Dasychoerus arvernensis ( Croizet & Jobert, 1828 )
|
publication ID |
https://doi.org/ 10.5252/g2016n1a5 |
|
publication LSID |
urn:lsid:zoobank.org:pub:7A44CAF1-59EB-4024-A261-6F69CBE8634E |
|
persistent identifier |
https://treatment.plazi.org/id/1E251311-B43F-3042-2C75-FD04FB8AF97D |
|
treatment provided by |
Felipe |
|
scientific name |
Dasychoerus arvernensis ( Croizet & Jobert, 1828 ) |
| status |
|
Dasychoerus arvernensis ( Croizet & Jobert, 1828)
Aper arvernensis Croizet & Jobert, 1828: 157-160 , pl. XIII, figs 3-5.
Sus provincialis var. minor Depéret, 1890: 84-88 , pl. V, figs 12-14.
Sus minor – Tobien 1951: 79-83; 1952: 191; Hünermann 1971: 213-222.
Sus arvernensis arvernensis – Guérin & Faure 1985: 22; Guérin et al. 1998: 442.
Sus arvernensis minor – Guérin & Faure 1985: 443-447.
For additional synonymy of the species see Fejfar (1964) and Hünermann (1971).
TYPE LOCALITY. — Les Étouaires, Perrier, France.
AGE OF TYPE LOCALITY. — MN 16, Lower Villafranchian (3.9- 3.4 Ma, Bout, 1968, 3.6-2.4 Ma, Steininger et al. 1990).
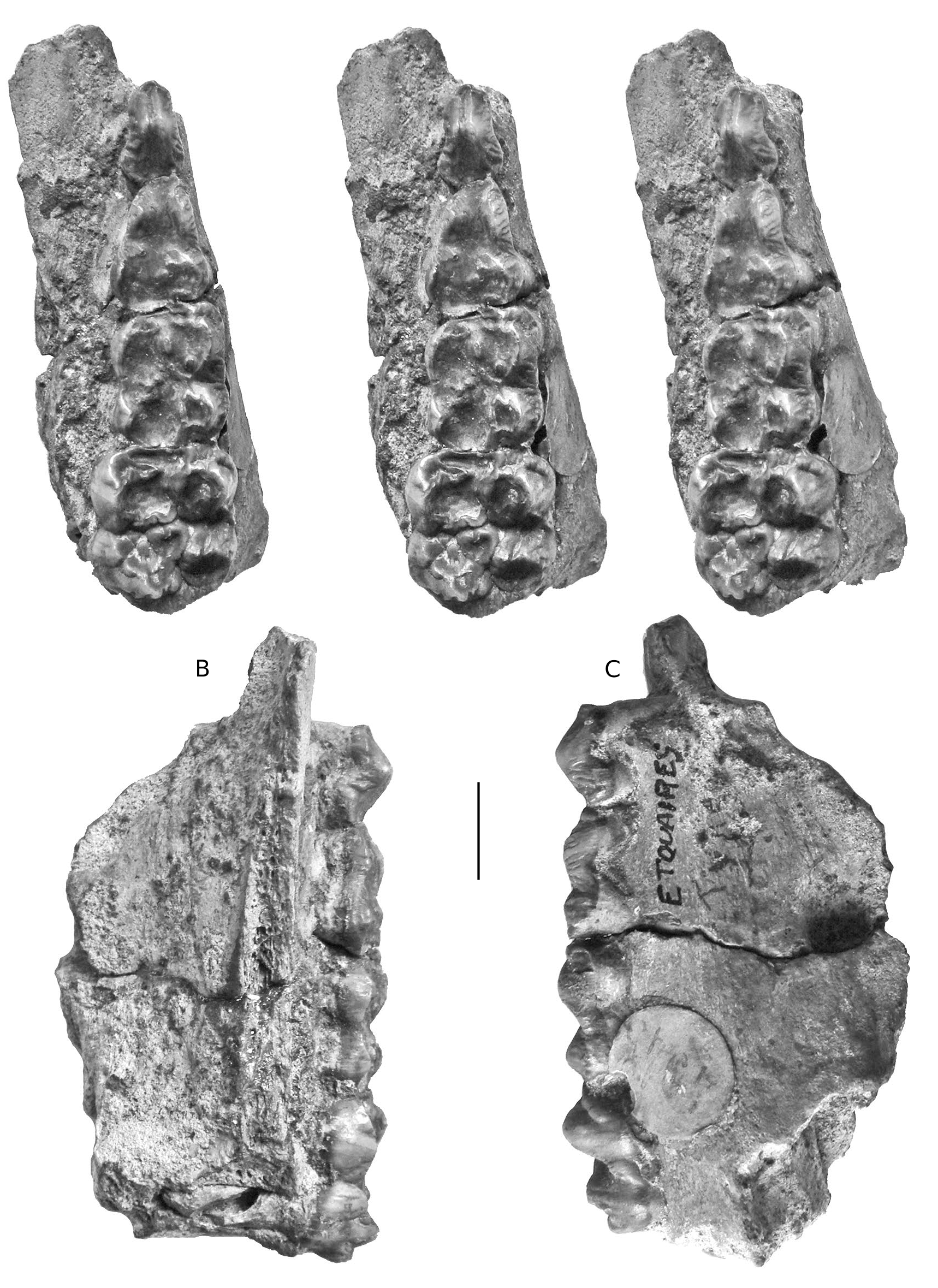
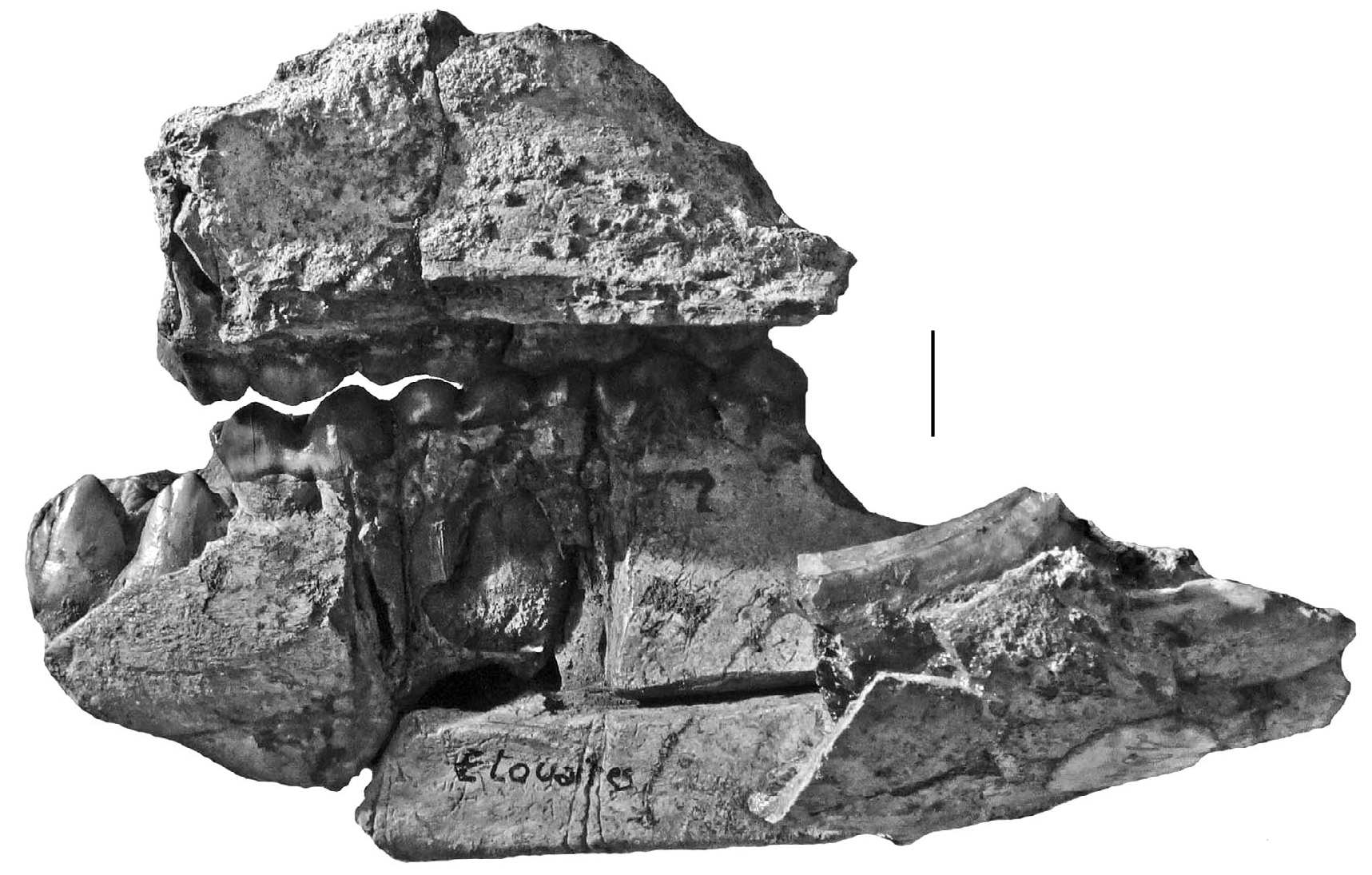
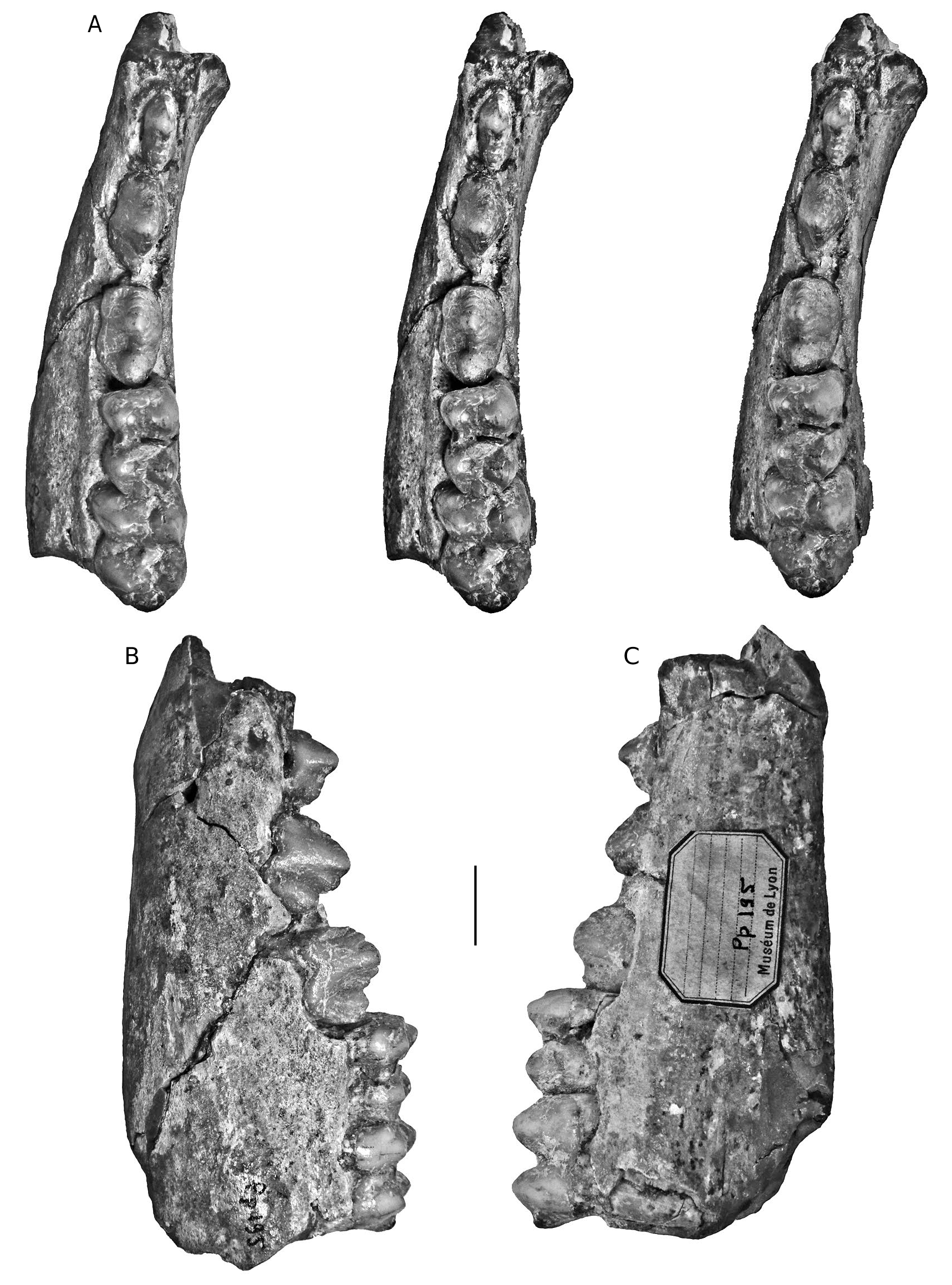
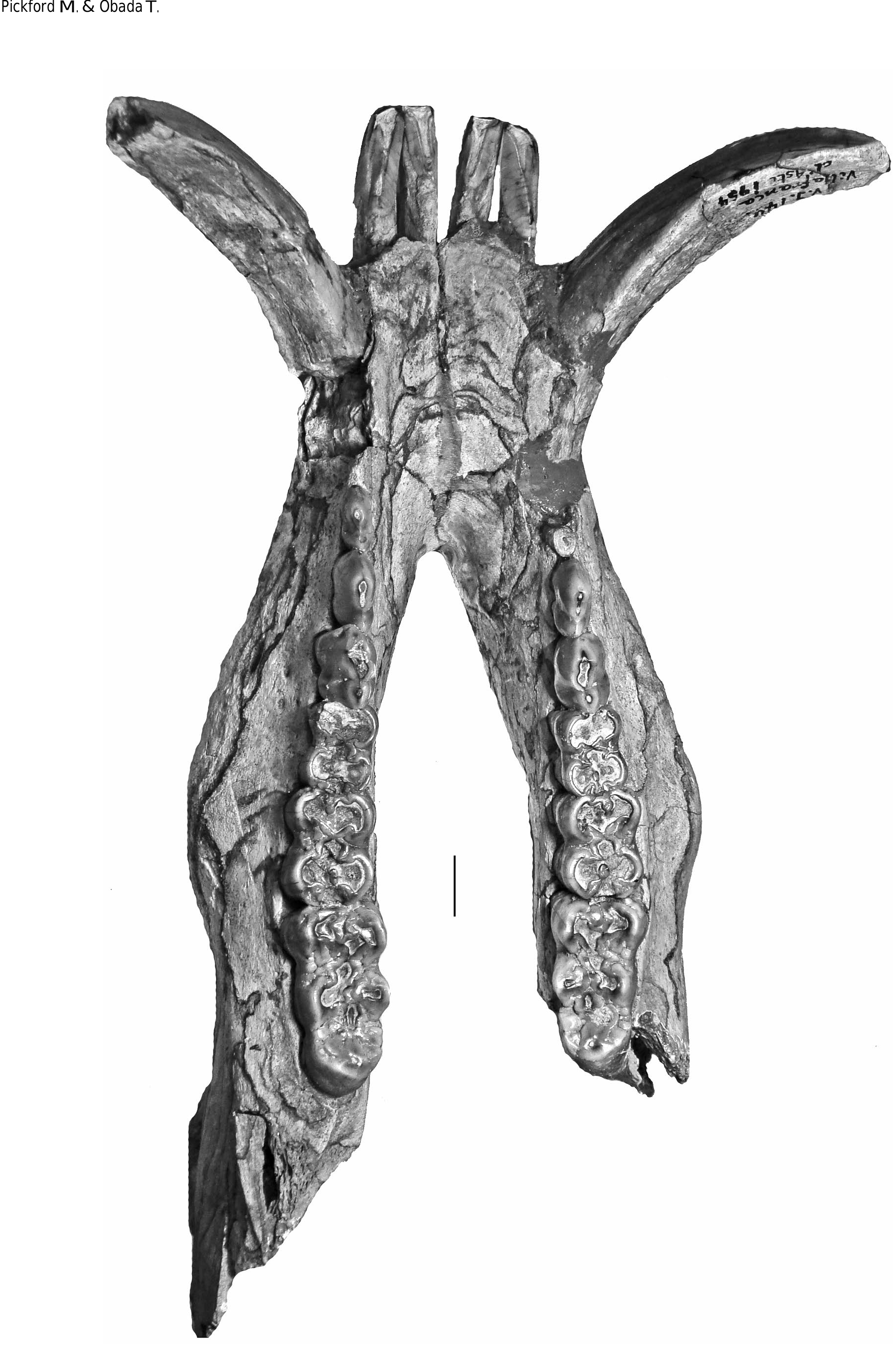
HOLOTYPE. — Specimen MNHN.F.PET2005 labelled “Étouaires”, comprising associated juvenile left maxilla containing D2/-D4/ and M1/ and a left mandible fragment and symphysis containing d/2- d/4 and m/ 1 in occlusion on both sides, and the germ of the left m/2 and p/ 4 in crypto (illustrated in mirror image by Croizet & Jobert [1828], and Blainville [1847] which has caused confusion about which side the specimens came from [ Guérin & Tsoukala 2013]). The symphyseal fragment which fits onto the left mandible contains parts of the left and right canines in situ (verrucosic male morphology) and the left i/ 2 in crypt (partly exposed by damage to the symphysis). The right mandible containing the d/2-d/4 and m/1 belongs to the same individual ( Figs 2-6 View FIG View FIG View FIG View FIG View FIG ).
DIAGNOSIS
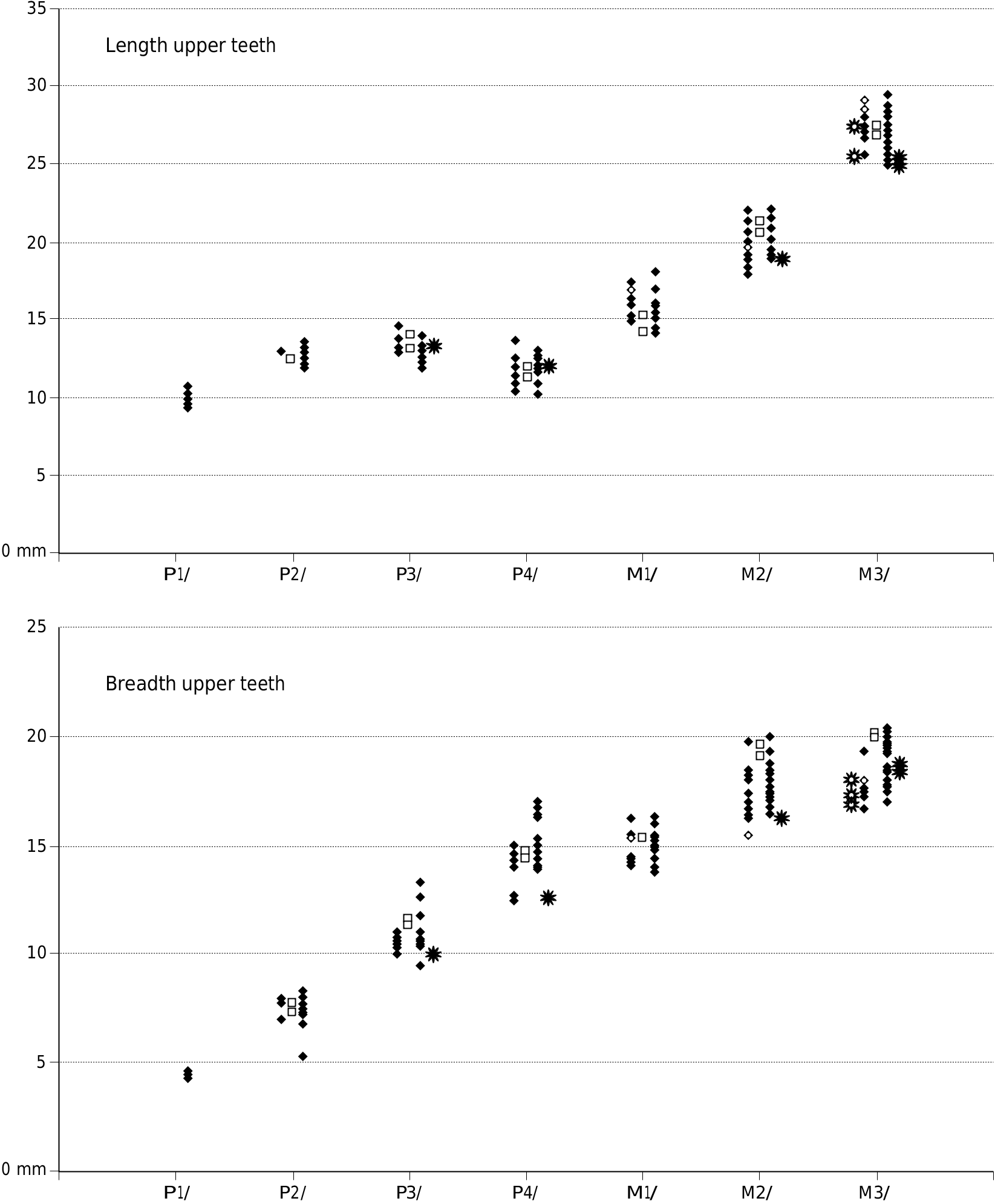
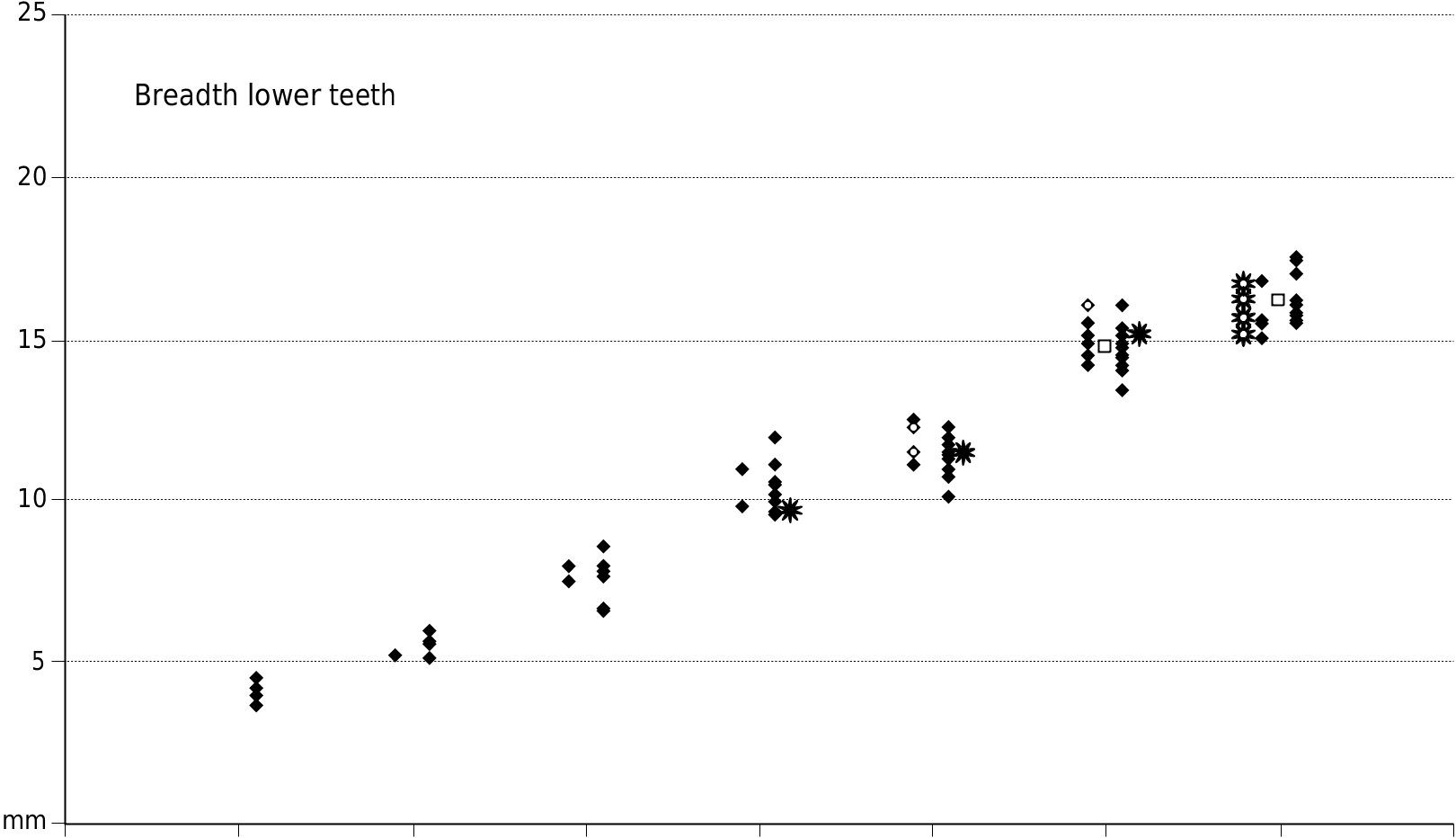
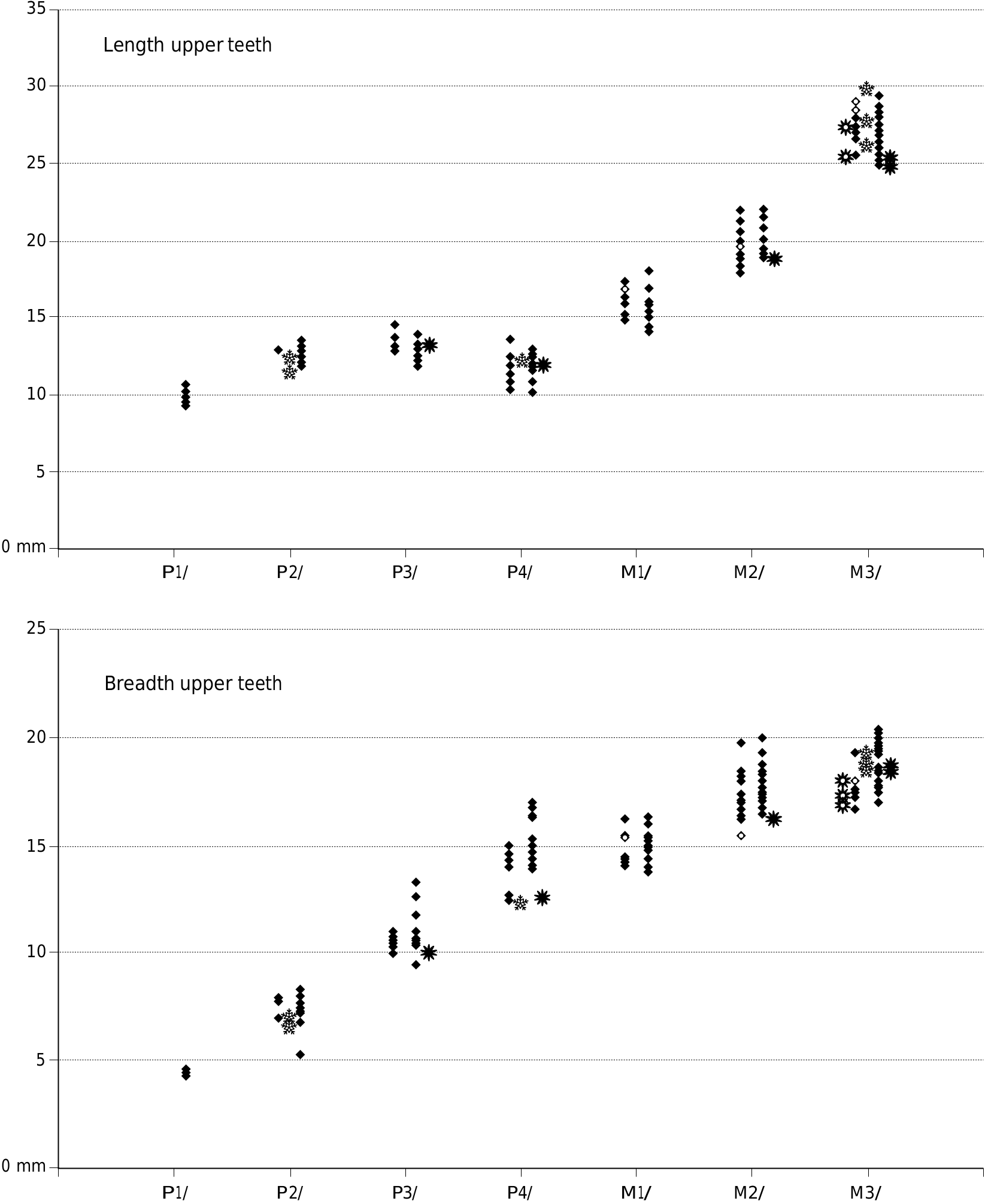
Small species of Dasychoerus , length M1/, 14.2-18 mm; length M2/, 18.5-22 mm; length M3/, 24.6-29.4 mm; length m/1, 14.7-18 mm; length m/2, 18- 24 mm; length m/3, 28.3-33.3 mm.
Note on nomenclature and lectotypes of Sus minor
