Thecidellina europa Logan, Hoffmann and Lüter, 2015
|
publication ID |
https://doi.org/10.11646/zootaxa.4013.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:72E2A94F-5F38-49A2-AB51-6406085A896E |
|
DOI |
https://doi.org/10.5281/zenodo.6098403 |
|
persistent identifier |
https://treatment.plazi.org/id/1F6E6A3A-D340-2D42-FF74-F88D6F6F0A92 |
|
treatment provided by |
Plazi |
|
scientific name |
Thecidellina europa Logan, Hoffmann and Lüter |
| status |
sp. nov. |
Thecidellina europa Logan, Hoffmann and Lüter View in CoL sp. nov.
( Fig. 1 View FIGURE 1 A–O)
2011 Thecidellina cf. blochmanni Dall—Baker and Logan , p. 121, pl. 2, figs 8–17. 2013 Thecidellina cf. blochmanni Dall—Logan and Baker , Fig. 4 View FIGURE 4 K–L.
Diagnosis. Medium-sized shell, large cardinal process, calcitic plate of hemispondylium attached to valve floor, long slender prongs, median septum broad, tuberculate, slightly grooved and widening at anterior end, ascending and tapering posteriorly, with prominent ridge at posterior end, no heavy development of calcitic deposits on anterior floor of dorsal valve, spiculate canopy rare.
Etymology. Named after the collecting locality of Europa Island in the Mozambique Channel, western Indian Ocean.
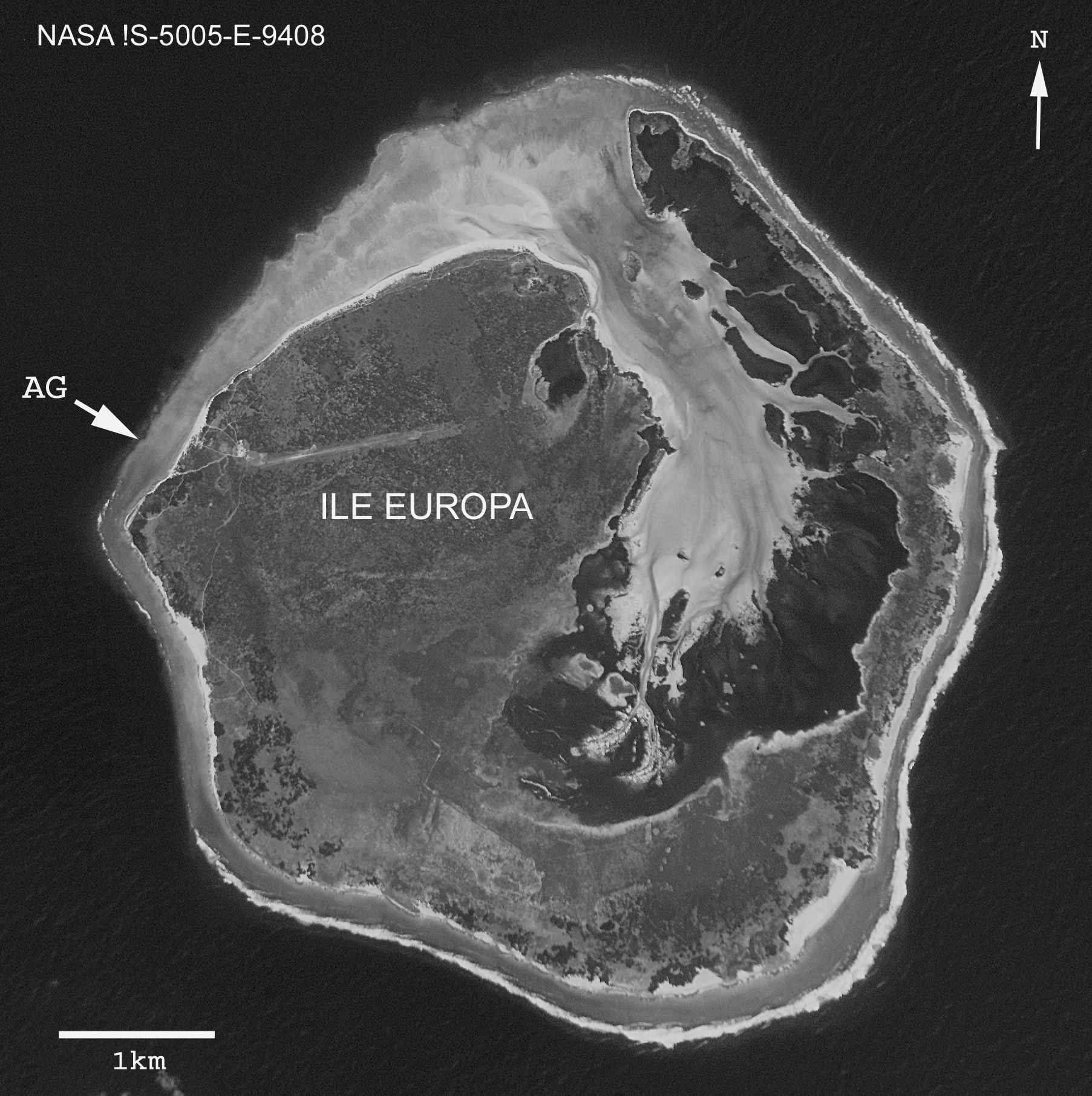
Type locality. Cave in Anse Gabriel, west side of Europa Island ( Fig 2 View FIGURE 2 ). All specimens were provided by Pierre Vasseur (Marseille) in 1975 from the floor of a cave at the base of a steep reef wall at 55 m depth at Anse Gabriel (see Gurgel & Vasseur 1973, their Figs 1 View FIGURE 1 and 2 View FIGURE 2 ).
