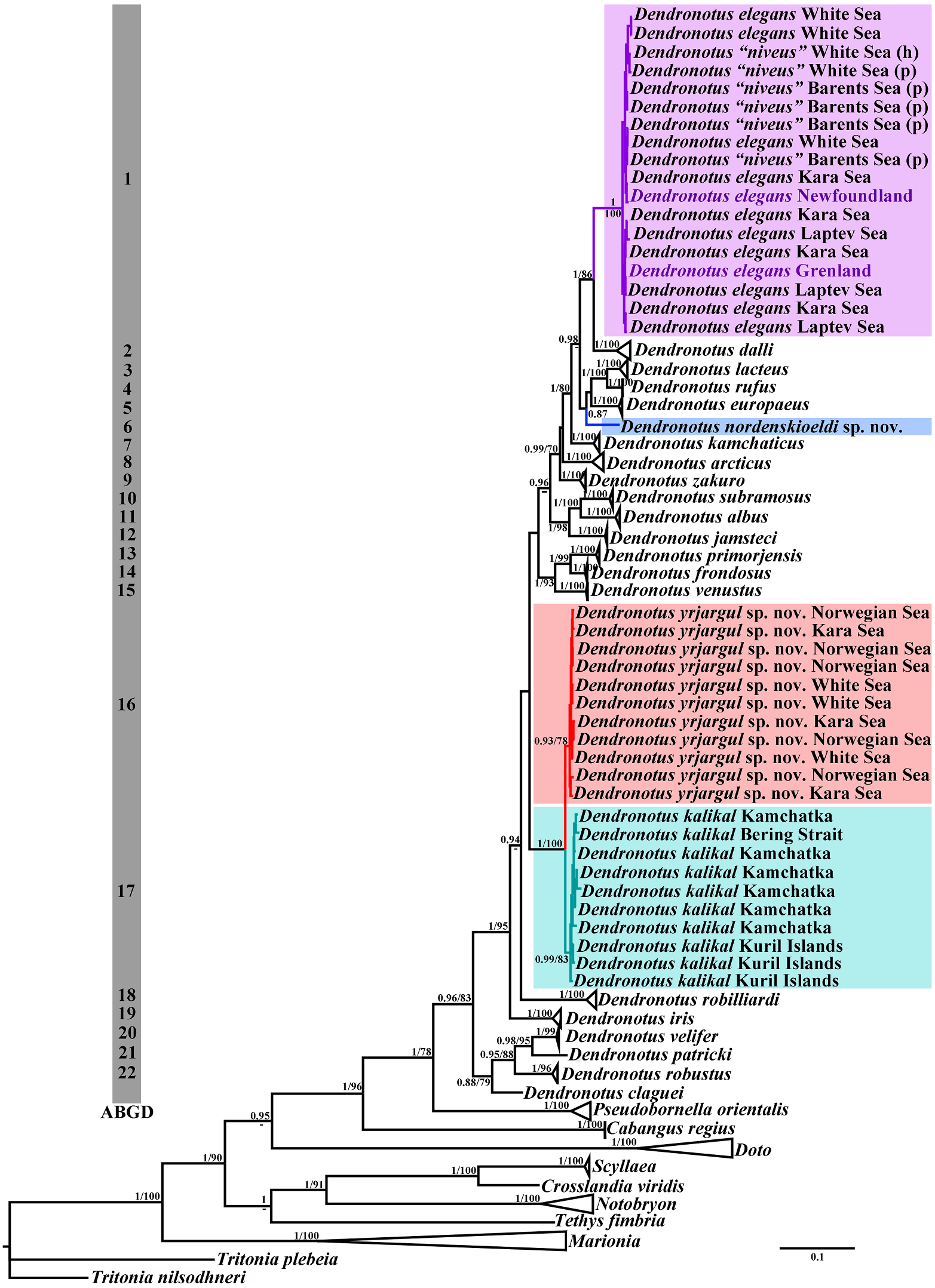
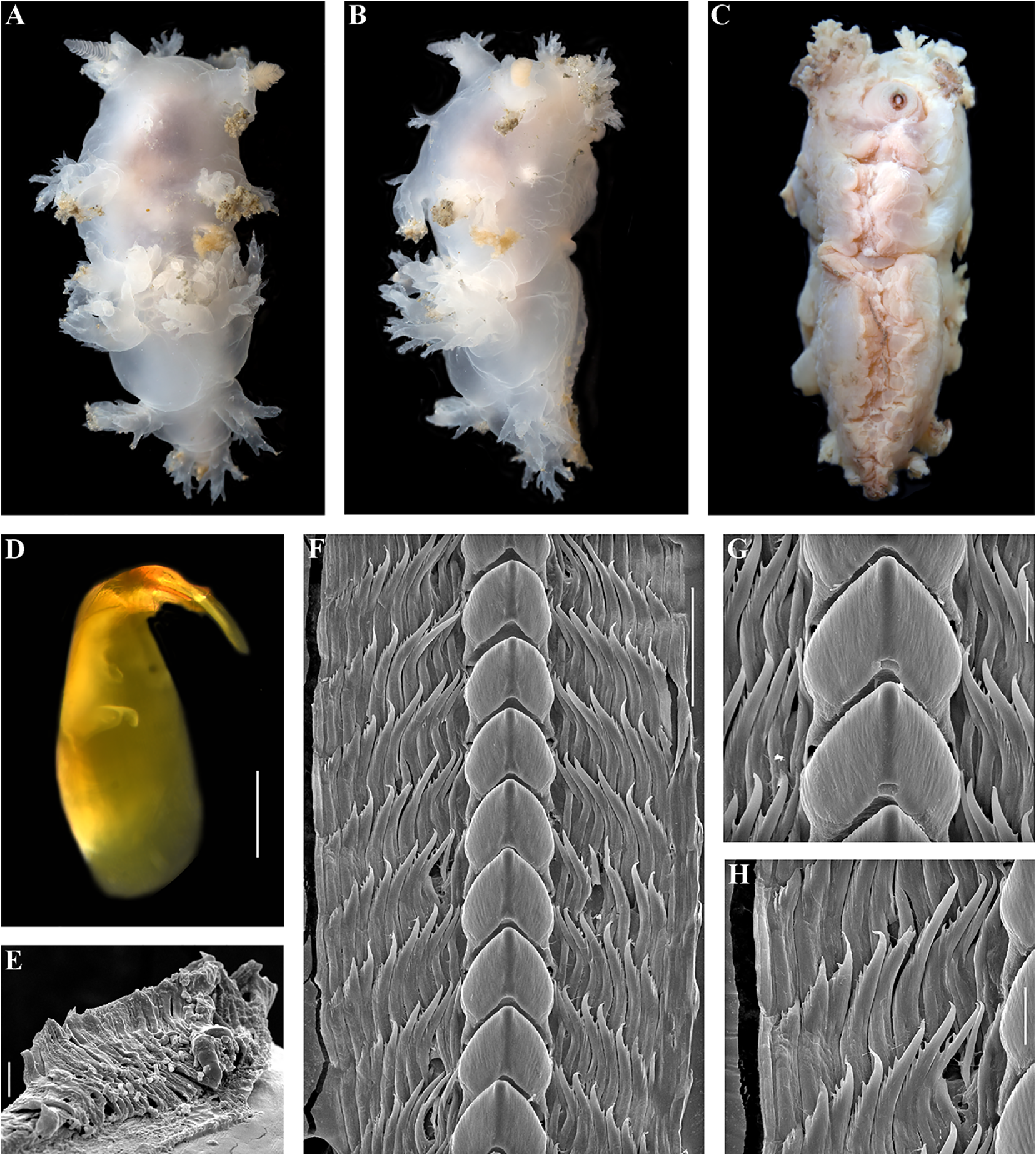
Dendronotus zakuro Martynov, Fujiwara, Bathymetry . Shallow
|
publication ID |
https://doi.org/10.1163/18759866-BJA10014 |
|
persistent identifier |
https://treatment.plazi.org/id/1E1A4C68-FFA2-FFCB-51C9-FABDFC35DE9D |
|
treatment provided by |
Felipe |
|
scientific name |
Dendronotus zakuro Martynov, Fujiwara, Bathymetry . Shallow |
| status |
|
Dendronotus zakuro Martynov, Fujiwara, Bathymetry. Shallow View in CoL waters, at depths of circa
Tsuchida, R. Nakano, N. Sanamyan, 7–20 m.
K. Sanamyan, Fletcher & Korshunova, 2020
Fig. 7 View FIGURE 7 Remarks. This species has recently been
Dendronotus zakuro Martynov, Fujiwara , described in detail (Martynov et al., 2020a).
Tsuchida, Nakano, Sanamyan, Sanamyan,
Fletcher, Korshunova, 2020a: 505–507, Figs 3 View FIGURE 3 , Genus Cabangus gen. nov.