Guérin & Faure (1985), Guérin et al. (1998) and Guérin & Tsoukala (2013) retained two subspecies Sus arvernensis arvernensis ( Croizet & Jobert, 1828) and Sus arvernensis minor ( Depéret, 1890) for these small Plio-Pleistocene European suids. Guérin et al. (1998) separated these two
A subspecies on the basis of the smaller dimensions of Sus arvernensis minor , and its relatively long premolars, narrower molars of simpler construction with fewer accessory tubercles. However, our own analysis of the dimensions and morphology of the teeth from the two type localities, Les Étouaires and Roussillon indicate significant morphometric overlap between the two subspecies. In this study, we therefore treat all the material as a single unit at the species level, rather than continue to deal with two subspecies.
Azzaroli (1954, 1975)considered that Sus arvernensis was a “ nomen dubium ”, and wrote that it could possibly be a synonym of Sus minor , in which case Sus arvernensis would be the valid name. Hünermann (1971) considered the name Sus arvernensis to be invalid due to the incomplete preservation of the type specimen (he agreed that Sus arvernensis and Sus minor were synonyms, but opted to support Sus minor even though this goes against the rule of priority). However, the type specimen of the former species is one of the more informative fossil suid specimens described, comprising associated upper and lower jaws, with well preserved elements of the deciduous and permanent cheek dentition, plus parts of both lower canines, and is thus more informative than the lectotype of Sus minor (CCECL Pp 195) which comprises a mandible fragment with p/2-m/2.
There is an unusual situation concerning the type specimen of Sus minor which has caused confusion to the extent that different specimens have been used by different authors as reference specimens. Four separate fossils have been proposed either as lectotypes or as syntypes. Azzaroli (1954: 58 and caption to pl. 1; fig. 6a, b) employed the term “ lectotype ” for the left mandible figured by Depéret (1890: pl. V, fig. 13). This is the earliest nomination of a type specimen for Sus minor and thus is the valid one. It is the specimen comprising a left mandible with unerupted (but mechanically exposed) p/2-p/4 and m/1-m/ 2 in occlusion, curated at the CCECL under number Pp 195. Azzaroli (1975) reiterated the nomination but made an erroneous reference (erroneous citation of figure as Azzaroli 1954: pl. V, fig. 11, 11a).
