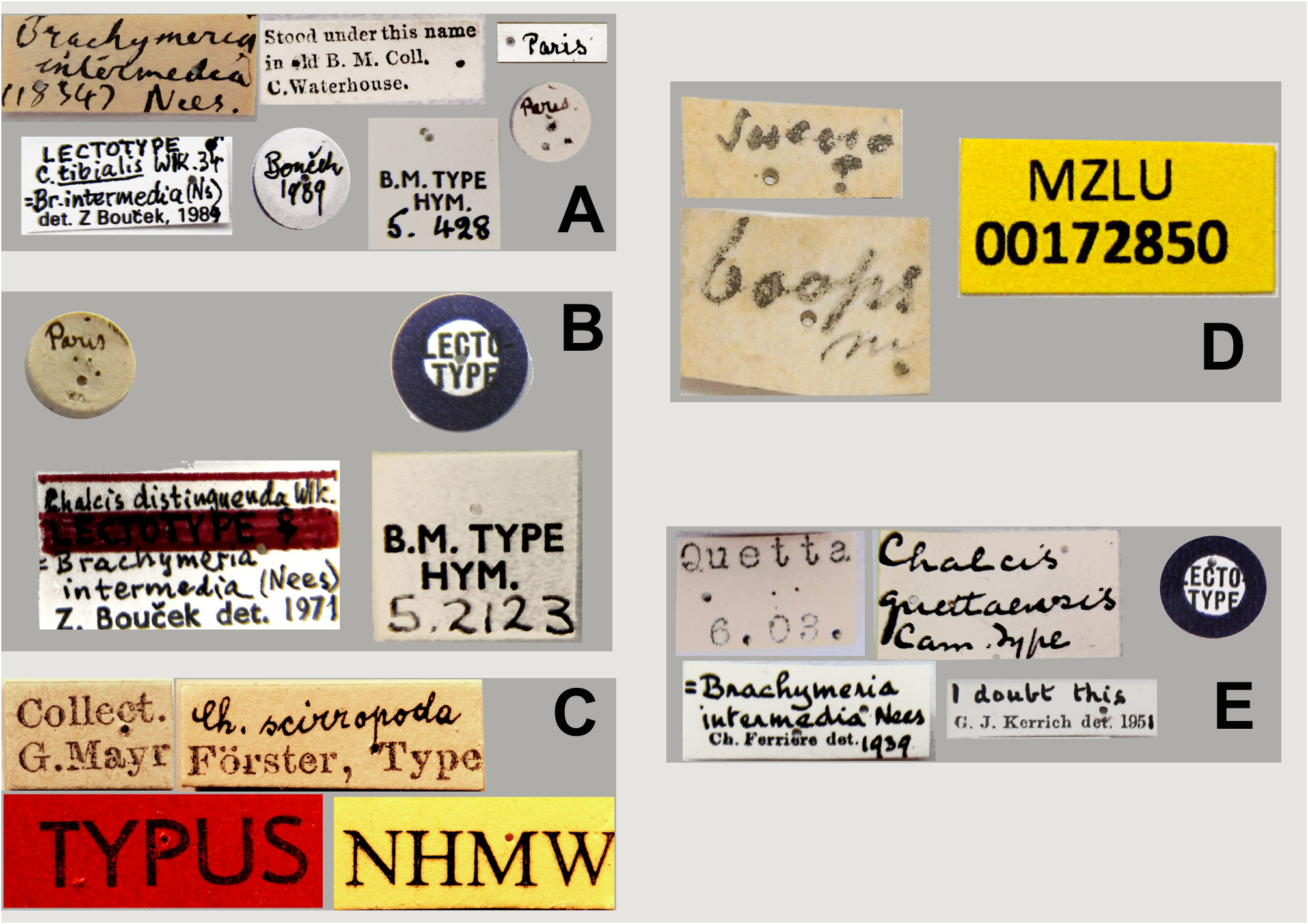
Material examined. TYPE MATERIAL. Chalcis tibialis : lectotype ♂ ( NHMUK Hym type 5-428) (labelling on Fig. 12A View FIGURES 12 ). Chalcis distinguenda : lectotype ♀ ( NHMUK Hym type 5.5123) (labelling on Fig. 12B View FIGURES 12 ). Chalcis cingulata : lectotype ♂ ( NHMUK Hym type 5-428). Chalcis scirropoda : three conspecific syntypes ( NHMW) (labelling on Fig. 12C View FIGURES 12 ). Oncochalcis quettaensis : lectotype, previously designated by Bouček, a ♀ on a micropin ( NHMUK Hym. 5-127) (labelling on Fig. 12E View FIGURES 12 ).