5B. ZooBank: http:// urn:lsid:zoobank.org:act:
Extended diagnosis. Body narrow. Six to seven Type species. Dendronotus regius Pola &
pairs of branched dorsolateral appendages. Stout, 2008 Downloaded from Brill.com 12/12/2023 04:12:08 PM via Open Access. This is an open access article distributed under the terms of the CC BY 4 .0 License. https://creativecommons.org/licenses/by/4.0 /
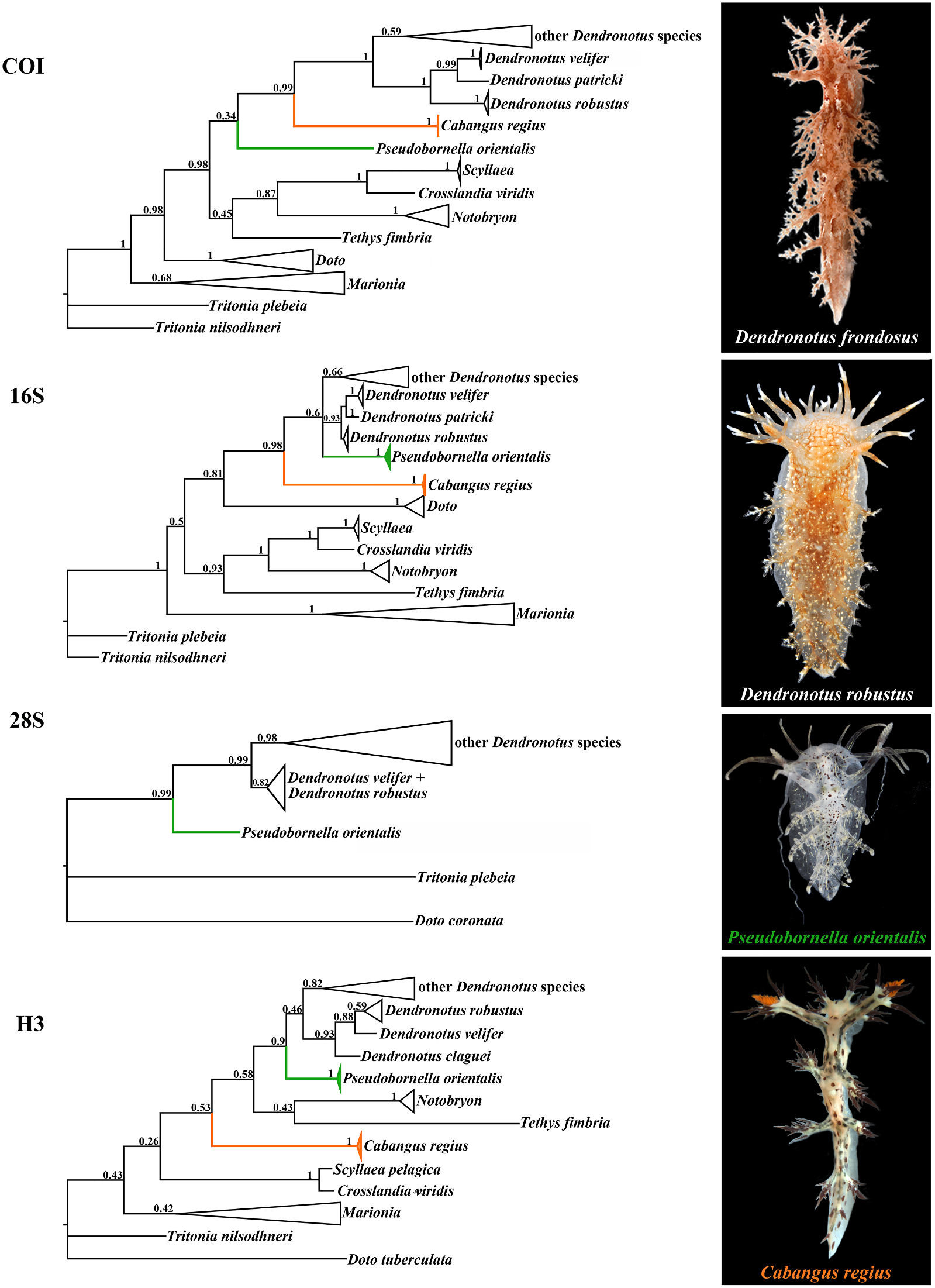
Etymology. From the Indonesian word denticle-bearing part of the central teeth is not “cabang” meaning “branch” in reference to separated from the lateral sides by a distinct this genus as “dendronotids of the tropics” shoulder.Thus the central teeth have a squarish and to respect the great contribution of base and a triangular top. This makes the the Indonesian fauna to global marine radula of both species more similar (though biodiversity (e.g., Hoeksema, 2007). not identical) to that of the family Bornellidae , whose species are also partly similar in external Diagnosis. Dorsolateral appendages with morphology. However, details of the stomach distinct tertiary branches. Oral veil with and the reproductive systems are different.
branched appendages. Radula with at least Therefore, taking molecular and morphological up to nine lateral teeth in adult specimens. evidence into account, we propose here the Central teeth with cusp integrated within new genus Cabangus gen. nov. with as type lateral denticles. Denticle-bearing part of species Dendronotus regius Pola & Stout, 2008 .
central teeth not separated from lateral sides, The holotype of the only other tropical species distinct shoulders absent, thus central teeth described so far, D. noahi , is likely a juvenile triangular in outline. Prostate represented by since it possesses only four rows of lateral teeth a thickened structure without evident alveolar and immature reproductive system. Although glands and disk. Copulative organ conical. this species is now included in the genus Cabangus gen. nov., this needs further study.
Remarks. According to the present molecular Externally, Cabangus spp. are different from the phylogenetic analysis, the tropical Dendronotus common external appearance of Dendronotus regius Pola & Stout, 2008 comes as a most basal spp. by a combination of a very narrow clade, distinct from all presently known species body and short dorsolateral appendages. By of the genera Dendronotus and Pseudobornella . these characters, Cabangus gen. nov. is also Morphologically, D. regius differs from any somewhat similar to the family Bornellidae , known species of the genus by the presence which is phylogenetically distant from the of a homogenous, fine prostate, which is Dendronotidae . Cabangus gen. nov. represents externally smooth and does not show any a basalmost clade within the Dendronotidae alveoles. Pola and Stout (2008: Fig. 4 View FIGURE 4 ) evidently and hence may retain some features of the did not indicate any alveols in the prostate common ancestors with phylogenetically more of their D. regius , but in the text they (Pola & distantly related families. Cabangus gen. nov.
Stout, 2008: 48) mentioned a “large prostate is also well supported by biogeographic data with the proximal limit being marked by a because the majority of its species inhabits closely set ring of alveolar glands”. To clear this Arctic and temperate waters,whereas Cabangus contradiction between this figure and the text gen. nov. represents a distinct tropical lineage.
in the original description, we checked several adult specimens of D. regius and confirm Cabangus noahi (Pola & Stout, 2008) comb.
that there are no externally or internally nov.
conspicous alveols, otherwise so characteristic Dendronotus noahi Pola & Stout, 2008: 55–63 ,
for a majority of true Dendronotus species. figs 6A, B.
There is another striking difference between both strictly tropical species of Dendronotus Diagnosis (original description). Body very
( D. regius plus D. noahi ) and any other narrow. Four pairs of branched dorsolateral known species of this genus, namely that the appendages Downloaded. Six appendages from Brill.com of oral 12/12 veil /2023. Four 04:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/ appendages(posterior longest) of rhinophoral stalks. Small lateral papilla of rhinophoral sheaths possibly present. Rhinophores with nine lamellae. Lip papillae not indicated in the original description. Colour semitransparent white with brownish branches of digestive gland. Masticatory processes with denticles. Radula with up to 18 rows of teeth. Central tooth quite narrow, with shallow furrows and with up to 15 distinct denticles. Up to four lateral teeth with up to eight denticles. Reproductive system reported as immature in the first description. Body length up to 4 mm (juvenile specimen).
seven denticles. Ampulla voluminous, folded. Bursa copulatrix large, pear-shaped to oval. Seminal receptaculum small placed distally at a short distance from the vaginal opening (in the original description entangled duct from ampulla was incorrectly identified as “receptaculum seminis”). Prostate non discoid, thickened, without evident alveolar glands.The vas deferens is moderate in length, penis tapered. Body length up to 15 mm.
Distribution. Tropical Indo-west Pacific.
Bathymetry. Up to 45 m deep.
Distribution. Papua New Guinea, north coast, Remarks. See under Cabangus gen. nov. outer barrier reef, Bagabag Island, Bismarck Sea. Genus Pseudobornella Baba, 1932
Type species. P. orientalis Baba, 1932 (fig. 2) Bathymetry. 30. 5 m depth.
Diagnosis. Dorsolateral appendages without Remarks. Molecular data unavailable, as well distinct (or with few very short) tertiary as additional specimens. Species is in need of branches. One of the rhinophoral stalk further study. appendages from both sides is extremely long,
usually exceeding body length. Oral veil with Cabangus regius (Pola & Stout, 2008) comb. long simple unbranched appendages. Radula nov. with very small number of lateral teeth Fig. 2 View FIGURE 2 (presently known to be no more than two rows Dendronotus regius Pola & Stout, 2008: 46 – of lateral teeth). Central teeth with protruding
54, Figs 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 . cusp distinctly separate from lateral denticles.