Fejfar (1964) seemingly unaware of Azzaroli’s (1954) prior selection of a lectotype, nominated a different specimen as lectotype, a left lower jaw containing m/2 and m/ 3 in situ, according to him figured by Depéret (1890: 85, 86, pl. V, fig. 12). However, figure 12 on the cited plate is an image of a left M3/, the mandible with two molars being figure 14 of the same plate.
Guérin et al. (1998) wrote that the syntypes (sic) of Sus minor consist of “an almost complete palate and a last upper molar”. These specimens from Roussillon, which comprise a left maxilla with the cheek dentition running from P3/-M3/, and an isolated M3/, the former of which was attributed in the first instance to Sus arvernensis by Depéret (1885: pl. V, fig. 1).
Thus various authors have employed four different specimens to typify Sus minor , and this has undoubtedly caused some fluidity in the interpretations that flowed from the choice of type material. The first nominated, and therefore the valid lectotype of Sus minor is the mandible nominated by Azzaroli (1954) “CCECL Pp 195”.
In order to clarify the situation, the type materials of Dasychoerus arvernensis ( Figs 2-7 View FIG View FIG View FIG View FIG View FIG View FIG ) and “ Sus ” minor ( Fig. 8 View FIG ) are illustrated. The holotype of Dasychoerus arvernensis possesses the following teeth in situ in the maxilla and mandible – left and right i/1, left i/2, parts of the left and right male canines (the tip of the left canine has gone missing since it was illustrated by Croizet & Jobert [1828]), left D2/-D4/, M1/, left d/2-d/4, m/1-m/2, p/ 4 in crypt, right d/2-d/4, m/1, p/ 4 in crypt. The lectotype of “ Sus ” minor is a left mandible containing p/2-p/4, m/1-m/2.
DESCRIPTION
Musaitu suid skull
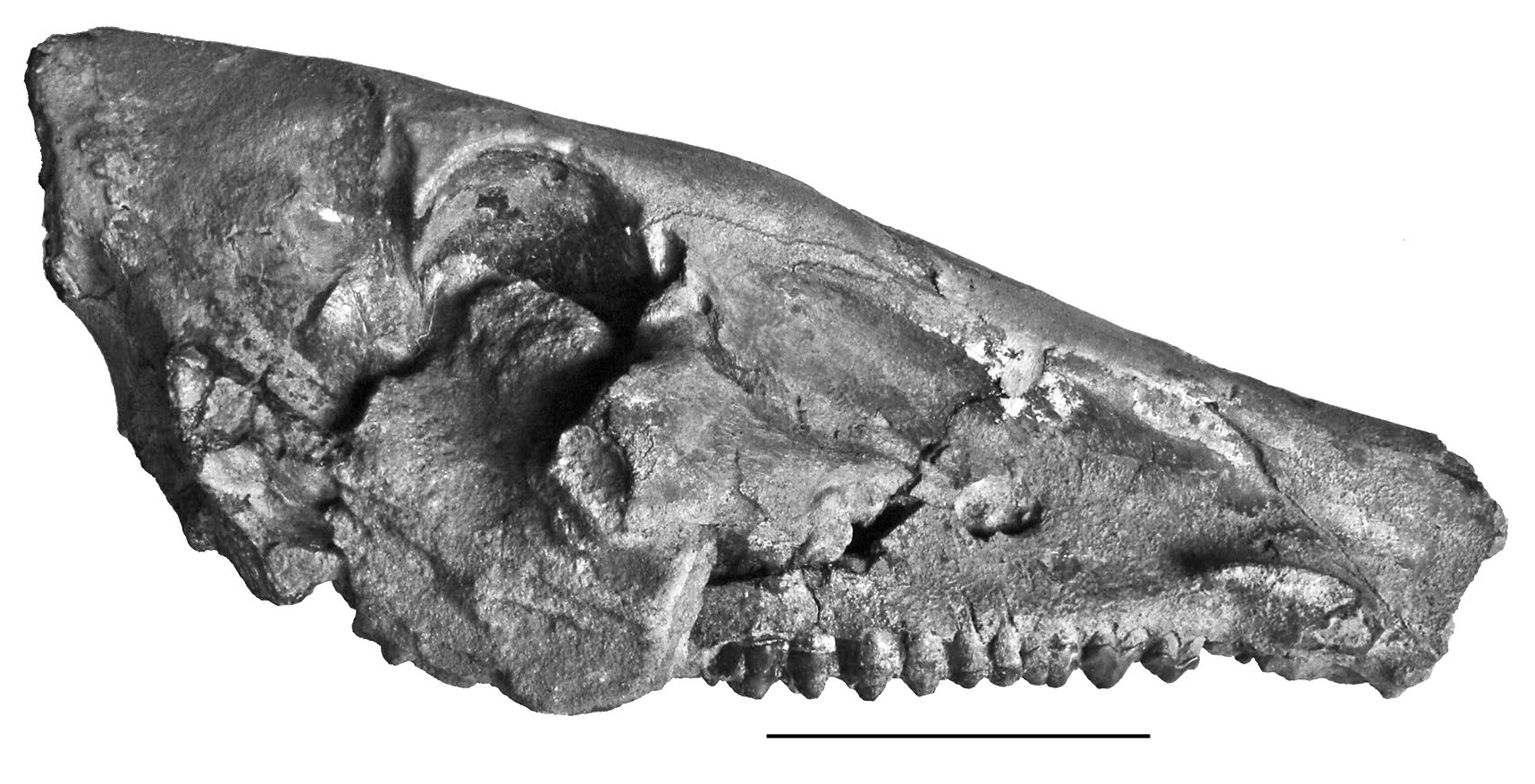
The Pliocene deposits at Musaitu, Moldova, yielded a remarkable skull of Dasychoerus arvernensis rivalling in completeness the material from Roussillon, France ( Azzaroli 1975; Berdondini 1992) ( Figs 9-14 View FIG View FIG View FIG View FIG View FIG View FIG ). The skull is notable for the narrow posterior cranial table, forming a narrow crest distally somewhat in the style of Euhys barbatus ( Müller, 1838) ( Gray 1868) and narrower than is usually the case in Dasychoerus verrucosus . The morphology of the brain case appears to be close to that of the Kvabebi suid described by Vekua (1972) although distortion of the latter specimen renders detailed comparisons difficult. A difference from the Kvabebi skull is the slightly convex longitudianl dorsal profile of the Musaitu skull, which is dished in the Kvabebi specimen (perhaps enhanced by crushing).