The male lectotype of Chalcis tibialis still has the base of the left flagellum. It was therefore possible to examine the distribution of the modified, spatulate setae on its underneath and determine that the setae are missing from F1 ( cf. Fig. 9A View FIGURES 9 ). The adscrobal area on the mesopleuron has only a moderate number of punctures and a smooth surface in front of the femoral depression, and the hind tarsus is relatively thick, all characters in accordance with the diagnosis provided below. Bouček (1992) erroneously stated that the type of C. distinguenda was lost but the putatively missing type is actually that of C. cingulata . The types of C. scirropoda and O. quettaensis show the distinctive feature of C. tibialis sensu stricto. Thanks to images sent to GD by M. Rune Bygebjerg, ( LBM) it was possible to confirm the identity of Chalcis boops (labelling on Fig. 12D View FIGURES 12 ). The synonymy between the above names established first by Ruschka (1922) and then by Bouček (1992) is also confirmed here.
The types of the varieties quoted by Masi as B. intermedia var. rufofemorata and B. intermedia var. turkestanica could not be located and the host name ( Genesis sp. ) could not be found in any catalogue or database of the Lepidoptera . Nevertheless our sampling included specimens that fit with the original descriptions. These varieties correspond to phenotypes that were mixed with specimens exhibiting the typical morphological characters of B. tibialis .
OTHER MATERIAL. EUROPE. CZECH REPUBLIC: CHKO Pálava: NPR Děvín-Kotel-Soutěska, Břeclav distr., Perná, 01vii.2010 (Delvare G.) ( 1♂, GDPC). FRANCE: Alpes-Maritimes: Belvédère, Castellarou, point 263, 1500 m sweeping, 24.vii.2009 (Delvare G.) ( 2♀, GDEL 0287 & GDEL 0288, CBGP); Breil-sur-Roya, GR52A, SW Cime du Bosc, 900 m, sweeping, 17.vi.2012 (Delvare G.) ( 1♀, GDEL 0338, CBGP); Levens, 10.x.1983 (Delvare G.) ( 1♀, GDPC). Ardèche: Berrias-et-Casteljau, Bois de Païolive, Montchamp, sweeping, 06.vii.2002 (Delvare G.) ( 2♀, GDEL 0217, GDEL 0218, CBGP); same locality, Malaise trap, 11.vi-07.vii.2006 (Aberlenc H-P.) ( 2♂, GDPC); same locality, ex pupae of Tortrix viridana (Delvare G.) ( 1♀ 1♂, GDPC); La Fontaine du Cade, on Juniperus , 24.iii.1990 (Foucart A.) ( 1♀, GDPC); Lagorce, 15.ix.1987 (Foucart A.) ( 5♂, GDPC); Saint-Laurent-les-Bains, 1100 m, N village, (Delvare G.) ( 1♂, GDPC). Bouches-du-Rhône: Aix-en-Provence, Europole de l’Arbois, 27.viii.2007 (Ponel P.) ( 1♂, GDEL 0471, CBGP); Var: Saint-Paul-Lez-Durance, 04.v.1986 (Matocq A.) ( 1♀, GDPC). Gard: Le Cailar, 15.v.1986 (Vayssières J.-F.) ( 3♀, GDPC); Saint-Félix-de-Pallières, L’Ourne, 02.x.1981 (Vayssières J.-F.) ( 1♀ 1♂, GDPC); Saint-Geniès-de-Malgoire, Mas de Mme Rouquette, on flower of Paliurus spina-christi , 22.vi.2000 (Delvare G.) ( 1♂, GDPC); Trèves, Gorges du Trévezel, 510 m, sweeping, 17.vi.2011 (Delvare G.) ( 2♀, GDEL 0329 & GDEL 0339, CBGP). Hérault: Aniane, Les Bernagues, on flower of Paliurus spina-christi , 29.vi.1985 (Maldès J.