Prostate represents by narrow tube, without Extended diagnosis. Body very narrow. alveols and disk. Copulative organ partly Three to four pairs of branched dorsolateral flattened and with a widened subcircular appendages. Circa six appendages of oral apical part. veil. Four to five appendages (posterior ones longest) of rhinophoral stalks. Rhinophores Remarks. The genus Pseudobornella differs from with 10–11 lamellae. Lateral papilla of both Dendronotus and Cabangus gen. nov. by rhinophoral sheaths present. Lip papillae a unique combination of external and internal possibly absent. Basal colour white or yellow, characters.These characters include a very long with blue, brown and reddish patches. appendage of the rhinophoral sheath, absence Masticatory processes with denticles. Radula of distinct tertiary branches of the dorsolateral with up to 36 rows of teeth. Central tooth appendages, resulting in the general appearance with up to 20 small distinct denticles with of dorsolateral appendages of Pseudobornella as furrows. Up to nine lateral teeth with up to ctenidium-like Downloaded. Instead from, all Brill.com species of 12/12/2023 the genus 04:12:08PM
via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License.
https://creativecommons.org/licenses/by/4.0/ Dendronotus possess well-defined tertiary coupled with the molecular data (including branchesonthedorsolateralappendages,andno presenting for the first time data on COI gene species has such a exceedingly long appendages for the type species Pseudobornella orientalis ,
of the rhinophoral sheaths. There is a deep-sea figs 1, 2) show that the genus Pseudobornella species, Dendronotus claugei (see above), for represents an early offshoot of the family which unbranched dorsolateral appendages are Dendronotidae . This evolution was fuelled reported, but this species is only known from by the paedomorphic reduction of the lateral a single specimen and needs further studies. teeth and juvenilization of the central teeth According to the present phylogeny D. claugei (for criteria of paedomorphosis (Korshunova may represent a separate genus, but more data et al., 2020a; Martynov et al., 2020b). A pre-
are needed to support this. Furthermore, so far vious morphological cladistic study (Pola et no single species of the genus Dendronotus and al., 2009) placed the genus Pseudobornella Cabangus gen. nov. have smooth unbranched outside the family Dendronotidae , thus appendages in the oral veil, whereas numerous highligthing the morphological peculiarities reported Pseudobornella specimens invariably of this genus as inconsistent with those of show strong unbranched appendages of the other dendronotid genera. However, a solely oral veil. Ultimately, the radular pattern of molecular study suggested to synonymize the genus Pseudobornella differs from that in Pseudobornella with Dendronotus (Pola &
the adult stage of species of Dendronotus and Gosliner, 2010). We confirm here the validity Cabangus gen. nov. by a very small number of of the genus Pseudobornella Baba, 1932 using lateral teeth (so far reported no more than two). integrative evidences. Specimens of the type Adult specimens of the genera Dendronotus species Pseudobornella orientalis from the Sea and Cabangus gen. nov. possess at least eight of Japan (fig. 2) in the present study matched lateral teeth, and only very rarely in poorly morphologically well with those in the origknown deep-sea lineages, the number of the inal description of P. orientalis (Baba, 1932) , lateral teeth rows can reach six in number. The including the characteristic small chocotropical C. noahi was reported to have only four late-brown spots and yellow lines on the dor-
rows of lateral teeth, but only a single juvenile sal side, and the shape of the radular teeth.
specimen is known (Pola & Stout, 2008). All investigated species of the genus Dendronotus Species composition. This presently monoshowed the presence of 1–3 rows of lateral teeth specific genus only contains P.orientalis Baba , during the early stages of their ontogeny (e.g., 1932.
Martynov et al., 2020a), while only in the genus
Pseudobornella such feature became apparent in adults stage. Furthermore, the shape of the Discussion central teeth of Pseudobornella is also different from that in any adult Dendronotus and General taxonomic and biogeographic
Cabangus gen. nov. species by the presence of a overview of the family Dendronotidae :
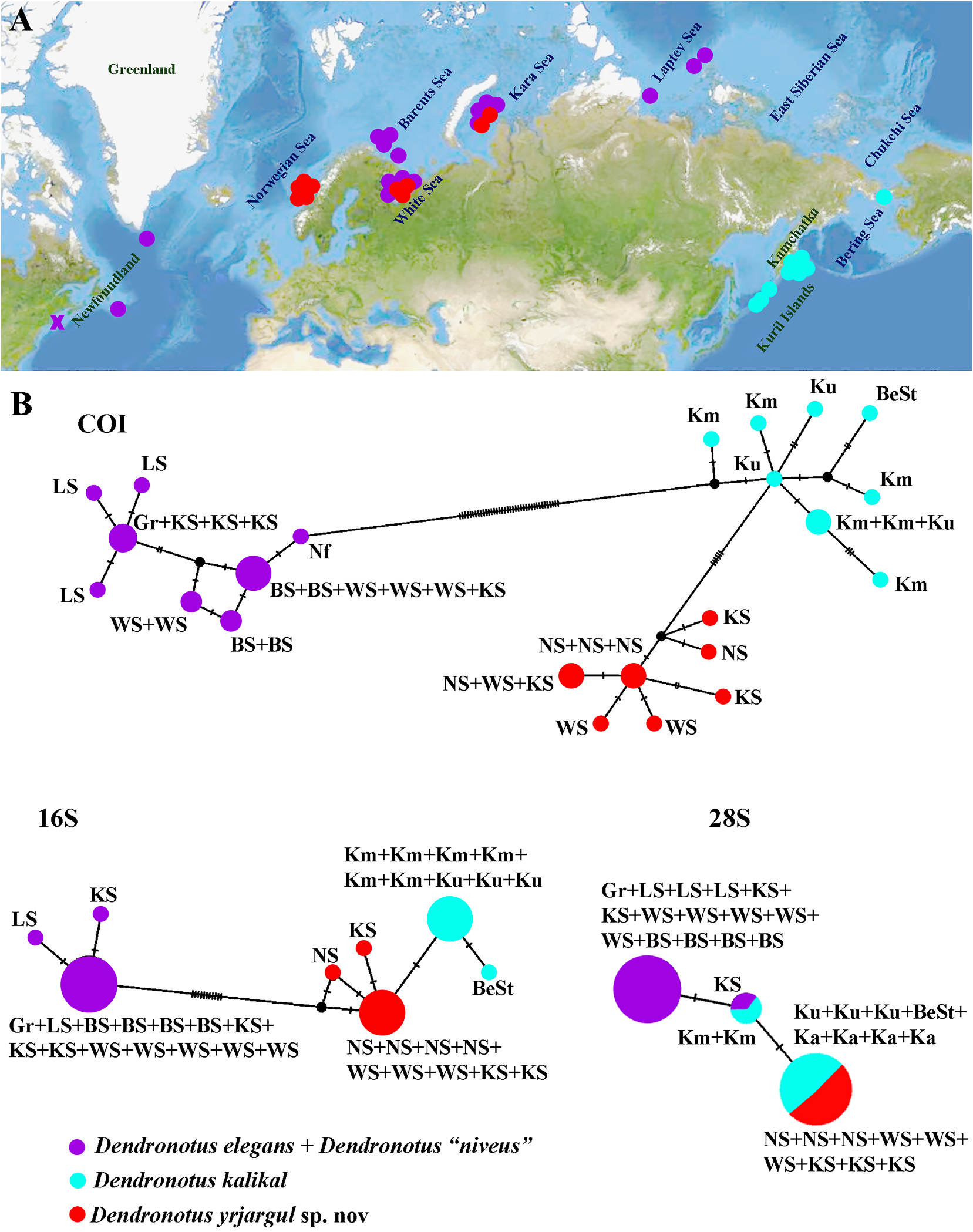
strongly protruding central cusp on the central a revolution of fine-scale defining of species teeth, which is distinctly separated from the The family Dendronotidae and its type genus lateral denticles. Dendronotus represent an emerging model ASEMimageoftheradulaof P.orientalis from for studying speciation, and ultimately for the Sea of Japan was presented in Martynov investigating general problems related to et al. (2015a: 60, fig. 5G). Radular characters the “species Downloaded concept from ”. The Brill.com total12number /12/2023 04of:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/ species belonging to the genus Dendronotus Dendronotus europaeus inhabits the north-
in restricted sense is 27 (a review of all the erneastern Atlantic in European waters only.
previously described and species consid- Dendronotus arcticus is reported only from ered valid with diagnoses is presented in the the true Arctic, e.g., Kara and Laptev seas.
Synopsis section). Though some new spe- Dendronotus lacteus occurs in northeastern cies were recognized using morphological Atlantic and Arctic with the Laptev Sea as methods (e.g., MacFarland, 1966; Robilliard, easternmost limit. Dendronotus yrjargul sp.