In lateral view the slightly convex dorsal transverse profile of the skull is evident, with a low swelling between the supra-orbital grooves in front of the supra-orbital foramina. The dorsal longitudinal profile of the nasals is very gently concave, almost flat. Although the anterior ends of the nasals are broken off, and the rear of the braincase is damaged, it is evident that the splanchnocranium was much longer than the neurocranium, as measured from the anterior margin of the orbit ( Azzaroli 1954). The orbit lies behind the level of the upper third molar and is high on the face, bordered anteriorly by the lacrimal in which there are two prominent foramina. The post-orbital process ends in a sharp point above the mid-height of the orbit. In front of the orbit there is a well developed fossa for the origin of the levator rostri snout musculature ( Ewer 1958). This fossa has a clear dorsal margin which leads forwards to the region above the canines, implying a well developed rooting habit in this species. The facial crest that separates the origin of the levator rostri from that of the dilator naris lateralis and the depressor rostri terminates short of the infra-orbital foramen. The infra-orbital foramen is large and lies at mid-height of the snout above the P4/-M1/. Above and just behind the canine alveolus there is a canine flange with a smooth concave dorsal surface over which passed the tendons for the snout musculature. At the canine level, the ventral part of the snout descends beneath the occlusal surface of the cheek teeth. The canine is inserted at the anterior ventral end of the supra-canine flange.
In dorsal view, the skull is observed to be long and narrow, broadest at the post-orbital processes, narrowing sharply distally, and narrowing more gently anteriorly. The infra-orbital foramina emerge from the skull roof in line with the front of the orbits, and the supra-orbital grooves follow a slightly curved course anteriorly along the dorsal surface of the skull, first approaching each other, and then running sub-parallel to each other. The temporal crests approach each other distally but do not form a true sagittal crest. The degree of narrowing of the braincase distally is comparable to that seen in Euhys barbatus . It is narrower than is usually the case in Dasychoerus verrucosus (Forsyth- Major 1897) and Sus scrofa , and quite similar to the fossil South African form Potamochoeroides hypsodon Dale, 1948 ( Pickford 2013b) . The zygomatic arches are broken, but the root of the right one shows that it departs from the face at an angle of c. 45° with its anterior end above the M2/, just behind the infra-orbital foramen.
The supra-canine flange projects from the face above and behind the canine alveolus, its dimensions and that of the canine alveolus suggest that this was probably a juvenile male in which the flange had not grown to its fully mature form. The dimensions of the canine root support this diagnosis. The snout is rectangular in section, with almost vertical sides.
In palatal view, the lingual edges of the two cheek tooth rows are sub-parallel. The teeth are well preserved with the P2/-M3/ forming a closed series separated by a short diastema from the P1/, represented by two alveoli just behind the canine alveoli which contain remnants of the root of the canine. The front parts of the palatines are broken so no information can be provided about the incisors. The supracanine flange is positioned such that the canines emerged from its anterior end, and its rear margin is opposite the P2/. The individual was a young adult at the time of death, as revealed by the fact that M3/ is not completely erupted but the cusp apices of the anterior loph have reached the same level as the occlusal surface of the teeth in front of it. The posterior choanae open up a short distance behind the rear of the M3/s. The basicranial area of the skull is badly damaged.
In anterior view, the section of the snout is visible where the anterior part has broken off. This shows that the lateral walls of the rostrum are vertical, the nasal cavity is comprised of two lobes, a large one dorsally and a smaller one ventrally. The dorsal surface of the rostrum shows a low ridge in the midline.
In posterior view, the nuchal zone is observed to be tall and relatively narrow. The basicranium is broken.
Dentition P1. The P1/ is missing on both sides but it had two roots. It is positioned just behind the canine alveolus and slightly lingual to the inner edge of the canine alveolus.
P2. The P2/ is subtriangular in occlusal outline, with rounded apices of the triangle. The main cusp is somewhat anterior to the midline of the tooth. It has a precrista that descends towards a small mesial cusplet, and a prominent, swollen postcrista that forms a separate cusplet distally. There is a slight incision in the postcrista which separates the main cusp from the distal one. Disto-lingually, there is a small cingular cusplet separated from the rest of the crown by the distal fovea. Thus the rear of the tooth is broader than the front half.
P3. The P3/ has a broad triangular occlusal outline. It is constructed along the same lines as the P2/ but all the structures are better defined and larger. Thus the mesial cusplet at the anterior end of the precrista is more prominent and has cingular folds extending medially and laterally from it for a short distance, but not reaching the buccal side of the crown. The distal cusplet on the buccal side is larger and is better separated from the main cusp by lingual and buccal grooves. The distal fovea is broader than in the P2/ and the disto-lingual cusplet is larger, connected to a low cingulum that extends along the lingual base of the crown.
P4. The P4/ is tricuspid, and rectangular in occlusal outline. There are two buccal cusps, the posterior one as tall as the main cusp but mesio-distally smaller than it. These two cusps are separated from each other on the buccal side by a prominent vertical groove that reaches almost to the cervix. There is a prominent protocone on the lingual side of the tooth, connected to cingula that extend anteriorly and posteriorly to join the mesial and distal cingula respectively. There is a sagittal cusplet on the lingual side of the paracone, but there is none on the lingual side of the metacone.
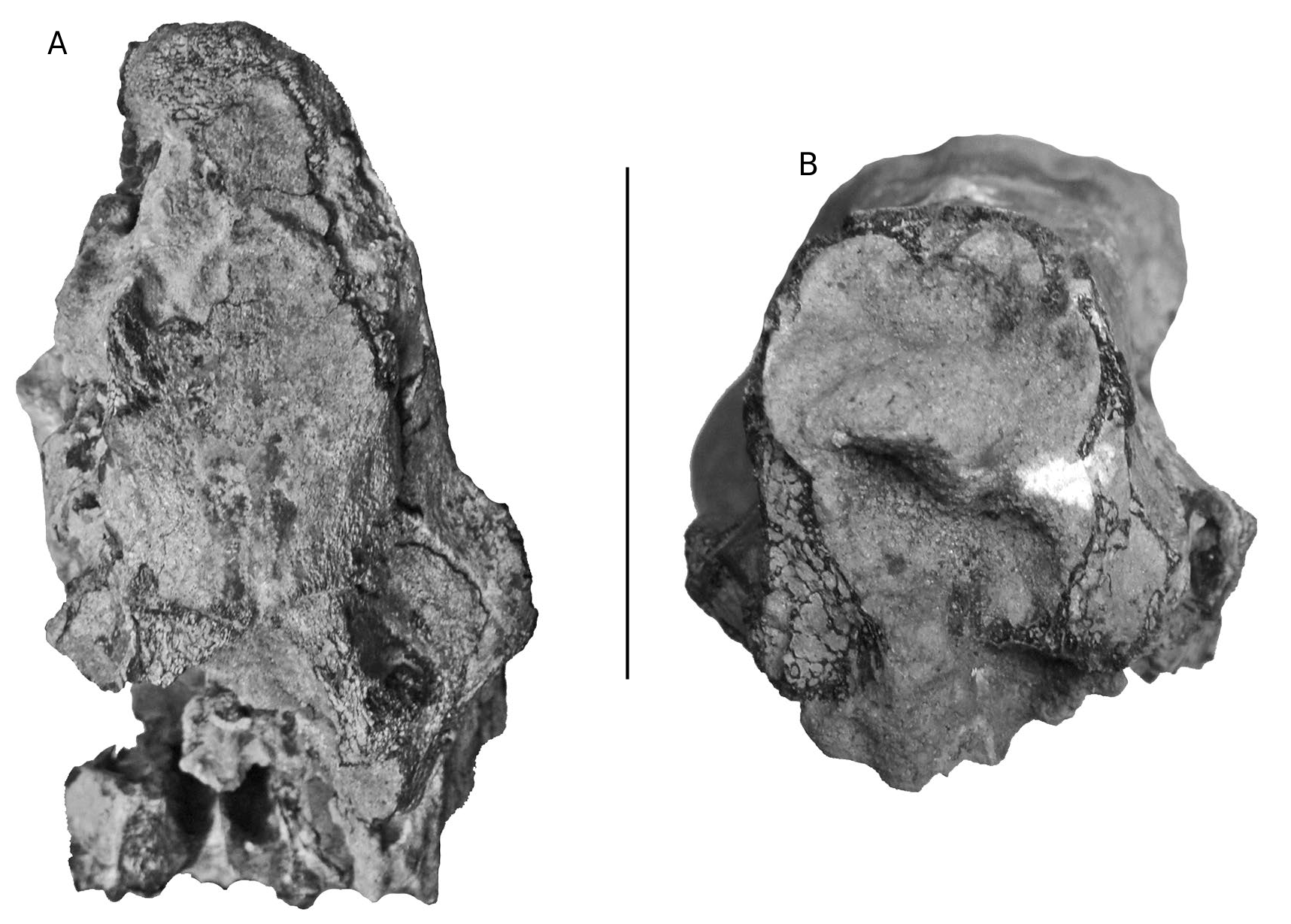
M1/ and M2/. The M1/ is deeply worn. The M2/ is also heavily worn, but shows four main cusps arranged in two lophs with a prominent median accessory cusplet blocking the median transverse valley. There are well formed mesial and distal cingula and there is a remnant of a lingual cingulum in the lingual end of the median transverse valley.
M3. The M3/s are lightly worn and show all the main structures well. This tooth has four main cusps and anterior, median and posterior accessory cusplets, as in the M2/ (the usual basic morphology found in suids) but in addition, at the rear of the crown, there is a small talon comprised of a low, pointed cusp slightly to the lingual side of the midline of the crown. The mesial cingulum is beaded and ends before reaching the buccal or lingual part of the crown. It ends lingually at a small cingular cusplet attached to the mesio-lingual corner of the protocone. The lingual end of the median transverse valley shows a low cingular remnant, and there are cingular beads of enamel at the lingual and buccal ends of the valley between the rear loph and the talonid of the tooth. The Fürchen are shallow apically but clearly discernible, especially towards the bases of the main cusps where they are deeper and broader.