- M.) ( 1♀, GDPC); same locality and collector, 17-19.vii.1984 ( 1♂, GDPC); Assas, on Prunus , 12.vi.1988 (Foucart A.) ( 1♀, GDPC); Clapiers, 01.vi.1984 (Delvare G.) ( 1♀, GDPC); Ganges ex pupa of Lymantria dispar , collect vi.1988 (Feltwell J.) ( 1♂, NMS); same data but collect vi.1989 emergence 24.vii.1989 ( 2♂, GDPC); Grabels; 10.vii.1989 (Tussac H.) ( 3♂, GDPC); 13.vii.1989 ( 2♂ GDPC); Lézignan, 20.v.1991 (Tussac H.) ( 1♂, GDPC); Mauguio, Mas de Roquefeuille, 26.v.2006 (Delvare G.) ( 1♀, GDPC); Montagnac, Mas de Linarès, 10.v.1980 (Maldès J.-M) ( 2♀, GDPC); Montferrier-sur-Lez, campus Csiro, within greenhouse, 26.v.2002 (Delvare G.) ( 1♀, GDPC); bord du Lez, 07.iv.1989 (Maldès J.-M) ( 1♀, GDPC); Montpellier, 03.iv.1979 (Maldès J.-M) ( 1♀, GDPC); same city, Cirad campus, 30.viii.1984 (Delvare G.) ( 1♂, GDPC); Saint-Gély-du-Fesc, 02.ix.2009 (Michel B.) ( 1♀, GDPC); SaintGeniès-de-Varensal, Albès, 785 m, 01.ix.1989 (Delvare G.) ( 1♀, GDPC); Saint-Guilhem-le-Désert, 16.vii.1986 (Vayssières J.-F.) ( 1♂, GDPC); same locality, 27.vi.1986 (Maldès J.-M) ( 1♂, GDPC); Saint-Martin-de-Londres, on flower of Echinops ritro , 02.viii.1988 (Foucart A.) ( 9♀, GDPC); same locality and collector, Uglas, 12.vii.1988 ( 3♀, GDPC); Frouzet, same locality and collector ( 6♂, GDPC); Sète, N112, on flower of Echinophora spinosa , 15.viii.1986 (Delvare G.) ( 1♂, GDPC); same locality and collector, on Arundo donax , 25.vii.2004 (Delvare G.) ( 1♂, GDPC); Valflaunès, 18.viii.2005 (Delvare G.) ( 1♂, GDEL 0179, CBGP) and 16.viii.2005 (Delvare G.) ( 1♀, GDEL 0180, CBGP); Vias, on flower of Pastinaca latifolia (Delvare G.) ( 2♀, GDPC); Viols-le-Fort, 13.iv.1985 (Delvare G.) ( 1♀, GDPC). Lot: Brengues, 21.vii.1984 (Tussac H.) ( 2♂, GDPC); Cabrerets, 13.vii.1983 (Tussac H.) ( 2♀ 4♂, GDPC); Cahors, 29.vii.1984 (Tussac H.) ( 1♀), 18.viii.1984 ( 1♂) (both GDPC); Marcilhac-sur-Célé, 30.vi.1983 (Tussac H.) ( 1♂, GDPC); Flaujac-Poujols, 28.ix.1984 (Tussac H.) ( 1♀, GDPC). Lozère: Belvezet, around Moure de la Gardille, 21.vii.2006 (Delvare G.) ( 1♂, GDPC); Le Monastier, on Prunus , 10.vii.1987 (Foucart A.) ( 1♀ 1♂, GDPC). Tarn-et-Garonne: Montauban, Villemade, 30.vii.1991 (Tussac H.) ( 1♂, GDPC). Var: Le Muy, Bois de Palaison, 13.vii.1995 (Delvare G.) ( 1♂, GDPC); Saint-Paul-en-Forêt, 15.vii.1995 (Delvare G.) ( 1♀, GDPC); Vidauban, Malaise trap, 08.vi.2015 (Rault P.-A.) ( 1♀, GDPC). GREECE: Arkadia: Tripoli, Piana road, 11.iv.2000 (Cocquempot C.) ( 1♀, GDPC). ITALY: Sicilia: Cesaro, Monte Nebrodi, Solazzo Buffali, 37.87305°N 14.67514°E 1300 m, sweeping on Opopanax chironium in Quercus cerris forest, 01.vii.2014 (Delvare G.) ( 1♀, GDEL 0404, CBGP). SPAIN: Bages, Alzimar de Sant Marti., 400 m, ex pupa of Euphydryas aurinia , collect 16.v.2003, emergence 07.vi.2003 (Stefanescu C. & Planas J.) ( 1♀, NMS); same data but collect on 22.iv.2006, emergence 2006 ( 1♀, NMS); same locality but ex pupa of Euphydryas desfontainii , collect 21.v.2005, emergence 05.vi.2005 ( 1♀, NMS); Bages, El Guix, 350 m, ex pupa of Euphydryas aurinia , collect 24.v..2004, emergence 09.vi.2004 (Stefanescu C. & Planas J.) ( 1♂, NMS). Zaragoza, Justibol, 200 m, ex pupa of Pleuroptya ruralis , collect 11.viii.1999, emergence 13.viii.1999 (King G.E.) ( 1♀, NMS).
NORTH AFRICA. MOROCCO: Larache , 17.v.1989 ( Tussac H.) ( 1♀, GDPC). Moulay Idriss, Antic city of Volubilis, 04.vi.1992 ( Delvare G.) ( 1♀ 8♂, GDPC) .