1970, 1972), the species composition of the nov. inhabits mostly the Arctic (with the Kara genus Dendronotus remained quite conserv- Sea as easternmost limit) and the neighbourative (Thompson & Brown, 1984; Roginskaya ing northern European regions with middle 1987, 1997). The genus Dendronotus was Norway as southernmost limit. Dendronotus represented by just about 10 recognised nordenskioeldi sp. nov. is so far only reported shallow-water species worldwide (plus one from the Arctic Laptev sea. Dendronotus deep-sea and two or three species of unclear kalikal is distributed in the northwestern status) until a considerable amount of hid- Pacific until the Bering Strait. Dendronotus den diversity was discovered recently in the kamchaticus is limited to the Northern Pacific shallow waters of both the North Pacific and only, with scattered records from the norththe North Atlantic (e.g., Korshunova et al., western and northeastern parts. Dendronotus 2017a; Martynov, 2020a). zakuro is reported from the northwestern The conservative approach in the tax- Pacific only, ranging from middle Honshu onomy of the family Dendronotidae relied to Kamchatka Peninsula. Dendronotus priconsiderably on Mayr’s (1942, 1969) polytypic morjensis is restricted merely to the Sea of species concept and allowed the presence Japan and neighbouring localities in Japan.
of broadly defined “species” distributed over Dendronotus venustus is restricted to the very large geographic distances. For example, northeastern Pacific. This considerable until about the year 2000 only D. frondosus regionalisation in a majority of the species is was recognised among the North Atlantic remarkable since all of them have planctonic narrow-bodied Dendronotus spp. (Thompson veliger larvae and may potentially disperse
& Brown, 1984). The wide geographic range of very widely. But in reality instead of a single D. frondosus at that time also encompassed “infinitely variable” species around whole the entire North Pacific up to subtropical Eurasia we have an enormously complicated regions of the middle of Honshu ( Japan) and radiation of evidently separate species. From China (e.g., Robilliard, 1970; Baba, 1993; Lin these species, so far only D. lacteus shows a et al., 1986). Later this has showed not to be maximum range from the northern European the case, and the supposedly super-polytypic seas to the Arctic. However, it also shows a and pan-geographic “species” D. frondosus considerable morphological variation comhas been split into at least 11 narrow-defined pared to other species, which may lead to this species (see below and figs 1, 7). Each of these species becoming further subdivided. A very species occupies a particular geographic broad geographic range (the North Atlantic, region. The natural range of true D. frondo- Arctic and North Pacific) was previously also sus sensu stricto is limited solely to the east- indicated for Dendronotus dalli (Robilliard, ern and western parts of the North Atlantic 1970; Roginskaya, 1987). Mutilevel data with the subarctic Barents Sea as easternmost for the putative North Atlantic and Arctic limit, while it is absent in the Arctic region. “ D. dalli Downloaded ” show instead from that Brill.com it represents a 04:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/ separate species D. elegans (as “ D. niveus ”). Notably, Robilliard (1970) noted differences in Dendronotus elegans is a predominatly Arctic the reproductive system between European species, which reaches cold water masses at D. frondosus and his NE Pacific specimens of the Canadian and US North Atlantic coasts. D. venustus that he at that time identified as Dendronotus dalli instead is a predominantly “ D. frondosus ”. At the same time, apparently North Pacific species, which reach Arctic key reproductive features (e.g., number of seas into the Bering Strait and neighbouring prostatic alveols and shape of ampulla) can waters, but it is absent in the subarctic North vary significantly, as in the cases of D. albus or Atlantic regions. D. elegans . The separate characters should be Importantly, many of these species could therefore used with considerable care to dis-
12/12/2023
be separated by a careful application of mor- tinguish particular species.
phological characters without aid of molec- All these cases clearly confirm that not ular data. The possibility of morphological only molecular data, per se, changed the separation is evident in the case of D. venustus modern landscape of biodiversity research.
from the NE Pacific. Dendronotus venustus was The fundamental changes have occurred described in considerable detail and already in the dominated paradigm of the species became delineated from the resembling D. description. A major modern feature of this frondosus in the mid-20th century (published is the general denial of huge polytypic and in MacFarland, 1966) without use of molecular pan-geographic species in favour of fine-scale techniques and scanning electron microscopy interspecific morphological differences in (SEM). SEM has been available to taxonomists combination with limited geographic ranges.
since the 1970s and its use would enable dif- Within restricted “narrow species” a polymorferentiation of some of the Dendronotus spe- phism (a basis for the polytypic concept) is cies that were described in the 21st Century. often manifested in the occurrence of parallel However, this did not happen because all these morphs, which may obscure species diversity characters could be considered as too subtle (see e.g., Korshunova et al., 2020b). Though from the perspective of the past dominant Mayr was among the major developers of the paradigm. For instance, Thompson and Brown population-based, variable, “non-typological” (1984) published an SEM image of radula of a species concept, he also admitted the possispecimen identified as D. frondosus but which ble existence of a hardly detectable diversity actually belongs to the recently described D. of “sibling species” (Mayr, 1942; Yoder et al., europaeus (Korshunova et al., 2017b) . Even 2005). Despite this, the gravity of “a morpho-
such morphologically well distinguished logically super-variable species concept” was species as D. albopunctatus (Robilliard, 1972) clearly unfavourable for seeking of fine-scale was commented as very similar to D. robus- differences. This was one of the reasons why tus (see Rudman, 2007). While data on the researchers for a long time omitted evidence multilevel diversity within Dendronotidae for morphological differences in the “ D. fron-
are currently accumulating, more and finer dosus megacomplex”. The polytypic concept cases will be discovered in the future among became popular more than five decades already restricted “species”. Nevertheless, a ago and at that time Mayr (1942) suggested majority of the recently separated species can a potential solution for how to unfold the either be differentiated by SEM radula data or apparent “chaotic species” of “typologists”
by a combination of the habitus data, radular into a polymorphic “biological” species.
data and features of the reproductive system. However this Downloaded concept from can Brill now.com be12considered /12/2023 04:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/ as rather an obstacle than an aid in the dis- not yet described diversity within the genus covery of real patterns of biodiversity. As a Dendronotus was discovered in the temperate clear evidence for that, until very recently waters of Southern Hemisphere (e.g., Burn, only drastically different species within the 2015).
genus Dendronotus have been recognised Most of the previously recognised without doubts (Robilliard, 1970; McDonald, Dendronotus species (seven out of nine, see 1983), despite uncertanties in their delimita- Robilliard, 1970, 1972) occur in the NE Pacific, tion (Robilliard, 1975). An especially remarka- while in the North Atlantic and NW Pacific ble example is the notorious problem of how only one or two species were commonly rec-
to distinguish “ D. albus ” from “ D. diversicolor ”, ognized. This caused a distorted picture of a which has persisted since Robilliard (1970). biogeographic asymmetry in the Dendronotus This problem was in a profound confusion of diversity between the North Atlantic, norththe morphological characters rather than in western Pacific and northeastern Pacific. One the absence of molecular data and was solved of the important results of the fine-scale speonly recently by the distinction of D. robil- cies definition is a considerable reduction liardi (Korshunova et al., 2016a). of the “biogeographic asymmetry” among The majority of the described species of the Dendronotus spp. between NE Pacific, NW genus Dendronotus inhabit cold and temper- Pacific, Arctic and North Atlantic localities.
ate regions of the Northern Hemisphere and The resulting numbers of shallow-water and their diversity clearly declines towards trop- shelf species in such regions, as the North ical localities. Pola and Stout (2008) assigned Atlantic and the NW Pacific, are impressive.
two tropical dendronotids first to the genus I.e., in the North Atlantic and western subarc- Dendronotus . However, the morphology of the tic regions (Barents Sea as limit) there are curmale part of the reproductive system of the rently seven species, in the true Arctic (Kara, these tropical representatives is different from Laptev and East Siberian seas) there are also those in the genus Dendronotus , and available seven species so far described and recorded, molecular data for“ D.regius ”show it to be a sep- while in the NW Pacific (Bering Strait as northarate with a more basal placement compared ernmost limit) there are eigth species and in to any other dendronotid (figs 1, 2). Therefore NE Pacific there are 10 species. In all regions “ D. regius ” has become the type species of the the number of species will likely increase furnew genus Cabangus gen. nov., which encom- ther. This analysis clearly suggests that not passes exclusively tropical dendronotids. This only geographical proximity to the rich warm corroborates well with the new general agenda water fauna (as in the case of NE Pacific) is of the multilevel organismal diversity, which important, but also that ecological and other proposes narrowly defined taxa not only on a factors (including the history of faunal forspecies level, but also at the genus and family mation) have contributed substantially to levels (Korshunova et al., 2017a, b, c; 2019a). an active speciation. The presence of at least This prevents to produce non-diagnosable seven species of Dendronotus in the Artic as huge taxa that not only lack support from mor- one of the world’s coldest regions is a clear eviphology but also from various other data, such dence for this. All these data and implications as biogeography. The taxonomic diversity of have contributed to rapid and revolutionary the family Dendronotidae undergoes a process advancements in the taxonomy of the family of understanding and more taxa in this family Dendronotidae (Thollesson, 1998; Stout et al., are expected to be discovered. For example, a 2010; Martynov Downloaded et from al. 2015 Brill.com a, c, 2020 12/12 /a 2023; Ekimova 04:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/ et al. 2015; Korshunova et al. 2016a, b, 2017a, genus Dendronotus implies the presence of 2019b; Lundin et al., 2017; present study). many taxa that are incomparably more close to D. frondosus as the type species of the genus Multilevel Dendronotidae diversity and (e.g., D. primorjensis , D. venustus ) than to the diFferent degrees of evolutionary separation much different wide-bodied D. robustus (and
The majority of dendronotids are putatively its closely related species, e.g., D. velifer , D.