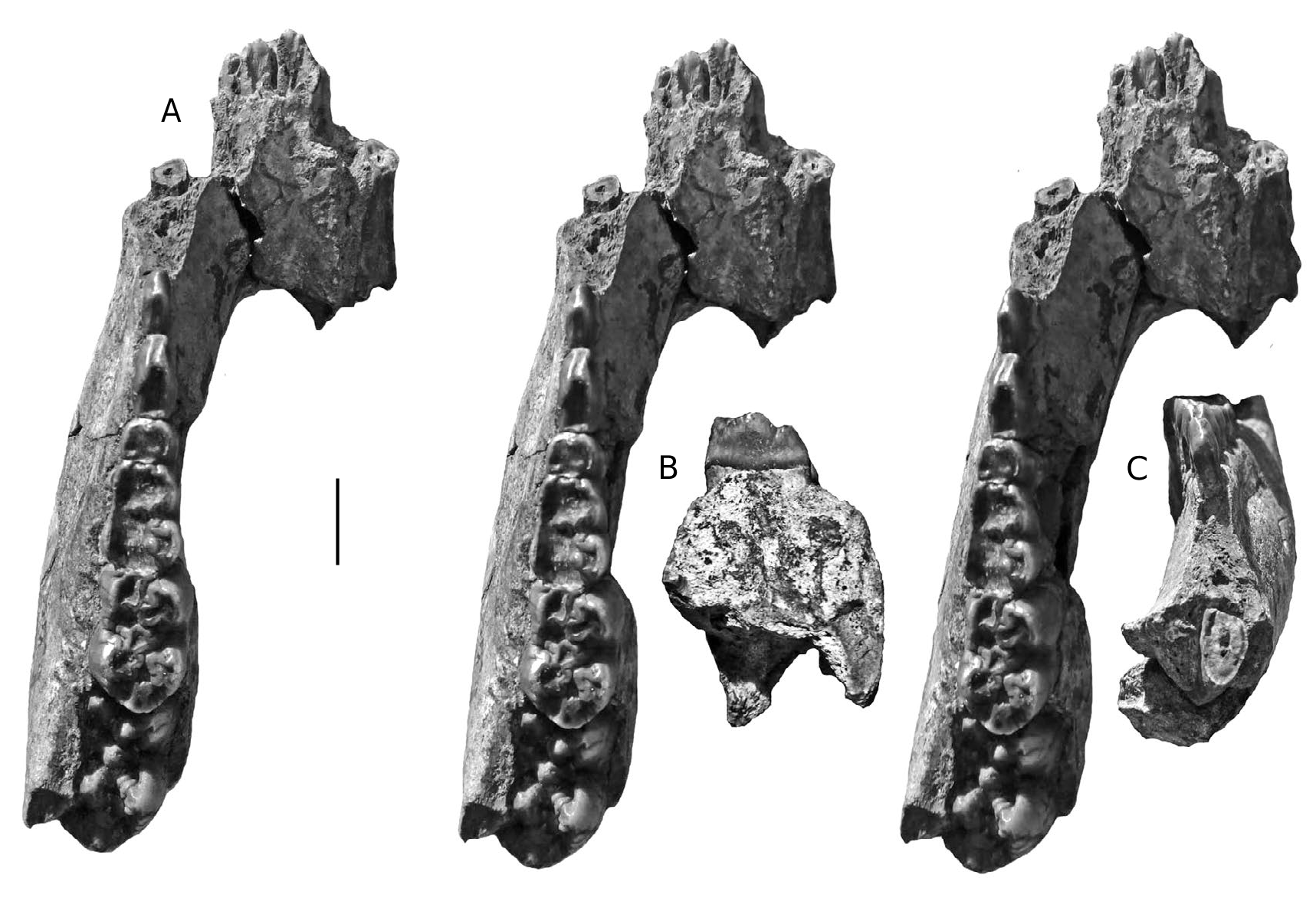
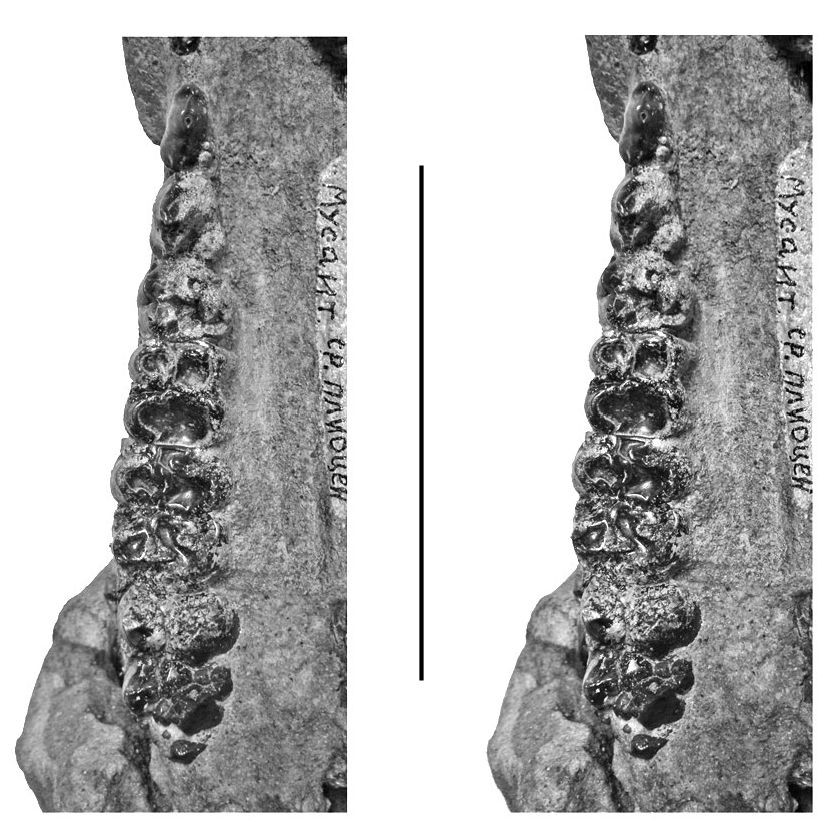
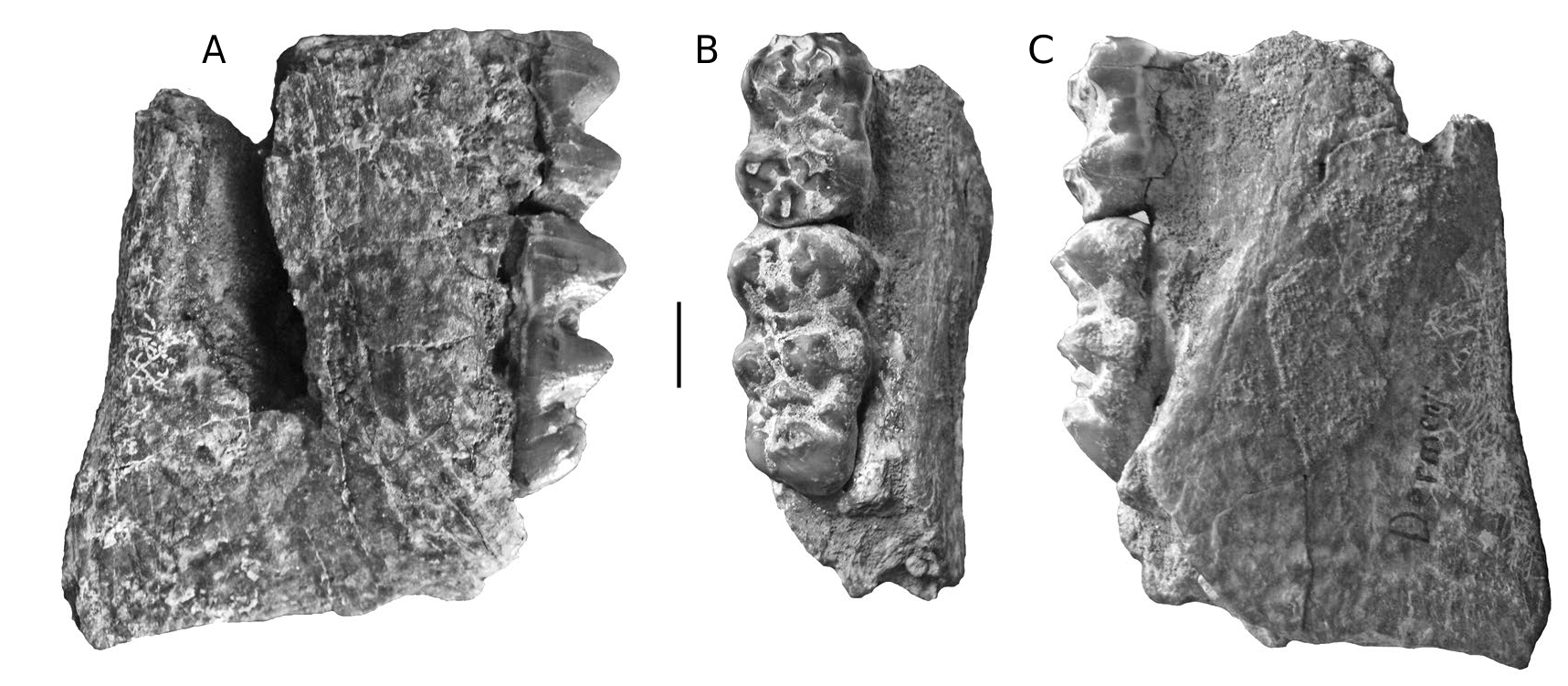
Dermenji mandible
A mandible fragment from Dermenji curated at the Musée des Complexes Faunistiques Fossiles de Moldova, Institut de Zoologie, Académie des Sciences de Moldova, comprises the rear part of the right ramus containing m/2 and m/ 3 in medium wear ( Fig. 15 View FIG ). The m/2 has four main cusps arranged in two lophs with clear anterior, median and posterior accessory cusplets in the midline of the crown. The two lophids are wide apart. The two anterior lophids of the m/3 have the same basic structure as the m/2, but in addition, there is a prominent talonid cusp behind the posterior accessory cusplet in the centre-line of the crown. The teeth in this specimen resemble those from Gödöllő, Hungary ( Mottl 1939) and Perpignan, France ( Depéret 1890).
Metric analysis
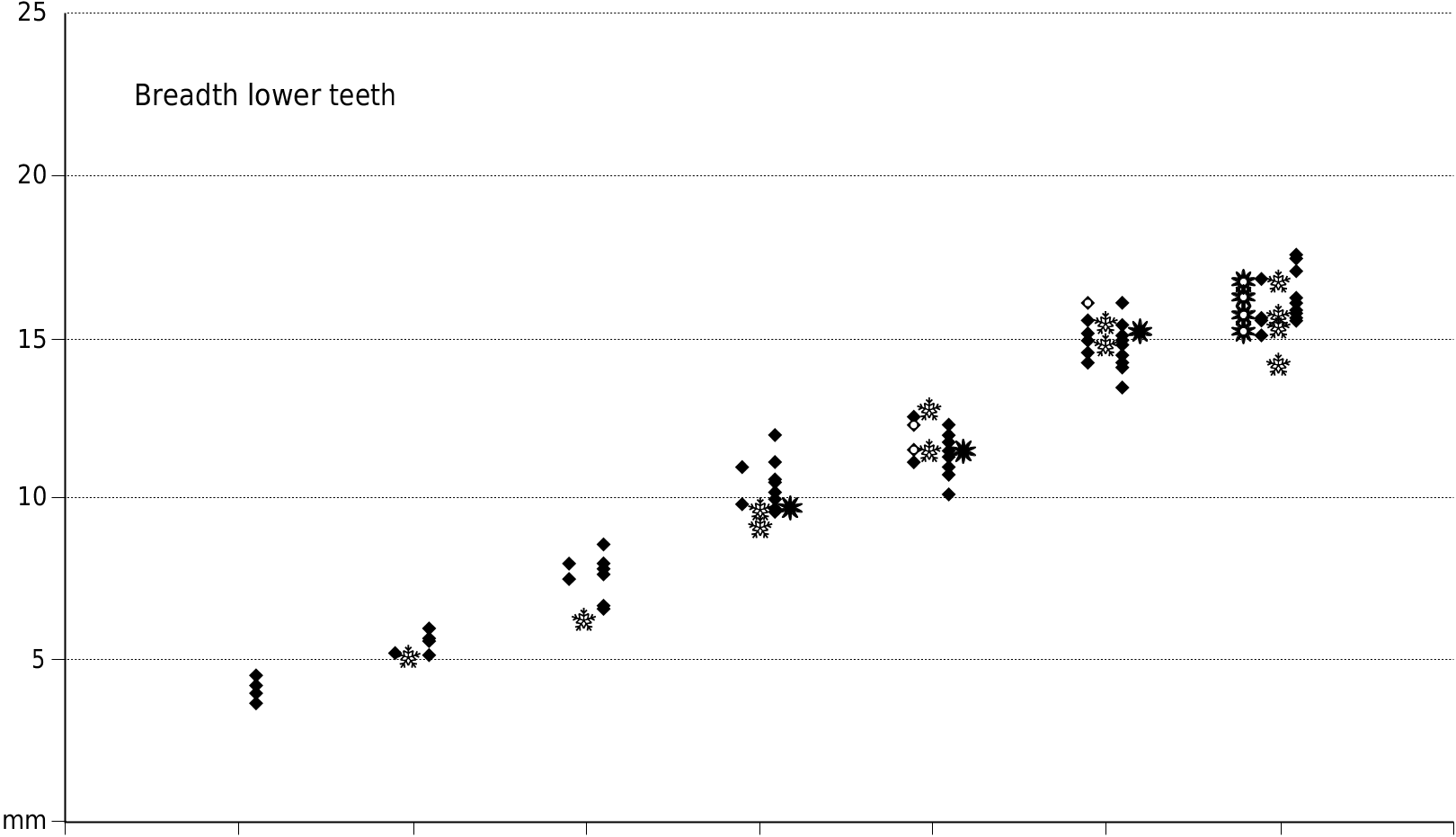
Appendices 1 (Europe) and 2 (Africa) provide measurements of the teeth used in the metric analysis.