NEAR EAST. IRAN: Kohmare , 29°32’°N 57°27’°E, Malaise trap in Quercus forest, 07.v.2006 ( Fallahzaded M.) ( 2♀, GDEL0378 & GDEL0379 , CBGP) . TURKEY: Ankara: Beynam , ex pupa of Tortricidae , collect 16.vi.1999, emergence 12.vii.1999 ( Shaw M. R.) ( 1♂, NMS) . Bingöl: Adakli, Genç, Karhova , Kiði , Merkez , Solhan , Yayladere , Yedisu , 975-1893 m, 17.v-20.vii.2019, on flowers of various plants ( Kaplan E.) ( 81♀ 5♂, BU) ; Diyarbakar: Ҫermik, Ҫinar, Ҫüngüs, Dicle, Eðil, Ergani , Hani , Hazro , Kokaköy , Kulp , Lice, Silvan, 545-1400 m, on flowers of various plants ( Kaplan E.) ( 80♀ 3♂, BU) .
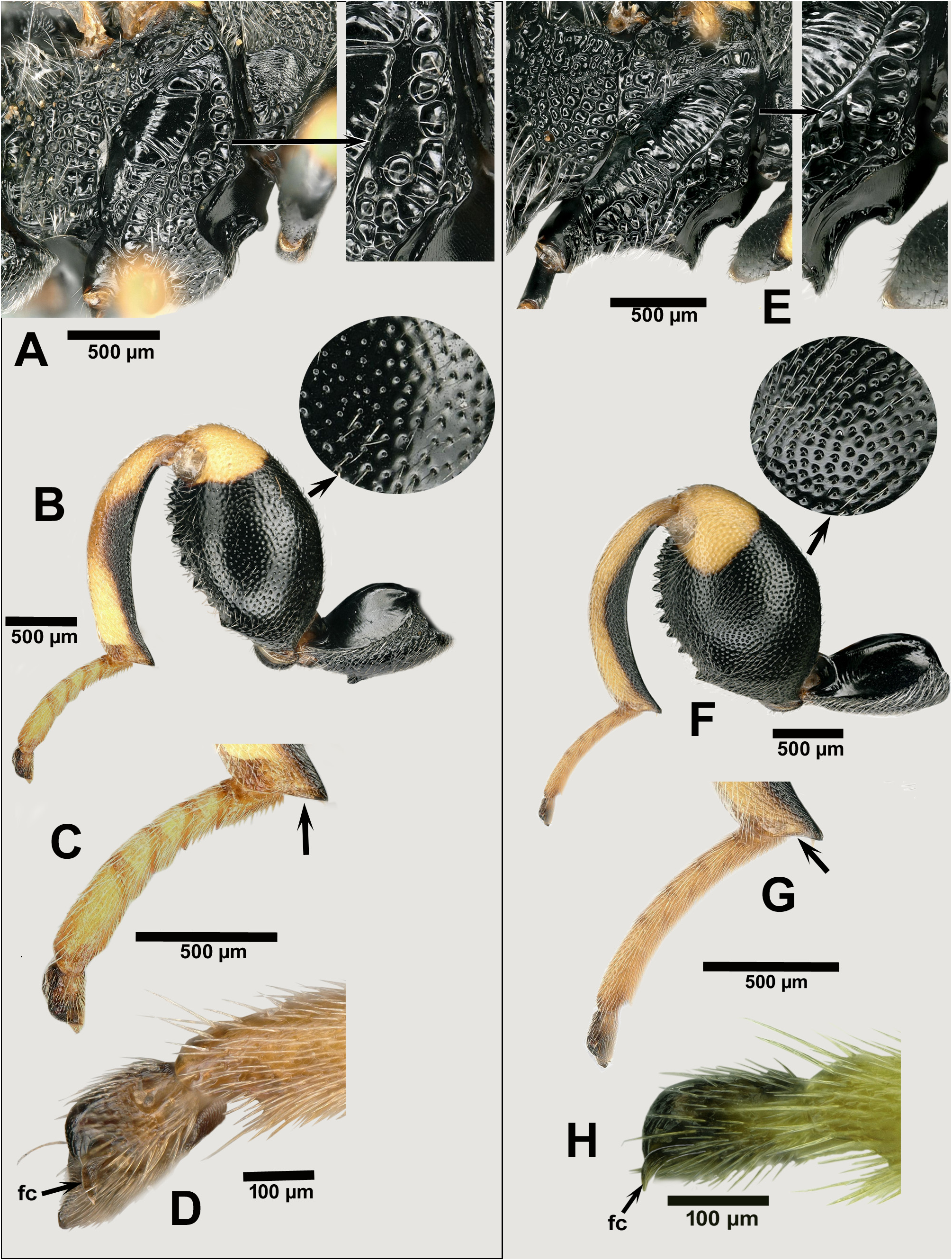
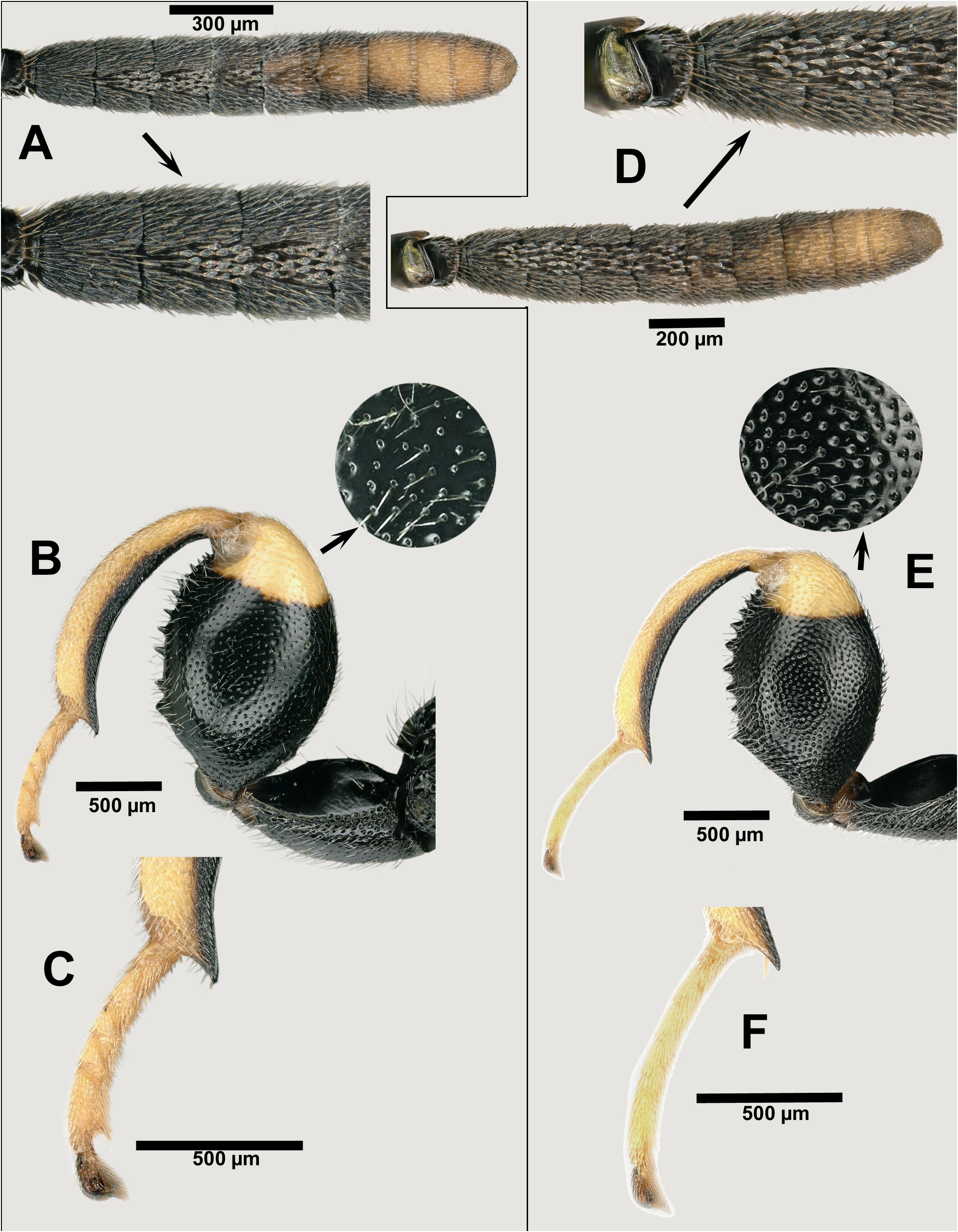
Description. FEMALE. See relevant parts above relative to tibialis species-group characters. Characters that distinguish females of B. tibialis from B. zygaenae include: mesepisternum with adscrobal area not completely areolate, with a smooth surface in front of femoral depression, which is either smooth or partly strigose ( Fig. 8A View FIGURES 8 ); metacoxa with well expanded inner ventral tooth ( Fig. 8B View FIGURES 8 ); metafemur more slender, about 1.8× as long as wide and sparsely punctulate, the interspaces larger than the diameter of the punctures ( Fig. 8B View FIGURES 8 ), the dorsal margin more strongly curved than ventral margin, and basal teeth on ventral margin not much projecting and with base broader than the following ones; metatibia somewhat thickened mesally (its dorsal margin more convex than the ventral one) ( Fig. 8B View FIGURES 8 ) and with edge of apical truncation straight ( Fig. 8C View FIGURES 8 ); hind tarsus incrassate, 3.4× as long as width of metatibia ( Fig. 8C View FIGURES 8 ); hind pretarsus with falcate seta lanceolate, somewhat broadening apically ( Fig. 8D View FIGURES 8 ); tegula and legs with light parts pale instead of bright yellow; propodeum without a postspiracular projection on the setose region.