united under the genus Dendronotus , which bathyvela ). Therefore, although a direct comrepresents a morphologically heterogenous parison between D. robustus sensus strictus assemblage of several distinct clades with a and D. frondosus sensus strictus is possible, significant molecular divergence (see figs 1, 2; this is not taxonomically meaningful any- Synopsis; Appendix, table A2). The case of the more considering the presence of such hardly family Dendronotidae is therefore particularly distinguishable complexes. The similarities illustrative of multilevel differences among between the “ Dendronotus robustus complex” apparently taxonomically equally recognized and the “ D. frondosus megacomplex” are thus “species”. These apparently natural units are similarities at genus or higher level.This needs not equal by their morphological and molecu- to be adressed in a further study.
lar properties, and broader, ontogenetic prop- The common formulation of a procedure erties. Hence, the term “species” is not equal to find differences within such multilevel over large phylogenetic distances but also organismal diversity, and hence the degree of among relatively small levels, such as genus separation between different groups of bio-
and family. To make taxonomy consistent logical organisms is a “species delimitation”
with the multilevel diversity among the fam- despite the species concept itself having no ily Dendronotidae , we separate a new genus universal fundamental agreement even in Cabangus gen. nov. and confirm a separate recent publications (Stanton et al., 2019). In status of the genus Pseudobornella . the present study we found a remarkable case Notably, even among the “core species” when the mean COI difference of a group is that is still constitute the genus Dendronotus slightly above 2% (normally indicative of
(fig. 1) our present study demonstrates various a single species) but when morphological, degrees of separation from species in a state of ontogenetic and biogeographic evidence being morphologically well distinguished but are examined the two Dendronotus groups molecularly low delineated (the D. yrjargul deserve to be taxonomically marked as dis-
sp. nov. – D. kalikal pair) to morphologically tinct species, namely D. kalikal and D. yrjarsubtle but molecularly well distinguished gul sp. nov. (figs 1–4, 6). At the same time species ( D. frondosus – D. primorjensis , D. dal- D. nordenskioeldi sp. nov., despite its habitus li– D. elegans , D. lacteus – D. europaeus and similarity with D. lacteus , differs substantially D. nordenskioeldi sp. nov.) (figs. 1–8). We can from that species by its central teeth with unambiguously present distinguishing char- very weak denticles devoid of rib-like strucacters only when these apparent groups tures (fig. 5) and intriguingly, (fig. 1) it demoncurrently known as species really belong to strates significant molecular distance from sufficiently distantly related subclades, as in all know species of the genus Dendronotus easily recognizable species such as D. iris or (Appendix, table A2). Despite the lack of D. subramosus , or D. frondosus and D. robus- consensus about the meaning of “species” we tus (figs 1, 7). A very complicated mutilevel can observe some “groups of genetically and morphological and molecular diversity of the ecologically Downloaded similar individuals from Brill.com” (12 e./ g 12.,/2023 Shapiro04:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/ et al., 2016) in nature, even though there are of live specimens. Our warm gratitude goes to no clear criteria about how to separate one Karin Fletcher for improvement of English in similar group from another similar group. an earlier version of the manuscript and for
A conclusion was reached that the “popula- providing us with the wonderful pictures of tion-to-species” level represents a continuum D. rufus and D. venustus . Electron Microscopy (Mallet et al., 2009; Coates et al., 2018). Using Laboratory MSU is thanked for support these numerous available data on different with electron microscopy. Reviewers are groups and our present data, we therefore dis- thanked for providing comments. The study agree with the most recent suggestion to save was supported by the Norwegian Taxonomy “species” as a universal concept using a “weak Initiative project #sneglebuss Barents Sea (19- realism” approach (Reydon & Kunz, 2019). 18_70184240). The work of AM was supported Because even in its weaker form, “species” as by the research project of MSU Zoological a reality still implies the universal application Museum (AAAA-A16-116021660077-3). The of this concept despite that it encompasses work of TK was conducted under the IDB RAS groups that have different degrees of separa- Government basic research program in 2020 tion, and hence different morphological and № 0108-2019-0002.
molecular properties. The big discrepance between “species” or other taxon as a systematic unit and “species/taxon” as a natu- References ral entity still persists (e.g., Ereshefsky, 1998;
Zachos, 2018). The recently proposed con- Agarwal, M. (2017) First record of Dendronotus cept of the multilevel organismal diversity orientalis (Baba, 1932) ( Nudibranchia : Den- (Korshunova et al., 2017b, 2019a) is helping dronotidae) in the temperate Eastern Pacific.
to reduce this discrepance and make a tran- BioInvasions Rec., 6, 135–138.
sit from the previous paradigm of “polytypic Alder, J. & Hancock, A. (1842) Descriptions of species”, by placing the organismal diversity several new species of nudibranchous mollusca in a broad ontogenetic framework, as a fine- found on the coast of Northumberland. Ann.
scale complex of the morphological, genetic Mag. Nat. Hist., 9, 31–36.
and epigenetic patterns and processes. Ascanius, P. (1774) Beskrivelse over en Norske sneppe og et sodyr. Det Kongelige Norske Videnskabelige Selskabs Skrifter Trondhejm, Acknowledgements 5, 153–158.
Baba, K. (1932) Pseudobornella orientalis , nov. gen.
Olga Zimina is warmly thanked for pro- et sp. from Japan. Annot. Zoolog. Japon., 13, 4, viding specimens from Arctic. The team of 369–376.
Gulen Dive Center (Christian Skauge, Ørjan Baba, K. (1949) Opisthobranchia of Sagami Bay Sandnes, Monica Bakkeli, and Guido Schmitz) collected by His Majesty The Emperor of Japan.
are generously thanked for their help during Iwanami Shoten, Tokyo.
fieldwork in Norway, as these Norwegian spec- Baba, K. (1993) A northern species of Dendronotus imens were used for comparative purposes (Mollusca: Nudibranchia : Dendronotidae ) from in this study. We are thankful to Yoshihiro Sado Island, Sea of Japan. Rep. Sado Mar. Biol.
Fujiwara, Kazunori Hasegawa, Doug Miller, Stat., 23, 29–33.
Karen Sanamyan, Nadezhda Sanamyan, and Bakken, T., Hårsaker, K. & Daverdin, M. (2020) Olga Zimina for providing some photographs Marine Downloaded invertebrate from collection Brill.com NTNU 12/12/2023 University 04:12:08 PM via Open Access. This is an open access article distributed under the terms of the CC BY 4 .0 License. https://creativecommons.org/licenses/by/4.0/ Museum.Version 1.414. NTNU University Museum. Colgan, N. (1914) The opisthobranch fauna of the Occurrence dataset https://doi.org/10.15468/ shores and shallow waters of County Dublin. Ir .
ddbs14 accessed via GBIF.org on 2020-02-25. Nat. J., 23 (8/9), 161–204.
Becher, E. (1886) Mollusken von Jan Mayen, Cooper, J. (1863) On new or rare Mollusca gesammelt von Dr. F. Fischer, Arzt der inhabiting the coast of California. No. II. Proc.
österreichishcen Expedition auf Jan Mayen. Calif. Acad. Nat. Sci., 3, 56–60.
Die internationale Polarforschung, 1882/83, 3, Couthouy, J. (1838) Descriptions of new species
1–16. of Mollusca and shells, and remarks on several Behrens, D. & Hermosillo, A. (2005) Eastern Pacific Polypi found in Massachusetts Bay. Boston J .
nudibranchs, a guide to the opisthobranchs from Nat. Hist., 2, 53–111.
Alaska to Central America . Sea Challengers, Crossman, C., Taylor, E., & Barrett-Lennard, L .
Monterey. (2016) Hybridization in the Cetacea: widespread Behrens, D. (1980) Pacific Coast Nudibranchs, a occurrence and associated morphological, Guide to the Opisthobranchs of the Northeastern behavioral, and ecological factors. Ecol. Evol., 6, Pacific. Sea Challengers, Los Osos, CA. 1293–1303.
Bergh, R. (1863) Campaspe pusilla , en ny Darwin, C. (1859) On the Origin of Species by Means Slaegstform af Dendronotidernes Gruppe, of Natural Selection. John Murray, London.
samt Bemaerkninger om Dotidernes Familie. de Queiroz, K. (2006) Ernst Mayr and the modern Naturhist. Tidsskr. Stiftet af Henrik Kroyer, 1, concept of species. Proc. Nat. Acad. Sci. USA, 471–483. 102, 6600–6607.