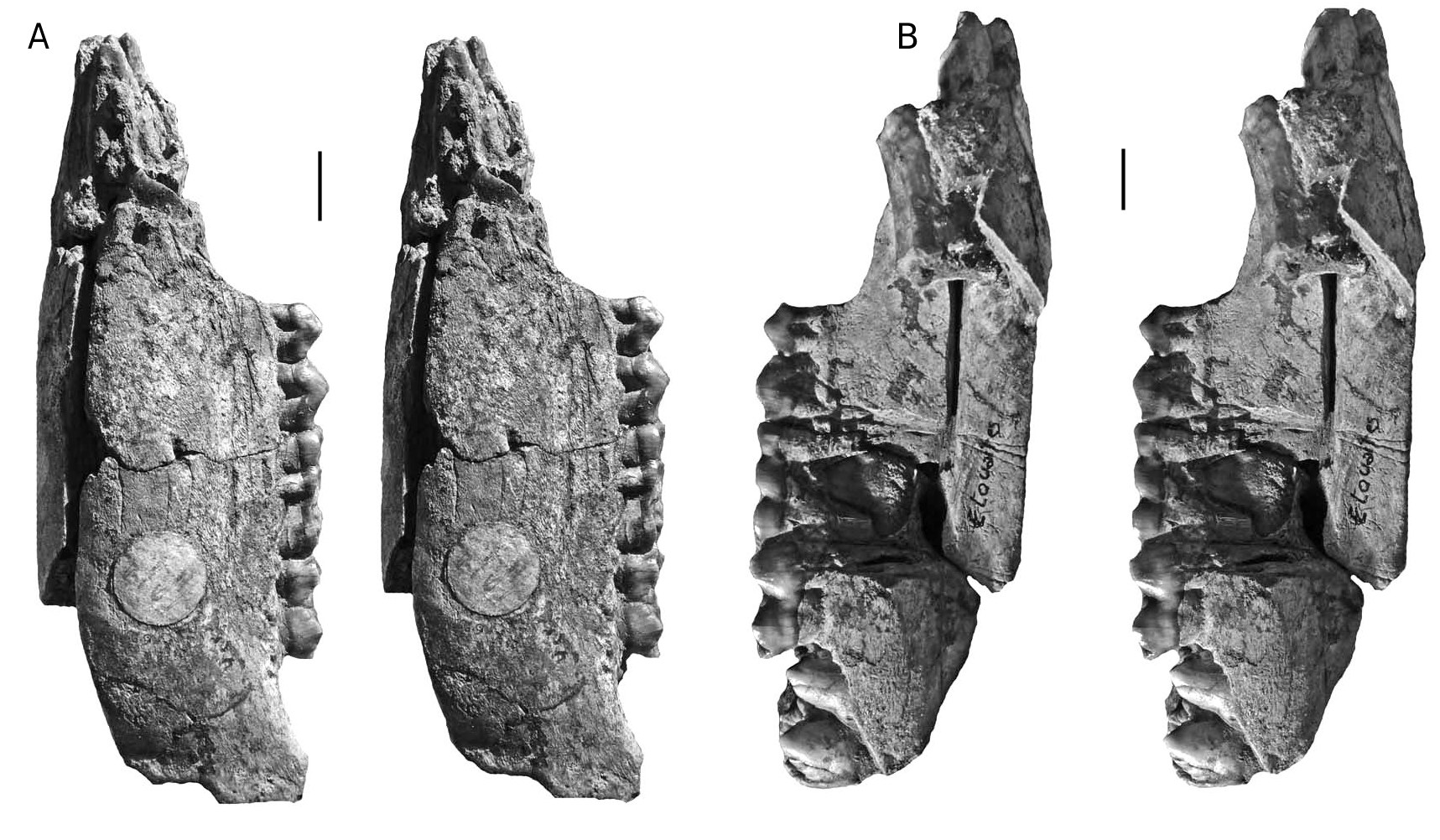
The dental metrics of the Musaitu and Dermenji suids show close correspondence with material of Dasychoerus arvernensis from other localities in Europe ( Figs 16-19 View FIG View FIG View FIG View FIG ). The specimens are smaller than the suids from Kvabebi, Georgia ( Vekua 1972) although there are some morphological resemblances in the skulls from Musaitu and Kvabebi, such as the presence of a narrow cranial table distally, almost forming a sagittal crest, and well developed supracanine flange. However, the dorsal surface of the Kvabebi skull has a dished profile in lateral view, and the specimen from Musaitu is slightly convex, almost straight. The Kvabebi specimen is distorted, and this may have altered the profile, but even so, the difference seems to be important. Similar dishing of the skull occurs in the African genus Kolpochoerus , and it could be that the Kvabebi morphology represents an intermediate stage between Dasychoerus arvernensis as exemplified by the almost straight, slightly convex condition seen not only in the Musaitu skull but also in the material from Roussillon and Villafranca d’Asti ( Azzaroli 1954, 1975; Berdondini 1992) and the dished condition observed in Kolpochoerus .
The Kvabebi suid ( Vekua 1972) has thickened mandibular rami, as do specimens from Roussillon and Villafranca d’Asti ( Figs 20 View FIG , 21 View FIG ), and the same condition occurs in Kolpochoerus . There can be little doubt that the African kolpochoeres, Dasychoerus arvernensis and the Kvabebi suid are closely related members of a lineage or clade.
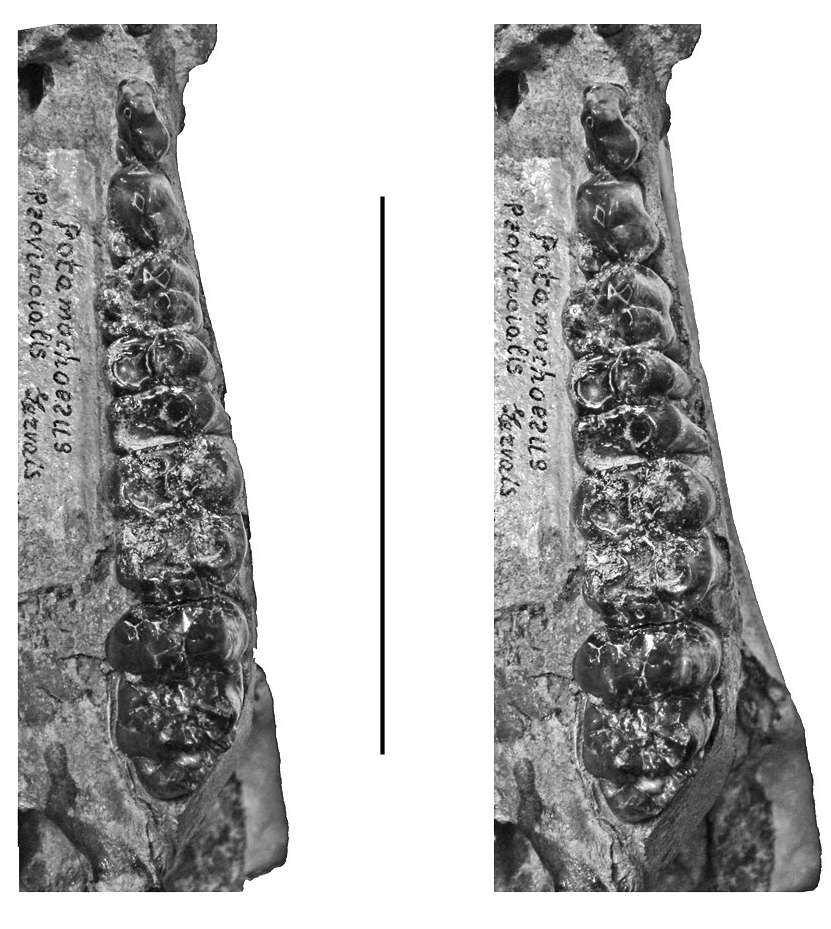
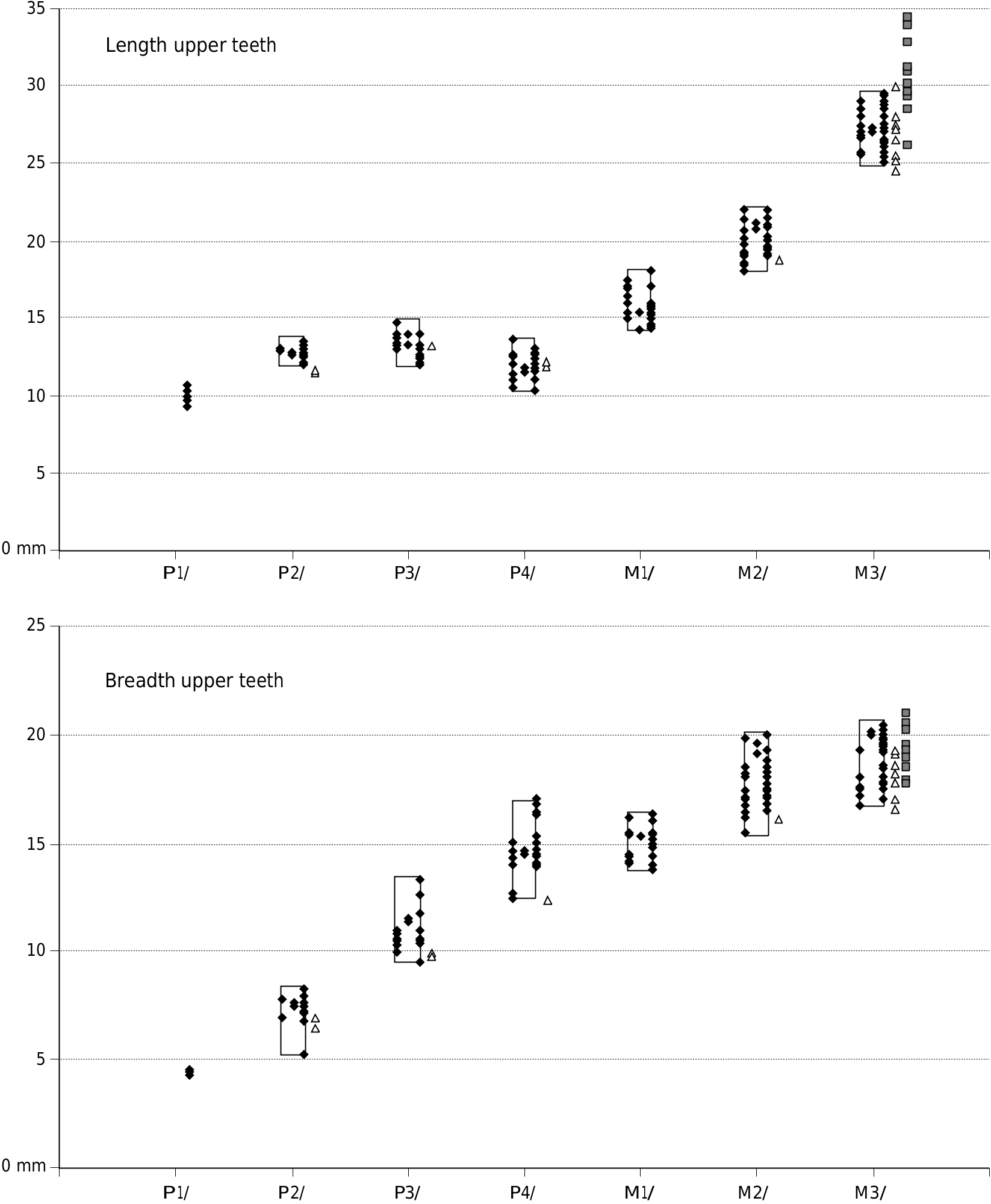
The larger of the specimens attributed to Kolpochoerus millensis are similar in size to the suid from Kvabebi, Georgia ( Vekua 1972) ( Figs 22 View FIG , 23).