MALE. Spatulate setae absent or present in very small number (2 or 3) on F1, present only on F2‒F5 their number decreasing from F2 ( Fig. 9A View FIGURES 9 ).
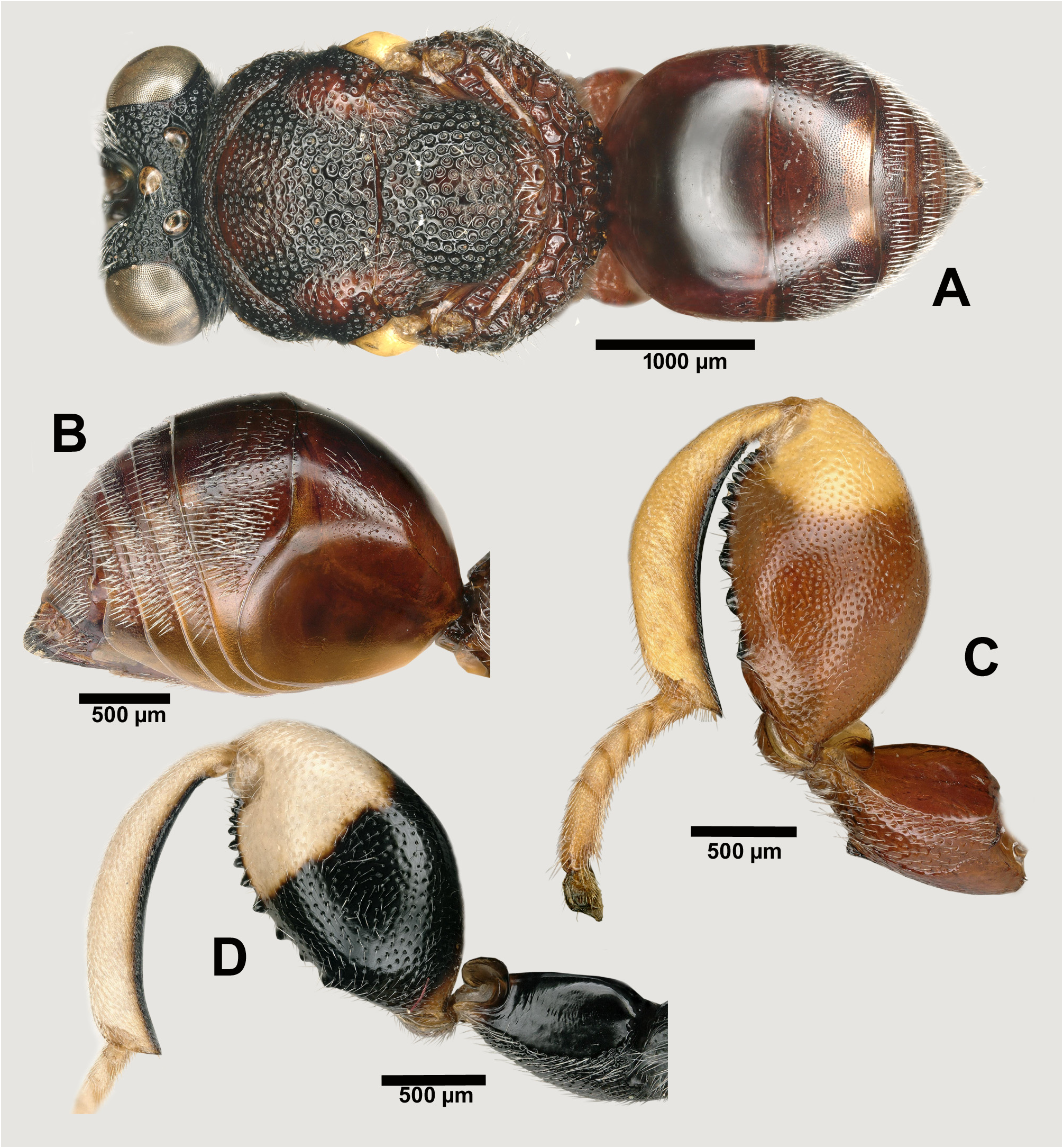
Morphological variation. The same variation in the sculpture of different parts of the body was seen as in B. zygaenae ; the punctulation of the metafemur is also somewhat variable, in some specimens approaching the condition exhibited by B. zygaenae . An outstanding variation in colour was found, mostly in the southern part of its distribution area ( Morocco, Turkey and Iran) but rarely also in southern France. In most cases this concerns the black part of the hind leg being reduced in comparison with the usual situation ( Fig. 11D View FIGURES 11 ); it corresponds to the variety turkestanica , found in Iran and Turkey. On the other hand, the black colour may be replaced by red or reddish, which corresponds to the variety rufofemorata found in Morocco, rarely in southern France, and frequently in Turkey. In one extreme variation concerning a female from Morocco the entire meso- and metasoma were dark red ( Figs 11A– C View FIGURES 11 ). In the southeast of Turkey the three phenotypes, the classical tibialis , turkestanica and rufofemorata, were seen in samples collected at the same places and time, which strongly suggests that they represent individual variation of no taxonomic value.