Bergh, R. (1879) On the nudibranchiate gasteropod de Queiroz, K. (2007) Species concepts and mollusca of the North Pacific Ocean, with species delimitation. Systematic Biology, 56, special reference to those of Alaska. Proc. Acad. 879–886.
Nat. Sci. Philadelphia, 31, 71–132. Ekimova, I., Korshunova, T., Schepetov, D., Bergh, R. (1886) Die Nudibranchien. Gesammelt Neretina, T., Sanamyan, N. & Martynov, A.
wärhrend der fahrten des “Willem-Barents” in (2015) Integrative systematics of northern and das Nördliche Eismeer. Bijdr. Dierk., 13, 1–37, pls. Arctic nudibranchs of the genus Dendronotus
1–3. (Mollusca, Gastropoda), with descriptions Bergh, R. (1904) Malacologische Untersuchungen. of three new species. Zool. J. Linn. Soc., 173, Reisen im Archipel der Philippinen von Dr. Carl 841–886.
Gottfried Semper, 9, 1–56. Ekimova, I., Schepetov, D., Chichvarkhina, O. & Bickford, D., Lohman, D.J., Sodhi, N.S., Ng, Chichvarkhin, A. (2016) Nudibranch molluscs P.K.L., Meier, R., Winker, K. & Das, I. (2006) of the genus Dendronotus Alder et Hancock, Cryptic species as a window on diversity and 1845 (Heterobranchia: Dendronotina) from conservation. Trends Ecol. Evol, 22, 148–155. Northwestern Sea of Japan with description of a Bleakney, J. (1996) Sea slugs of Atlantic Canada and new species. Invertebr. Zool., 13, 15–42.
the Gulf of Maine. Nimbus Publishing & The Eldredge, N. & Gould, S.J. (1972) Punctuated
Nova Scotia Museum, Halifax. equilibria: an alternative to phyletic gradualism.
Burn, R. (2015) Nudibranchs and Related Molluscs. In Schopf T.J.M. (Ed.) Models in Paleobiology, Museum Victoria, Melbourne. pp. 82–115. Freeman Cooper, San Francisco.
Coates, D., Byrne, M. & Moritz, C. (2018) Genetic Eliot, C. (1910) A Monograph of the British diversity and conservation units: dealing with Nudibranchiate Mollusca: with Figures of the the species-population continuum in the age of Species. pt. VIII (supplementary). Ray Society, genomics. Front. Ecol. Evol., 6, 165. London. Downloaded from Brill.com 12/12/2023 04:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/
Ereshefsky, M. (1998) Species pluralism and anti- Katoh, K., Misawa, K., Kuma, K. & Miyata, T. (2002)
realism. Philos. Sci., 65, 103–120. MAFFT: a novel method for rapid multiple
Evertsen, J. & Bakken, T. (2005) Nudibranch sequence alignment based on fast Fourier diversity ( Gastropoda, Heterobranchia) along transform. Nucleic Acids Res., 30, 3059–3066.
the coast of Norway. Fauna Norvegica, 25, 1–37. Kijewski, T., Zbawicka, M., Väinölä, R. & Wenne,
Farmer WM. 1980. Sea-slug Gastropods. W.M. R. (2006) Introgression and mitochondrial
Farmer Enterprises, Tempe, AZ. DNA heteroplasmy in the Baltic populations
Fraïsse, C., Belkhir, K., Welch, J. & Bierne, N. of mussels Mytilus trossulus and M. edulis. Mar.
(2016) Local interspecies introgression is the Biol., 149, 1371–1385.
main cause of extreme levels of intraspecific Knipowitsch, N. (1902) Zoologische ergebnisse differentiation in mussels. Mol. Ecol. Evol., 25, der russischen expeditionen nach Spitzbergen,
269–286. Mollusca und Brachiopoda. Ann. Mus. Zool.
Friele, H. (1879) Catalog der auf der norwegischen Acad. Imp. Sci. St. Pétersbourg, 7, 355–459.
Nordmeerexpedition bei Sptizbergen Korshunova, T., Fletcher, K., Picton, B., Lundin,
gefundenen Mollusken. Jahrb. Deutsch. K., Kashio, S., Sanamyan, N., Sanamyan, K.,
Malakozool. Gesellsch., 6, 264–286. Padula, V., Schrodl, M. & Martynov A. (2020a)
Gmelin, J. (1791) In: Linnaeus (Ed.) Systema The Emperor Cadlina , hidden diversity and gill
Naturae, per regna tria naturae. 13, 1, 6, 3103– cavity evolution: new insights for the taxonomy
3107, 3147–3148. and phylogeny of dorid nudibranchs (Mollusca:
González, A., Thompson, P. & Loreau, M. (2018) Gastropoda). Zool. J. Linn. Soc., 189, 762–827.
Spatial ecological networks: planning for Korshunova, T., Malmberg, K., Prkić, J., Petani, A.,
sustainability in the long-term. Curr. Opin. Fletcher, K., Lundin, K. & Martynov, A. (2020b)
Environ. Sust., 29, 187–197. Fine-scale species delimitation: speciation in
Harrison, R. & Larson, E. (2014) Hybridization, process and periodic patterns in nudibranch introgression, and the nature of species diversity. ZooKeys, 917, 15–50.
boundaries. J. Hered., 105, 795–809. Korshunova, T., Martynov, A. & Picton, B. (2017c)
Hoeksema, B.W. (2007) Delineation of the Ontogeny as an important part of integrative
Indo-Malayan Centre of Maximum Marine taxonomy in tergipedid aeolidaceans
Biodiversity: The Coral Triangle. In: Renema ( Gastropoda: Nudibranchia ) with a description
W. (ed.) Biogeography, Time and Place: of a new genus and species from the Barents
Distributions, Barriers and Islands, pp 117–178. Sea. Zootaxa, 4324, 1–22.
Springer, Dordrecht. Korshunova, T., Martynov, A., Bakken, T. & Picton,
ICZN. (1999) International Code of Zoological B. (2017b) External diversity is restrained
Nomenclature. The International Trust for by internal conservatism: New nudibranch
Zoological Nomenclature, London. mollusc contributes to the cryptic species
Jančúchová-Lásková,J.,Landová,E.&Frynta,D.(2015) problem. Zool. Scr., 46, 683–692.
Experimental crossing of two distinct species of Korshunova, T., Martynov, A., Bakken, T., Evertsen,
leopard geckos, Eublepharis angramainyu and J., Fletcher, K., Mudianta, I., Saito, H., Lundin, K.,
E. macularius: Viability, fertility and phenotypic Schrödl, M. & Picton, B. (2017a) Polyphyly of the variation of the hybrids. PLoS ONE, 10, e0143630. traditional family Flabellinidae affects a major
Johnson, R.F. (2010) Breaking family ties: group of Nudibranchia : aeolidacean taxonomic taxon sampling and molecular phylogeny reassessment with descriptions of several of chromodorid nudibranchs (Mollusca, new families, genera, and species (Mollusca,
Gastropoda). Zool. Scr., 40, 137–157. Gastropoda Downloaded). ZooKeys from Brill, 717.com, 1–139 12./12/2023 04:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/
Korshunova, T., Nakano, R., Fletcher, K., Sanamyan, museum collections and modern systematics:
N. & Martynov, A. (2019b) First molecular a relict population of the Arctic nudibranch confirmation of the presence of Dendronotus Dendronotus velifer G.O. Sars, 1878 in a Swedish primorjensis Martynov,Sanamyan&Korshunova , fjord. Contrib. Zool., 86, 303–318.
2015 in Japan and new distributional records MacFarland, F.M. (1966) Studies of of Dendronotus species in the North Pacic opisthobranchiate mollusks of the Pacific coast ( Nudibranchia : Dendronotidae ). Venus, 77, 1–14. of North America. Mem.Calif.Acad.Sci., 6, 1–546.
Korshunova, T., Picton, B., Furfaro, G., Mariottini, Mallet, J. (2005) Hybridization as an invasion of
P., Pontes, M., Prkić, J., Fletcher, K., Malmberg, the genome. Trends Ecol. Evol., 20, 229–237.
K., Lundin, K. & Martynov, A. (2019a) Multilevel Mallet, J., Meyer, A., Nosil, P., & Feder, J. (2009) fine-scale diversity challenges the ‘cryptic Space, sympatry and speciation. J. Evol. Biol., 22, species’ concept. Sci. Rep., 9, 6732. 2332–2341.
Korshunova, T., Sanamyan, N. & Martynov, Marcus, Ev. & Marcus, Er. (1967) American
A. (2016b) Morphological and molecular opisthobranchmollusksPartI,TropicalAmerican evidence indicate Dendronotus primorjensis is opisthobranchs, Part II, Opisthobranchs from the a valid species that has priority over D. dudkai Gulf of California. Stud. Trop. Oceanogr., Miami, ( Nudibranchia ). ZooKeys, 634, 15–28. 6, 1–256.