Holotype. The complete specimen (both valves) illustrated in Fig. 1 View FIGURE 1 A–H, NBM- 010311, with dimensions: length of ventral valve (LV) 4.6 mm, length of dorsal valve (LD) 3.7 mm, width of both valves (W) 4.5 mm, thickness (T) 3.3 mm.
Paratypes. 7 specimens figured in Fig. 1 View FIGURE 1 I–O, Paratype 1 NBM-010312.1 ( Fig. 1 View FIGURE 1 I): LD 3.0 mm, WV 3.4 mm. Paratype 2 NBM-010312.2 ( Fig. 1 View FIGURE 1 J): LD 3.1 mm, WD 3.4 mm. Paratype 3 NBM-010312.3 ( Fig. 1 View FIGURE 1 K): LV 5.2 mm, WV 4.7 mm. Paratype 4 NBM-010312.4 ( Fig. 1 View FIGURE 1 L): LD 4.2 mm, WD 4.7 mm. Paratype 5 NBM-010312.5 ( Fig. 1 View FIGURE 1 M): LV 4.9 mm, WV 4.2 mm. Paratype 6 NBM-010312.6 ( Fig. 1 View FIGURE 1 N): LD 4.2 mm, WD 4.0 mm. Paratype 7 NBM- 0 10312.7 ( Fig. 1 View FIGURE 1 O): LV 4.1 mm, WV 4.0 mm.
Material. A total of 29 specimens (14 ventral valves and 15 dorsal valves) from Anse Gabriel. Also, specimens from the type locality representing a series of ontogenetic stages are figured in Baker and Logan (2011, Plate 2, figs 8–17; NBM 007098.1-5) and a single bored ventral valve figured in Logan and Baker (2013, Fig. 4 View FIGURE 4 K; NBM 007098.6). Representative topotypes were sent to G.A. Cooper in 1975 as T. cf. blochmanni and are housed in the U.S. National Museum.
Associated biota. 23 species of cave-dwelling bryozoans and other invertebrate groups such as sponges, hydroids, octocorals, scleractinian corals and ascidians are listed in Gurgel and Vasseur 1973 from the Anse Gabriel cave. It is likely that lacazelline brachiopods are also present on the reefs in the area ( Zezina 1987; Simon and Hoffmann 2013).