Distribution. In addition to the countries given under ‘Material examined’ the species has been recorded from several others. Its distribution extends from North Africa to the North of India (Kashmir) through the Iberian, Italian and Balkan Peninsulas, Western and Central Europe, Anatolia, Russia and SW Asia. The species is apparently absent from Great Britain, Scandinavia, the Arabian Peninsula and Japan. Brachymeria tibialis was introduced to North America in the first half of the 20th century for the control of Lymantria dispar ( Erebidae : Lymantriinae ).
Hosts and biology. Brachymeria tibialis is a solitary endoparasitoid of lepidopteran pupae ( Burgess & Crossman 1929). The recorded hosts are summarized in Table 1 View TABLE 1 . The host families cited only a single time may be doubtful, especially Gelechiidae ( Madl 2008) and Depressariidae (Thomson 1954) in Lepidoptera , Diprionidae ( Madl 2008) in Hymenoptera , Cecidomyiidae (Nanu et al. 1980; Andriescu 1988) and Sarcophagidae ( Georgiev 1996) in Diptera . The other Diptera cited (all Tachinidae ) were reared in laboratory culture, never recovered in the field; Dowden (1935) pointed out that the parasitism was constrained and thus artificial in those cases. Parasitism of Cricula trifenestrata is erroneous according to the images provided by Tikader (2012), which are those of B. criculae (Kohl) a well-known parasitoid of that Saturniidae . Finally, the Brachymeria species quoted by Sacharov (1913) as a parasitoid of Pieris rapae and Pontia daplidice is certainly B. femorata (Panzer) , a frequent parasitoid of these Pieridae ; at that time it was mixed with B. tibialis before Masi (1916) and then Ruschka (1922) provided characters for recognizing them as distinct.
In most of the papers the parasitoid is mentioned as B. intermedia even after its synonymy with B. tibialis by Bouček (1992). In our list, two lepidopteran families are predominant, Tortricidae (17 hosts) and Erebidae (14 hosts), which possibly is a result of a bias towards moths of economic importance or as pests of forests.
Largely because of interest in using the species for the control of invasive Lymantriinae such as Lymantria dispar North America, there is a large body of literature on various aspects of the biology of B. tibialis , which is certainly the most frequently studied chalcidid species and may figure as a model for the family. Its biology was particularly extensively researched by Dowden (1935) and has been summarised by Villemant (1989). Seasonal occurrence was studied in the USA by Kerguelen & Cardé (1997a). It is plurivoltine with two or three generations occurring each year, the last generation hibernating at the adult stage under bark or inside the litter ( Burgess & Crossman 1929; Waldvogel & Brown 1978), sometimes in aggregation ( Schaefer 1993). Only the females hibernate as the males die at the end of the summer; they are able to oviposit again after winter even if they had oviposited before ( Villemant 1989). The female is synovigenic, capable of producing eggs successively. The duration of oviposition activity ranges from 20‒40 days ( Burgess & Crossman 1929) and starts 2‒3 days after mating ( Villemant 1989). The reproductive system was studied by Barbosa & Frongillo (1979), Barbosa et al. (1986), Drost & Cardé (1992a) and Dindo et al. (1996). The female shows behavioral and physiological responses according to its immediate history: those permanently deprived of hosts accumulate eggs but have a low level of host acceptance when again in contact with suitable pupae, in contrast with those having been initially in contact with a host and regularly ovipositing thereafter ( Drost & Cardé 1992a, 1992b; Kerguelen & Cardé 1996 a). Progeny production, development time, sex ratio and parasitoid-related host mortality are dependent on female age and host exposure ( Barbosa et al. 1986). The female uses olfactory cues in searching for the host, though visual cues are involved in the recognition of the host microhabitat, and some learning is exhibited in this respect ( Leonard et al. 1975; Minot & Leonard 1976 c; Tucker & Leonard 1977; Barbosa et al. 1978; Mohamed 1986; Cardé & Lee 1989; Drost & Cardé 1990, 1992a; Kerguelen & Cardé 1996 b, 1997b, 1998). Host acceptance and host suitability both depend on the identity of the host ( Dindo et al. 2013). An optimal fresh age of the pupae (around 4 days) is important for suitability and B. tibialis selects its hosts accordingly ( Lashomb et al. 1983; Rotheray & Barbosa 1984). The female is strongly reluctant to oviposit into already parasitized pupae ( Dowden 1935; Goodwin & Odell 1979).