The upper and lower third molars attributed to the Ethiopian species Kolpochoerus millensis (Haile-Selassie & Simpson 2012) have a somewhat greater range of length variation than the closely related taxon Dasychoerus arvernensis . Many of the former specimens fall within the range of variation of the latter species but there are a few more elongated teeth. Whether this is related to a real increase in variability of the length of the teeth, or whether it is due to the mixing of two closely related taxa needs to be examined. The authors did not provide measurements of the other cheek teeth of Kolpochoerus millensis , so it is not possible to determine whether they too show an increase in variability over the situation in Dasychoerus arvernensis . It is clear however, that the larger of the specimens attributed to Kolpochoerus millensis overlap in length dimensions with the Kvabebi suid, although the latter material has broader third molars than K. millensis .
Boreal, sub-tropical and tropical suids
As in other artiodactyl groups there are suids adapted to high latitudes and others to low latitudes. A major difference between these groups concerns the timing of reproduction related to seasonal changes in climate. Boreal taxa time their reproduction to winter-summer cycles whereas more tropical lineages time their reproduction to wet season – dry season cycles. The metabolic triggers for reproductive activity differ between the groups, in high latitudes the hormonal changes are linked to variations in day length which are highly predictable. The Wild Boar ( Sus scrofa ) times its reproductive cycle such that litters are born in spring ( MacDonald 2001) whereas in the tropics suid reproduction is linked to changes in humidity which are much more variable in timing, partly due to the El Niño phenomenon. In the humid tropics where the seasons are not marked, reproduction may occur all the year round, but where there is marked dry-wet cyclicity, birth is usually timed to occur near the beginning of the wet season, but with marked variability due to vagaries in the onset of the different seasons.
This translates into divergent controls of reproductive behaviour in Sus Linnaeus, 1758 and Dasychoerus , the Wild Boar ( Sus scrofa ) being adapted to boreal environments with marked day length changes through the year, whereas the more tropical Warty Pigs ( Dasychoerus ) and their relatives the Wart Hog ( Phacochoerus Cuvier, 1826 ) the Red River Hog ( Potamochoerus ) and the Forest Hog ( Hylochoerus ) are adapted to tropical environments where day length changes are not as marked as they are at high latitudes. Thus not only do reproductive parameters link Dasychoerus , Phacochoerus , Potamochoerus and Hylochoerus , to the exclusion of Sus , but so do the presence of warts, lower canine morphology (verrucosic), style of intraspecific combat during the rutting season among others ( MacDonald 2001).The definition of verrucosic canines is based on the sub-equal dimensions of the lingual and labial enamel-covered surfaces of the tooth. In scrofic canines the labial surface is appreciably shorter than the lingual surface. The distal, enamel-free surface can be broad or narrow in both types. Of course, the measurements should be taken on the unworn part of the tooth, because wear can give a false scrofic section to a verrucosic underlying morphology. By these criteria, the canines of Potamochoerus are verrucosic.
0 p/1 p/2 p/3 p/ 4 m / 1 m / 2 m /3
Reproductive potential of Suidae
Species of Sus (sensu lato) differ from most other artiodactyls by their reproductive potential. They are the only group to give birth to several offspring at a time (litters range from one to twelve in Sus ) ( Table 1). Other suids tend to give birth to fewer offspring (one to six in Porcula Hodgson, 1847 and one to two in Babyrousa Perry, 1811 , usually two in Phacochoerus (but as many as four have been observed by the senior author), and perhaps two in Hylochoerus ). Peccaries usually give birth to fewer offspring (one to four, usually two) and most other artiodactyls only one or two offspring at a time. Given that in Sus scrofa sexual maturity is attained at 18 months, gestation is 115 days and life span ranges from 15 to 20 years, a sow that starts breeding at an age of two years may leave behind an astonishing number of descendents, especially if she breeds twice each year ( White 1788).
Rates of evolution
A consequence of having large litters and relatively short generation times ( Table 1) is that evolution seems to have occurred more slowly when compared with other artiodactyls which have only one or two offspring per pregnancy and successive pregnancies more spaced apart. Suids with fewer offspring per pregnancy and longer gestation periods, such as Phacochoerus and Hylochoerus , appear, from their fossil record, to have evolved more rapidly than those lineages that have larger numbers of offspring ( Sus scrofa , for example). The same probably applies to Babyrousa , which departs greatly from the basic suid grundplan as exemplified by Sus . Possessing warts, the babyrussa may be more closely related to Dasychoerus than to other suids.
There is debate about the possibility of a link between rates of evolution and reproductive strategies (K-strategy, r-strategy) in mammals, with evolution often occurring more rapidly in K-strategists such as proboscideans, than in r-strategists such as many rodent taxa. However, some K-strategists, including rhinocerotids, underwent slow evolution. For this reason, rates of evolution in suines requires further study,but at present,the fossil record seems to support the notion that r-strategists such as Sus scrofa evolved more slowly than K-strategists such as Phacochoerus .
PHYLOGENY
Molecular evidence
Molecular techniques have thrown some light on relationships among extant suid lineages, although the results are actively debated without any firm consensus emerging from
0
p/1 p/2 p/3 p/ 4 m / 1 m / 2 m /3
the studies ( Wu et al. 2006; Funk et al. 2007; Gongora et al. 2010; Frantz et al. 2013). The genus Porcula , for example, was for many years included in the genus Sus as a sister taxon of Sus scrofa (sometimes as a subgenus Porcula [ Herre 1962]) with other species of Sus more distantly related. Porcula is now considered by some researchers ( Funk et al. 2007) to represent a genus distinct from Sus , which means that some of the other species traditionally included in the genus Sus need to be reclassified into a separate genus or genera. From a morphological perspective two or three subgroups have been commonly recognised among the species hitherto included in Sus ( Groves 1981, 1997; Groves & Grubb 1993): 1) non-warty pigs ( Sus scrofa and Porcula salvania Hodgson, 1847 ); and 2) warty pigs (all other species of “ Sus ”). Subdivision on the basis of lower canine morphology yields almost the same categories except that the “warty” subgroup is capable of further subdivision into two clusters: 2a) the philippensis group; and 2b) the verrucosus group. “ Sus ” philippensis poses particular problems because it is a “warty” pig, whereas some molecular analyses suggest closer affinities to Sus scrofa than to the other warty pigs ( Funk et al. 2007). Warts occur in the African warthog, indicating possible phylogenetic links with the “warty” pigs, and so does the Giant Forest Hog ( Hylochoerus ) ( Ewer 1970). The bush pig ( Potamochoerus ) has thickened skin patches on the face ( Ewer 1958) and some authors ( MacDonald 2001) report that males have these structures. This suggests that the presence of facial warts represents a derived (autapomorphic) feature of the group.
On the basis of whole-genome analyses, Frantz et al. (2013) recognised a deep split between Sus verrucosus and Sus scrofa . Their cladogram shows a single specimen of “ Sus scrofa ” plotting within the clade comprising Sus verrucosus + Sus barbatus Müller, 1838 + Sus celebensis Müller & Schlegel, 1845 , and this is the subspecies Sus scrofa vittatus Boie, 1828 ( Müller & Schlegel 1845), from Sumatra, a tropical island form long ago considered by some authors ( Lydekker 1915) (possibly in the basis of misidentified specimens – see Forsyth-Major [1897]) to be specifically distinct from boreal populations of Sus scrofa . This raises questions about the taxonomic affinities of Sus scrofa vittatus – is it really a subspecies of Sus scrofa? Old literature refers this species to the genus Aulacochoerus Gray, 1873 , of which it is the type species. However, there has been confusion about which specimens belong to “ Sus ” vittatus , who the author of the species name was ( Boie 1828 or Müller & Schlegel 1845) and where the type material came from (Java or Sumatra). According to Mees (1957) the lectotype of Sus vittatus is the skeleton numbered RMNH 13508, and the type locality is Tjikao aan de Tjitaroem, West Java, but according to Hardjasasmita (1987) the holotype is a skull from Padang, Sumatra (RMNH Ost “d” in image page 51, but erroneously cited as Ost “c” in the text). Forsyth-Major (1897) reidentified four of the six skulls that Gray (1873) attributed to Aulacochoerus vittatus – NHMUK 1362f as a juvenile Sus verrucosus, NHMUK 1362c and 1362d as Sus verrucosus amboinensis (Forsyth-major, 1897) and NHMUK 1362g as Sus verrucosus celebensis . Thus from its inception, the concept of the genus Aulacochoerus was based on misidentifications of at least four out of six skulls.
In summary, recent molecular biology studies of suids indicate that the so-called Warty Pigs ( Dasychoerus ) and Bearded Pig ( Euhys ) are more closely related to each other, than any of them are to the Wild Boar ( Sus scrofa ) ( Gongora et al. 2010; Frantz et al. 2013) or to Phacochoerus (the outgroup in the study by Frantz et al. 2013). On the basis of the fossil record, Pickford (2013e) postulated that the Wart Hog lineage ( Phacochoerus ) split from the Warty Pig lineage ( Dasychoerus ) during the Late Miocene which would accord with the molecular data.
Fossil evidence
The morphometric resemblances between European and African Dasychoerus arvernensis , the Kvabebi suid, and Kolpochoerus phillipi are flagrant. The Kvabebi suid has pachygnathous mandibles, the dorsal profile of the skull is moderately dished, the lower incisors are well beneath the occlusal surface of the cheek teeth, and the lateral edges of the supra-canine flanges project upwards, all features observed in species of Kolpochoerus . Souron et al. (2013) listed two of these characters as unambiguous synapomorphies of the group comprising Potamochoerus , Hylochoerus and Kolpochoerus . The lateral extent of the zygomatic arches cannot be determined in the Kvabebi skull, due to damage, but what remains appears to be pneumatised. A dished cranial profile is listed by these authors as a synapomorphy of Hylochoerus and Kolpochoerus , but such a morphology also occurs in the Kvabebi skull. Furthermore, Dasychoerus arvernensis and the Kvabebi suid have procumbent lower incisors that are positioned well beneath the level of the occlusal surface of the cheek teeth. The latter character was listed by Souron et al., (2013) as a potential synapomorphy of Kolpochoerus . Because of all this, it is concluded that Dasychoerus arvernensis and the Kvabebi suid are closely related to Kolpochoerus , Potamochoerus and Hylochoerus .