Description. Medium-sized thecideide brachiopod, biconvex, inequivalve, endopunctate, rectimarginate, attached by ventral valve cicatrix to substrate, shell usually longer than wide, maximum shell dimensions observed 6.3 mm LV, 4.4 mm LD, 5.0 mm W. Ventral valve interarea striated, with flat pseudodeltidium (planodeltidium of Logan and Baker 2013), the central triangular area often faintly marked and occasionally outlined by endolithic borings ( Logan & Baker 2013, Fig, 4K). Hemispondylium of ventral valve flat or slightly concave, development controlled by degree of valve concavity, rectangular, connected to valve floor ( Fig. 1 View FIGURE 1 C–D, M) affording attachment for diductor and submedian muscles, flanked by two long divergent curved prongs which also act as diductor muscle attachment sites ( Figs. 1 View FIGURE 1 B–D, K, M, O); lateral adductor muscles large, elongate, postero-lateral to teeth near ends of hinge line ( Fig. 1 View FIGURE 1 M); ventral valve floor smooth ( Fig. 1 View FIGURE 1 B) or with broad median ridge ( Fig. 1 View FIGURE 1 K). Dorsal valve slightly convex, sloping upwards posteriorly ( Fig.1 View FIGURE 1 H), cardinal process large, with central lobe and lateral inner socket ridges ( Fig. 1 View FIGURE 1 E–F, N), diductor muscles attached to cardinal process, lateral adductor muscles adjacent ( Fig. 1 View FIGURE 1 N); median septum broad, slightly grooved and widening at anterior end, ascending and tapering posteriorly, with prominent ridge at posterior end ( Fig.1 View FIGURE 1 E), tuberculate; intrabrachial ridge present, outer margins slightly dentate, posterior margin with small marsupial orifice on either side ( Fig. 1 View FIGURE 1 E–F), prominent brachial cavities, canopying spicules usually absent (but see Fig. 1 View FIGURE 1 L with fine mesh-like cover); peribrachial ridge with tubercles, brachial bridge smooth, supported by robust calcitic pole ( Fig. 1 View FIGURE 1 G, J) with occasional outgrowth forming a well-defined spur ( Fig. 1 View FIGURE 1 E–F); visceral