Low humidity, high temperatures and light intensity increase the activity, fecundity and biotic potential of B. tibialis ( Minot & Leonard 1976a, 1976b; Barbosa & Frongillo 1977, 1979b; Weseloh 1979). Consequently the wasp is typically found in sunny and open places.
The description of the courtship behaviour and interaction with pheromones were dealt with by Leonard & Ringo (1978), Askari (1979), Simser & Coppel (1980) and Mohamed & Coppel (1987a, 1987b).
Brachymeria tibialis is the only Chalcididae to have successfully been reared on an artificial medium ( Thompson 1980; Dindo & Campadelli 1992, 1993; Dindo & Luciano 1995; Dindo et al. 1995, 1997, 2001, 2002). This procedure allowed the authors to precisely observe the host-parasitic larva relationships, particularly the effect of parasitism on the host’s activity.
Natural and biological control of Lymantria dispar . Numerous assessments have been made, as listed in Table 1 View TABLE 1 . The assessment of parasitism depends on the method used as shown by Gould et al. (1992). Different authors operating in various countries and continents discovered that the level of parasitism depends on the density of the host and is higher during outbreaks ( Ticehurst et al. 1978; Luciano & Prota 1982, 1984; Fuester & Taylor 1996; Hoch et al. 2006). In addition, the pupae of L. dispar originating from an outbreak population are even more suitable for the development of B. tibialis ( Greenblatt & Barbosa 1980) . Consequently the chalcidid may effectively destroy its host population as shown with L. dispar in Morocco by Lépiney (1930). In addition to parasitism, injuries from the sting in cases of host feeding increase host mortality both directly and possibly further through introductions of pathogenic microorganisms ( Villemant 1989).
Brachymeria tibialis was introduced in North America to control the gypsy moth after its involuntary introduction at the end of the 19th century ( Howard & Fiske 1911; Burgess & Crossman 1929). The introduction of B. tibialis was in two phases, respectively before and after the First World War. Finally, 22,197 parasitoids were introduced from some Mediterranean countries ( Clausen 1978). Brachymeria tibialis was nevertheless recovered only much later ( Leonard 1966, 1967, 1977). In fact the release of the chalcidid usually had no apparent effect on the population dynamics of the gipsy moth ( Blumenthal et al. 1979). In some cases, however, the presence and abundance of alternate hosts ( Tortricidae ) can allow a quick increase of the parasitoid population which is then able to destroy a large number of the gypsy moth pupae ( Minot & Leonard 1976a).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Brachymeria tibialis ( Walker, 1834 )
| Delvare, Gérard & Shaw, Mark R. 2022 |
Brachymeria intermedia var. turkestanica
| Boucek, Z. 1992: 92 |
Chalcis intermedia
| Boucek, Z. 1992: 93 |
| Masi, L. 1916: 78 |
Oncochalcis quettaensis
| Boucek, Z. 1992: 92 |
| Narendran, T. C. 1989: 251 |
| Cameron, P. 1906: 94 |
Chalcis boops
| Boucek, Z. 1992: 92 |
| Ruschka, F. 1922: 225 |
| Thomson, C. G. 1876: 19 |
Chalcis scirropoda Förster, 1859: 97
| Boucek, Z. 1992: 92 |
| Ruschka, F. 1922: 225 |
| Forster, A. 1859: 97 |
Chalcis intermedia var. rufofemorata
| Boucek, Z. 1992: 92 |
| Masi, L. 1951: 45 |
| Rosenhauer, W. G. 1856: 175 |
Chalcis tibialis
| Boucek, Z. 1992: 92 |
| Ruschka, F. 1922: 225 |
| Walker, F. 1834: 29 |
Chalcis distinguenda
| Boucek, Z. 1992: 92 |
| Ruschka, F. 1922: 225 |
| Walker, F. 1834: 29 |
Chalcis cingulata
| Boucek, Z. 1992: 92 |
| Ruschka, F. 1922: 225 |
| Walker, F. 1834: 30 |