Korshunova, T., Sanamyan, N., Zimina, O., Fletcher, Martinsson, S. & Erséus, C. (2018) Cryptic
K. & Martynov, A. (2016a) Two new species and diversity in supposedly species-poor genera a remarkable record of the genus Dendronotus of Enchytraeidae (Annelida: Clitellata). Zool. J.
from the North Pacific and Arctic oceans Linn. Soc., 183, 749–762.
( Nudibranchia ). ZooKeys, 630, 19–42. Martynov, A., Fujiwara, Y., Tsuchida, S., Nakano, Kröyer, H.N. (1847) Zoologisches. I. Aus R., Sanamyan, N., Sanamyan, K., Fletcher, K. & Königlichen Musäen. Amtlicher Bericht über Korshunova, T. (2020a) Three new species of die Versammlung Deutscher Naturforscher und the genus Dendronotus from Japan and Russia
Ärzte in Kiel, 24, 109–115. (Mollusca, Nudibranchia ). Zootaxa, 4747,495–513.
Kumar, S., Stecher, G., &Tamura, K. (2016) MEGA7: Martynov, A., Ishida, Y., Irimura, S., Tajiri, R., Molecular evolutionary genetics analysis O’Hara, T. & Fujita, T. (2015b) When ontogeny version 7.0 for bigger datasets. Mol. Biol. Evol., matters: a new Japanese species of brittle star
33, 1870–1874. illustrates the importance of considering both Lafont, A. (1871) Note pour servir a la faune de la adult and juvenile characters in taxonomic Gironde contenant la liste des animaux marins practice. PLOS ONE, 10, e0139463.
dont la présence a été constatée a Arcachon Martynov, A., Lundin, K., Picton, B., Fletcher, pendant les années 1869–70. Act. Soc. Linn. K., Malmberg, K. & Korshunova, T. (2020b) Bordeaux, 28, 237–280. Multiple paedomorphic lineages of soft- Lamb, A. & Hanby, B.P. (2005) Marine Life of the substrate burrowing invertebrates: parallels Pacific Northwest, a Photographic Encyclopedia in the origin of Xenocratena and Xenoturbella.
of Invertebrates, Seaweeds and Selected Fishes, PLoS ONE, 15, e0227173.
Harbour Publishing, Madeira Park, BC. Martynov, A., Sanamyan, N. & Korshunova ,
Lin, G., Zhang, F. & Ma, S. (1986) Opisthobranchia. T. (2015a) New data on the opisthobranch
In: Chinese animal atlas: Mollusca, volume 3. molluscs ( Gastropoda: Opisthobranchia) of Science Publishing Company, China. waters of Commander Islands and Far-Eastern Lundin, K., Korshunova, T., Malmberg, K. & seas of Russia. Conservation of biodiversity of Martynov, A. (2017) Intersection of historical Kamchatka Downloaded and coastal from Brill waters.com–12Proceedings /12/2023 04:12:08 PM via Open Access. This is an open access article distributed under the terms of the CC BY 4 .0 License. https://creativecommons.org/licenses/by/4.0/ of the XV International Scientific Conference Nakano, R. (2018) Field Guide to Sea Slugs and Petropavlovsk-Kamchatsky, pp. 55–69. Kamchat Nudibranchs of Japan. Bun-ichi Co., Ltd., Press , Petropavlovsk-Kamchatsky. Tokyo .
Martynov, A., Sanamyan. N. & Korshunova, NMNH (National Museum of Natural History,
T. (2015c) Review of the opisthobranch Smithsonian Institution) (2020) Dendronotus mollusc fauna of Russian Far Eastern seas: elegans Verrill, 1880 Holotype USNM Pleurobranchomorpha, Doridida and 842116. Accesssed through http://n 2t.net/ Nudibranchia . Bull. Kamchatka State Technol. a r k: / 6 5 6 6 5 /3 9 f c 3a c7 d -5 c a a -4 7 a e -b 0 c b - Univ., 34, 62–87. 60371b864c4d
Martynov, A.V. & Korshunova, T.A. (2011) Nordsieck, F. (1972) Die europäischen Opisthobranch Molluscs of the Seas of Russia. Meeresschnecken (Opisthobranchia mit
A Colour Guide to their Taxonomy and Biology. Pyramidellidae; Rissoacea), Vom Eismeer bis Fiton, Moscow. Kapverden, Mittelmeer und Schwarzes Meer.
Mayer, F. & von Helversen, O. (2001) Cryptic Gustav Fischer Verlag, Stuttgart.
diversity in European bats. Proc. R. Soc. B, 268, Nylander, J., Ronquist, F., Huelsenbeck, J. & Nieves- 1825–1832. Aldrey, J. (2004) Bayesian phylogenetic analysis Mayr, E. (1942) Systematics and the Origin of Species of combined data. Syst. Biol., 53, 47–67.
Columbia University Press, New York. O’Donoghue, C. (1921) Nudibranchiate Mollusca Mayr, E. (1969) Principles of Systematic Zoology. from the Vancouver Island region. Trans. R. Can.
McGraw-Hill, New York. Inst., 13, 147–209.
Mayr, E. (1982) The Growth of Biological Thought: O’Donoghue, C.(1922) Notes on the nudibranchiate Diversity, Evolution, and Inheritance. Harvard Mollusca from the Vancouver Island region. III.
University Press, Harvard, MA. Records of species and distribution. Trans. R.
Mcdonald, G. (1983) A review of the nudibranchs Can. Inst., 14, 145–167.
of the California coast. Malacologia, 24, 114–276. Odhner, N.(1907)Northern and Arctic invertebrates McDonald, G. (2009) Nudibranch Systematic Index. in the collection of the Swedish state museum.
Second edition. Long Marine Laboratory, UC Det Kongelige Norske Videnskabers Selskabs Santa Cruz. Skrifter, 41, 1–114.
McDonald, J., Koehn, R. (1988) The mussels Odhner,N.(1936)NudibranchiaDendronotacea—A Mytilus galloprovincialis and M. trossulus on revision of the system. Mém. Mus. R. Hist. Nat.
the Pacific coast of North America. Mar. Biol., Belg. (Ser. 2), 3, 1057–1128.
99, 111–118. Odhner, N. (1939) Opisthobranchiate Mollusca MØller, H.P.C. (1842) Index Molluscorum from the western and northern coasts of Groenlandiae.C.A. Reitzelii, Hafniae. Norway. Det Kongelige Norske Videnskabernes Mörch, O. (1875) Prodromus Faunae Molluscorum Selskabs Skrifter, 1, 1–93.
Groenlandiae,. In: Rupert Jones, T. (Ed.) Manual Platania, L., Vodă, R., Dincă, V., Talavera, G., Vila, R.
of the Natural History, Geology, and Physics of & Dapporto, L. (2020) Integrative analyses on Greenland and the Neighbouring Regions, 2014 Western Palearctic Lasiommata reveal a mosaic Edition, pp. 124–135. Cambride University Press, of nascent butterfly species. J. Zool. Syst. Evol.
Cambridge. Res., doi:10.1111/jzs.12356
Müller, O.F. (1776) Zoologiae Danicae. Prodromus Pola, M. & Stout, C. (2008) Description of the seu animalium Daniae et Norvegiae ingenarum first two tropical Indo-Pacific species of characteres, nomina, et synonyma imprimis Dendronotus ( Gastropoda: Nudibranchia ) popularium. Hallageriis, Havniae. with new Downloaded data from of the Brill poorly.com 12known /12/2023species 04:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/ Dendronotus gracilis Baba, 1949 . Zootaxa, 1960, Roginskaya, I.S. (1990) Additional data on the food 45–66. and feeding of Dendronotus robustus Verrill ,
Pola, M., & Gosliner, T.M.(2010) The first molecular 1879 ( Nudibranchia : Dendronotidae ) from phylogeny of cladobranchian opisthobranchs Dvinsky Bay of the White Sea. In: Kuznetsov (Mollusca, Gastropoda, Nudibranchia ). Mol. A.P. (Eds) Feeding and Bioenergetics of Marine
Phyl. Evol., 56, 931–941. Bottom Invertebrates, pp. 93–110, 158. P.P.
Pola,M.,Rudman,W.&Gosliner,T.(2009)Systematics Shirshov Institute of Oceanology, Academy of and preliminary phylogeny of Bornellidae Sciences of the U.S. S.R., Moscow.
(Mollusca: Nudibranchia : Dendronotina) based Roginskaya, I.S. (1997) Predation by a nudibranch on morphological characters with description of Dendronotus robustus from the Sea of Japan on four new species. Zootaxa, 1975, 1–57. Oweniid polychaetes. Opisthobranch Newsl., Potkamp, G. & Fransen, C.H.J.M. (2019) Speciation 23, 21–23.
with gene flow in marine systems. Contrib. Zool., Ronquist, F., Teslenko, M., van der Mark, P., Ayres,
88, 133–172. D.L., Darling, A., Höhna, S., Larget, B., Liu, Pruvot-Fol, A. (1954) Mollusques Opisthobranches. L., Suchard, M.A. & Huelsenbeck, J.P. (2012) Faune de France, 58, 1–460. MrBayes 3.2: Efficient Bayesian phylogenetic Puillandre, N., Lambert, A., Brouillet, S. & Achaz, inference and model choice across a large
G. (2012) ABGD, Automatic Barcode Gap model space. Syst. Biol., 61, 539–542.
Discovery for primary species delimitation. Rudman, W. (2007) Comment on Dendronotus
Mol. Ecol., 21, 1864–1877. robustus from Spitzbergen, Nth Atlantic by Ratnasingham, S. & Hebert, P. (2013) A DNA- Erling Svensen. Message in Sea Slug Forum.