gap between bridge and intrabrachial ridge ( Fig. 1 View FIGURE 1 G, J); median lobe of cardinal process connected to supporting pole of brachial bridge ( Fig. 1 View FIGURE 1 G, J); two small, oval, almost imperceptible submedian adductor muscle scars on valve floor anterior to central part of bridge.
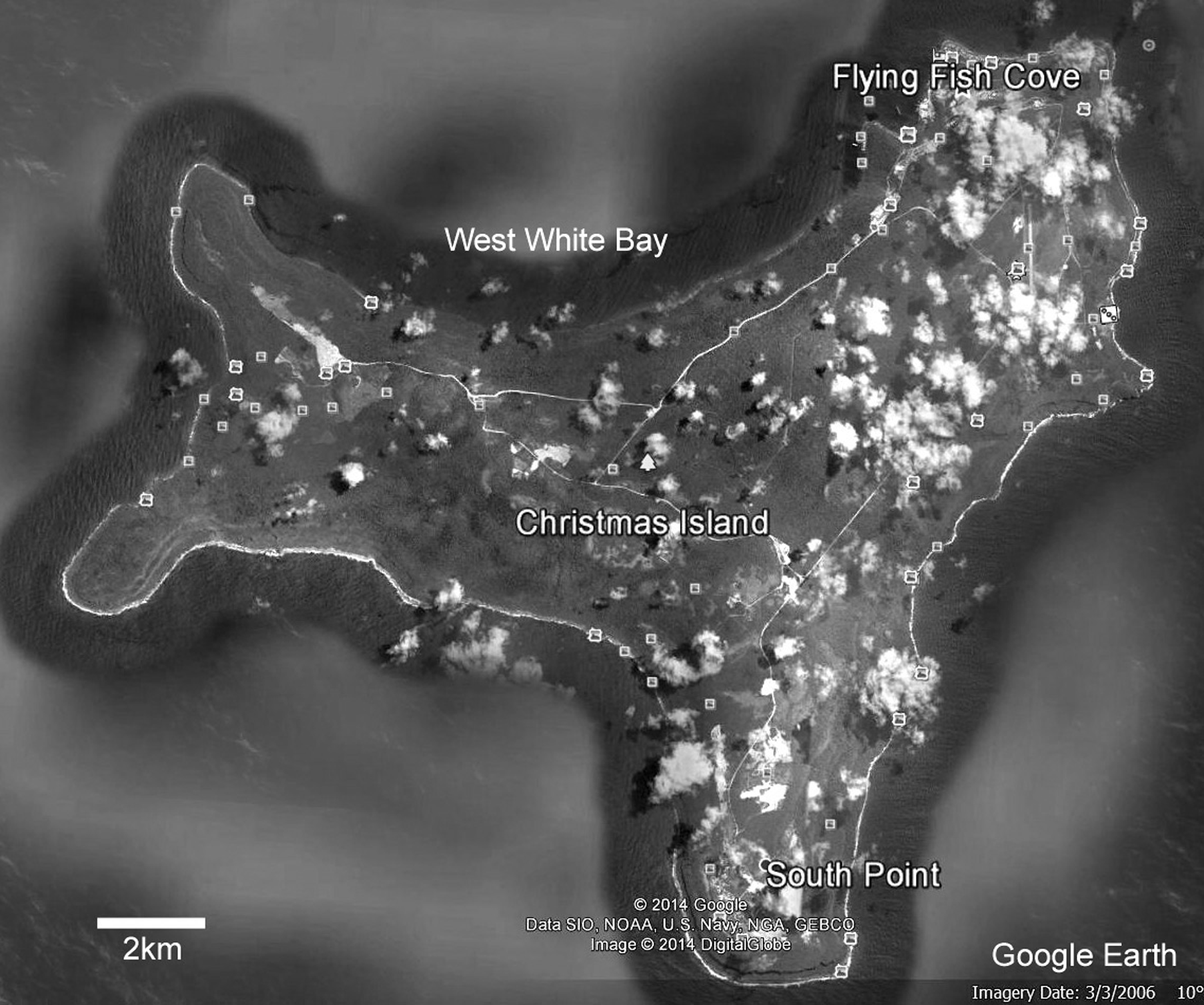
Remarks. Lee & Robinson (2003) have discussed the difficulties in separating “real” species from variants in thecideide brachiopods. Hoffmann & Lűter (2010) have recognized an Atlantic-Caribbean group which appears to be distinguishable from a Pacific group on morphological and molecular grounds, as might be anticipated, given their great geographic separation. Indian Ocean species might be expected to have affinities with Pacific species, such as T. maxilla ( Hedley, 1899) and T. congregata Cooper, 1954 . Whether the Europa species is a truly valid species can only be reliably determined from molecular studies in the future but in the meantime we separate it on purely morphological criteria. This species was tentatively identified as Thecidellina cf. blochmanni by Baker and Logan (2011) and Logan and Baker (2013). However, the current revision of T. blochmanni in this report, based on detailed examination of topotypes from the type locality of Flying Fish Cove, Christmas Island, in both the London and Berlin Museums have convinced us that the Europa material is not conspecific with T. blochmanni , hence its re-description as a new species.
In addition, based on the abundance and excellent preservation of T. europa juveniles, together with those of T. congregata from Saipan (Logan 2008), Baker & Logan (2011) were able to re-construct the sequence of ontogenetic stages regarded as typical of Recent thecidellinines and compare them with those seen in fossil forms from the Mesozoic, suggesting a common developmental pattern.
| NBM |
New Brunswick Provincial Museum, Saint John (Herbarium) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Thecidellininae |
|
Genus |