The importance of Dasychoerus arvernensis
The fossil suids from Ethiopia and Chad previously attributed to Kolpochoerus deheinzelini by Brunet & White in 2001, are so similar to material of Dasychoerus arvernensis , that Pickford (2012) proposed that the two species were synonymous, with Dasychoerus arvernensis ( Croizet & Jobert, 1828) having priority over Kolpochoerus deheinzelini Brunet & White, 2001 . The metric analysis ( Figs 16 View FIG , 17 View FIG ) indicates how close the African and European material is, as does the morphology of the cheek teeth. The fact that the African and European fossils span the same time range strengthens the taxonomic conclusions, otherwise one would need to explain how Lower Pliocene suids from Africa and Europe which are morphometrically compatible with each other and which occurred
p/1 p/2 p/3 p/ 4 m / 1 m / 2 m /3
FIG. 23. — Comparison of dimensions of the lower cheek teeth of Dasychoerus arvernensis ( Croizet & Jobert, 1828) from Europe () and African fossils attributed to the same species (previously as Kolpochoerus deheinzelini Brunet & White, 2001 []) and the fossils attributed to Kolpochoerus millensis Haile-Selassie & Simpson, 2012 () in the right hand column (measurements of only the m/3 are available, Haile-Selassie & Simpson 2012).
as contemporaries, could belong to two distinct genera. Furthermore, such a decision would once again leave the African kolpochoeres without an ancestral group which runs against the almost universally accepted scenario that their ancestors must have dispersed into Africa from Eurasia during the basal Pliocene ( Cooke & Wilkinson 1978; Pickford 2012).
BIOGEOGRAPHY OF EXTANT AND EXTINCT SUIDAE
AND DISTRIBUTION OF DASYCHOERUS SPECIES
As a consequence of their high potential for demographic increase, extant suid species are capable of rapidly expand-
ing their range, and the same appears to have been the case in the past. The fossil record reveals that suids were often in the vanguard of mammalian lineages dispersing to newly available territory, as for example when passage between two continents, or between continents and islands, became possible ( Pickford 1993). Feral pigs have often expanded rapidly into suitable environments, even when subjected to human intervention (hunting, trapping) aimed at reducing or controlling their populations.
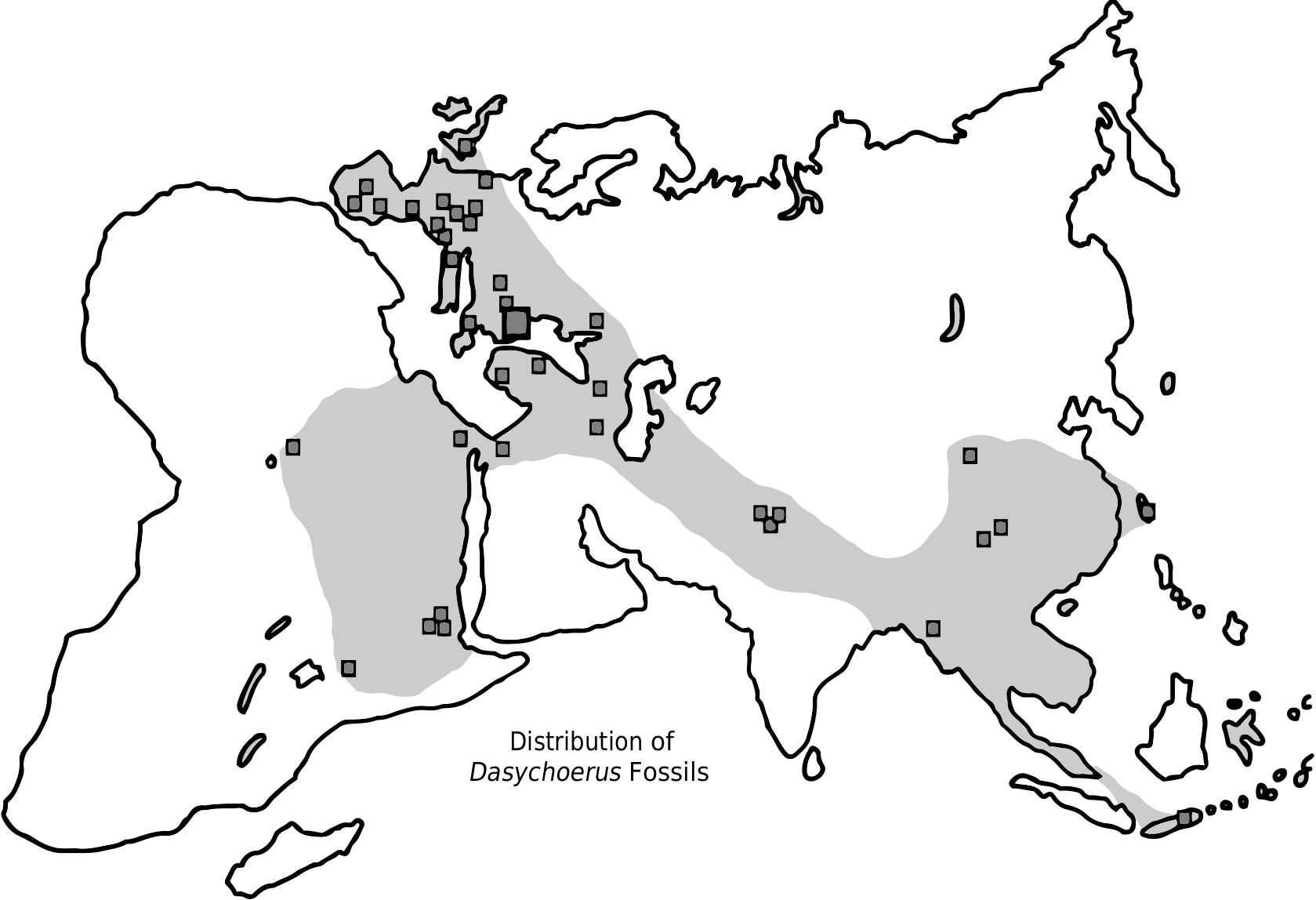
Today confined to Java and the Celebes (and some nearby islands [Forsyth-Major 1897]), the genus Dasychoerus was extremely widespread during the Pliocene, a period during which a diversity of mammals and other vertebrates spread widely over mid-latitude Eurasia which was tropical to subtropical at the time. Among the dispersals from the Far East to Europe were the tapir, the panda, the peafowl, bunodont otters, some bovids, hyaenids, giraffids, rhinocerotids, camels and monkeys, some of which managed to disperse southwards into Africa, such as some bovids, bunodont otters and other carnivores, Dasychoerus , the pea-fowl, giraffids and camels ( Pickford 2012).
The poor Pliocene record in Peninsular India and the Iranian-Arabian corridor represents a void in our knowledge. A similar fossil void extends over much of Africa. Further palaeontological discoveries in these regions may fill some of the blank areas, but the overall picture that emerges is that the Far East, mid-latitude Eurasia and Africa were for a while (MN 14-MN 15: 6-3 Ma) part of a widespread tropical to subtropical biogeographic province over the extent of which dispersal by mammals was relatively easily accomplished. The high latitude limit of this palaeobiogeographic province was probably much as shown on the map ( Fig. 24 View FIG ) (many fossil sites are known from this high latitude zone, but none have yielded Dasychoerus ). Instead they contain proto-boreal taxa, including Sus sensu stricto ( Sus scrofa and close relatives).
Dasychoerus has been found in Pliocene and Pleistocene deposits in Asia, Europe and Africa ( Pickford 2012, 2013a, b) ( Fig. 24 View FIG ). In Eurasia it has usually previously been identified as Sus View in CoL , whereas in Africa early representatives of the genus were previously attributed to Kolpochoerus and/or Potamochoerus View in CoL .
This paper recognises several species of the genus Dasychoerus in the fossil record, as well as the survival of at least two extant species, Dasychoerus verrucosus and Dasychoerus celebensis which, among extant suids, are most closely related to Euhys barbatus and more distantly related to Sus scrofa ( Frantz et al. 2013) View in CoL .
At present Dasychoerus is restricted to the Island of Java (and small islands close to Java) ( D. verrucosus ) and Sulawesi ( D. celebensis ). However, the genus was widespread through Asia, Europe and Africa during the Pliocene and into the Pleistocene (Appendix 3). Hardjasasmita (1987) reported that Sus verrucosus is also present in Malasia and Sumatra.
The small extinct species Dasychoerus arvernensis was the most widespread member of the genus, being common in Europe, Asia and Africa. The large extinct species Dasychoerus strozzii appears to have been confined to Europe and the Middle East, although Gallai (2007) thought that it might have spread to Africa to give rise to Kolpochoerus . The extinct Pleistocene species Dasychoerus macrognathus has been reported from Java, Myanmar and China, and it could represent the ancestor of the extant species Dasychoerus verrucosus .
Fossils attributed to Dasychoerus have been reported from many localities throughout the Old World (Appendix 3).
Origin of the Kolpochoerus lineage
Wherever its ultimate centre of origin is determined to have been (the Far East? – similar species are known from Java ( Hardjasasmita 1987), Myanmar ( Pickford 2013b) and China ( Han 1987; Pickford 2013b), as well as the Siwaliks of Indo- Pakistan ( Pickford 1988) – Dasychoerus arvernensis was in the right place (mid-latitude Eurasia) at the right time (basal Pliocene) to spread to Africa ( Fig. 24 View FIG ) whereupon it gave rise to the Kolpochoerus and subsequently to the Hylochoerus lineage ( Pickford 2013a, d) and possibly to Potamochoerus as well, an old idea (Cooke 1978; Bishop 2010), resurrected by Souron et al. (2013). The earliest record of this genus in Africa is Dasychoerus natrunensis Pickford, 2012 , which either evolved into Dasychoerus arvernensis , or was replaced by a second wave of dispersal by the latter species – it did not change its generic status while entering the continent, but only later, when it was subjected to a variety of selective pressures which led to modifications in its dimensions (generally increasing in size), skull morphology (cranial dishing, expansion of the cranial table, swelling and drooping of the zygomatic arches) and dental morphology (increased hypsodonty, elongation of the third molars by addition of loph(id)s to the talon(id), hypertrophy of the canines, reduction of anterior cheek teeth).
Gallai (2006, 2007) postulated a slightly different scenario for the origin of Kolpochoerus , which he considered could be descended from Sus arvernensis via Sus strozzii . Whilst we agree that Kolpochoerus afarensis and Dasychoerus strozzii are related to each other and are comparable in dimensions and some details of morphology, we prefer to derive the genus Kolpochoerus earlier than is implied by the hypothesis of Gallai (2007). We estimate that the dichotomy between the Dasychoerus arvernensis-strozzii lineage on the one hand and the Dasychoerus arvernensis-Kolpochoerus lineage on the other occurred during the Pliocene c. 3.5 million years ago ( Pickford 2012). Nevertheless, there could have been some gene flow among suid populations of Europe and Africa during the Lower Pliocene.
What the new interpretations of the fossil record reveal is that in mid-latitude Eurasia and Africa during the Plio- Pleistocene there was a widespread clade of suids related to the extant species Dasychoerus verrucosus which was adapted to tropical and sub-tropical regions which differed in several features from the Wild Boar ( Sus scrofa and relatives) which is well adapted to boreal regions. During the Pleistocene the latter lineage populated mid-latitude regions formerly occupied by Dasychoerus species , either displacing Dasychoerus from its former more northern habitats, or simply moving in after Dasychoerus had died out in those regions consequent to climatic change (increased severity of Plio-Pleistocene cooling), with its descendants surviving only in the tropical parts of Asia, and in Africa.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dasychoerus arvernensis ( Croizet & Jobert, 1828 )
| Pickford, Martin & Obada, Théodor 2016 |
Sus arvernensis arvernensis
| GUERIN C. & FAURE M. & SEN S. 1998: 442 |
| GUERIN C. & FAURE M. 1985: 22 |
Sus arvernensis minor
| GUERIN C. & FAURE M. 1985: 443 |
Sus minor
| HUNERMANN K. A. 1971: 213 |
| TOBIEN H. 1952: 191 |
| TOBIEN H. 1951: 79 |
Sus provincialis var. minor Depéret, 1890: 84-88
| DEPERET C. 1890: 88 |
Aper arvernensis
| CROIZET J. B. & JOBERT A. 1828: 160 |