Based Registry for All Animal Species: Australian Museum, Sydney. Available from
The Barcode Index Number (BIN) System. http://www.seaslugforum.net/find/19087
PLoS ONE, 8, e66213. doi:10.1371/journal. Rybakova, E., Galkin, S., Gebruk, A., Sanamyan, pone.0066213 N. & Martynov, A. (2020) Vertical distribution Reydon, T. & Kunz, W. (2019) Species as natural of megafauna on the Bering Sea slope based entities, instrumental units and ranked on ROV survey., PeerJ, 8, e8628. doi 10.7717/
taxa: new perspectives on the grouping and peerj.8628
ranking problems. Biol. J. Linn. Soc., 126, Sars, G. (1878) Bidrag til kundskaben om Norges 623–636. Arktiske fauna. I. Mollusca regionis Arcticae Robilliard, G. (1970) The systematics and some Norvegiae,oversigtoverdeiNorgesArktiskeregion aspects of the ecology of the genus Dendronotus . forekommende. BlØddyr. Universitetsprogram, Veliger, 12, 433–479. Christiania.
Robilliard, G. (1972) A new species of Dendronotus Shapiro, B.J., Leducq, J. -B. & Mallet, J. (2016) What from the northeastern Pacific with notes on is speciation?. PLoS Genet., 12, e1005860.
Dendronotus nanus and Dendronotus robustus Shaw, G. (1991) Chemotaxis and lunge-feeding (Mollusca: Opisthobranchia). Can. J. Zool., 50, behaviour of Dendronotus iris (Mollusca, 421–432. Opisthobranchia). Can. J. Zool., 69, 2805–2810.
Robilliard, G. (1975) The nudibranch Dendronotus Stamatakis, A., Hoover, P. & Rougemont, J. (2008) frondosus – one species or four? Festivus, 6, Rapid bootstrap algorithm for the RAxML web- 44–47. servers. Syst. Biol., 75, 758–771.
Roginskaya, I.S. (1987) Order Nudibranchia Stanton, D., Frandsen, P., Waples, R., Heller, R., Blainville, 1814 . In: Molluscs of the White Sea. Russo, I., Orozco-terWengel, P., Pedersen, C.,
Keys to the Fauna of USSR, 151, 155–201. Siegismund Downloaded, H. & Bruford from Brill, M.com. (2019 12/)12 More /2023grist 04:12:08PM via Open Access. This is an open access article distributed under the terms of the CC BY 4.0 License. https://creativecommons.org/licenses/by/4.0/
for the mill? Species delimitation in the genomic Gastropoda: Heterobranchia) with the era and its implications for conservation. description of a new species. J. Mar. Biol. Ass.
Conserv. Genet., 20, 101–113. U.K., 97, 303–319.
Stout, C., Pola, M. & Valdés, Á. (2010) Phylogenetic Verril, A. (1880) Notice of recent additions to the analysis of Dendronotus nudibranchs with marine Invertebrata, of the northeastern coast emphasis on northeastern Pacific species. J. of America, with descriptions of new genera
Mollus. Stud., 76, 367–375. and species and critical remarks on others.
Stout, C., Wilson, N. & Valdés, Á. (2011) A new Part II.—Mollusca, with notes on Annelida, species of deep-sea Dendronotus Alder & Echinodermata , etc., collected by the United
Hancock (Mollusca: Nudibranchia ) from States Fish Commission. Proc. US Nat. Hist.
California, with an expanded phylogeny of the Mus., 3, 356–405.
genus. Invertebr. Syst., 25, 60–69. Verrill, A. (1870) Contributions to zoology from the
Swennen, C. (1961) Data on distribution, Museum of Yale College. No.8.—Descriptions reproduction and ecology of the nudibranchiate of some New England Nudibranchiata. Am. J.
molluscs occurring in the Netherlands. Neth. J. Sci. Arts (Ser. 2), 50, 405–408.
Sea Res., 1, 191–240. Vervoort, W. (1942) Northern Hydroida in the
Talavera, G. & Castresana, J. (2007) Improvement Collections of the Rijksmuseum van Natuurlijke of phylogenies after removing divergent and Historie and the Zoological Museum at ambiguously aligned blocks from protein Amsterdam, with notes on their distribution.
sequence alignments. Syst. Biol., 56, 564–577. Zool. Meded,, 23, 275–312.
Thollesson, M. (1998) Discrimination of two Wägele, H., Willan, R. (2000) Phylogeny of the
Dendronotus species by allozyme electro- Nudibranchia . Zool. J. Linn. Soc., 130, 83–181.
phoresis and the reinstatement of Dendronotus Wiemers, M. & Fiedler, K. (2007) Does the DNA lacteus (Thompson, 1840) ( Nudibranchia , barcoding gap exist? – a case study in blue
Dendronotoidea). Zool. Scr., 27, 189–195. butterflies. Front. Zool., 4, 8.
Thompson, T. & Brown, G. (1984) Biology of Willis, S. (2017) One species or four? Yes!…and, no.
Opisthobranch Molluscs (vol. 2). The Ray Society Or, arbitrary assignment of lineage to species
Publishing, London. obscures the diversification processes of
Thompson, W. (1840) Contributions towards a Neotropical fishes. PLoS ONE, 12, e0172349.
knowledge of the Mollusca Nudibranchia and Wobber, D. (1970) A report on the feeding of
Mollusca Tunicata of Ireland, with descriptions Dendronotus iris on the anthozoan Cerianthus sp.
of some apparently new species of invertebrata. from Monterey Bay, California. Veliger, 12,383–387.
Ann. Mag. Nat. Hist., 5, 84–102, Wollscheid-Lengeling, V., Boore J., Brown,
Valdés,Á.& Bouchet, P. (1998) Naked in toxic fluids: W. & Wägele, H. (2001) The phylogeny of a nudibranch mollusc from hydrothermal vents. Nudibranchia (Opisthobranchia, Gastropoda,
Deep-sea Res. Pt II, 45, 319–327. Mollusca) reconstructed by three molecular
Valdés, Á., Lundsten, L. & Wilson, N. (2018) markers.. Org. Divers. Evol., 1, 241–256.
Five new deep-sea species of nudibranchs Yale Peabody Museum. (2020) Dendronotus
( Gastropoda: Heterobranchia: Cladobranchia) elegans Verrill, 1880 YPM IZ 010761.GP. Accessed from the Northeast Pacific. Zootaxa, 4526, 4, through https://collections.peabody.yale.edu/
401–433. search/Record/YPM-IZ-010761.GP
Valdés, Á., Murillo, F., McCarthy, J. & Yedinak, N. Yang, Z. (2015) The BPP program for species tree (2017) New deep-water records and species estimation and species delimitation. Curr. Zool.,
of North Atlantic nudibranchs (Mollusca, 61, 854Downloaded –865. from Brill.com 12/12/2023 04:12:08 PM via Open Access. This is an open access article distributed under the terms of the CC BY 4 .0 License. https://creativecommons.org/licenses/by/4.0/
Yoder, A., Olson, L., Hanley, C., Heckman, K., Rasoloarison, R., Russell, A., Ranivo, J., Soarimalala, V., Praveen Karanth, K., Raselimanana, A. & Goodman, S. (2005) A multidimensional approach for detecting species patterns in Malagasy vertebrates. In: Hey, J., Fitch, W.M., Ayala, F.J., (Eds) Systematics and the origin of species: on Ernst Mayr’s 100th anniversary, pp. 203–228 The National Academies Press, Washington, D.C..
Zachos, F.E. (2016) Species concepts in biology. Historical Development, Theoretical Foundations and Practical Relevance. Springer Nature Switzerland, Cham.
Zachos, F.E. (2018) Mammals and meaningful taxonomic units: the debate about species concepts and conservation. Mammal Rev., 48, 153–159.
Zachos, F.E., Apollonio, M., Bärmann, E.V., Festa-Bianchet, M., Göhlich, U., Habel, J.C. et al. (2013) Species inflation and taxonomic artefacts—A critical comment on recent trends in mammalian classification. Mamm. Biol., 78, 1–6.
RECEIVED: 2 MARCH 2020 | REVISED AND
ACCEPTED: 21 JULY 2020
EDITOR: B.W. HOEKSEMA
| R |
Departamento de Geologia, Universidad de Chile |
| PM |
Pratt Museum |
| CC |
CSIRO Canberra Rhizobium Collection |
| T |
Tavera, Department of Geology and Geophysics |
| NTNU |
National Taiwan Normal University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.