Mimagoniates sylvicola, Menezes & Weitzman, 1990
|
publication ID |
https://doi.org/10.5281/zenodo.11050899 |
|
DOI |
https://doi.org/10.5281/zenodo.11051708 |
|
persistent identifier |
https://treatment.plazi.org/id/1B3B8785-FFA2-FFE3-812E-FC24FD56FAB8 |
|
treatment provided by |
Juliana |
|
scientific name |
Mimagoniates sylvicola |
| status |
sp. nov. |
Mimagoniates sylvicola View in CoL , new species
Figs. 7—16 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig , Table 1
Species A. — Weitzman et al., 1988:figs. 6, 10 [phylogeny and biogeography].
Holotype. — MZUSP 36612 , male, SL 30.2 mm, Brazil, Bahia, Município de Prado, forest stream tributary to Atlantic Ocean , near Fazenda Embaçuaba . approximately 8-9 km NW of Cumuruxatiba , 17°05 ' S, 39° 13 ’ W, 20 Mar 1985; N. Menezes, R. M. C. Castro, M. Weitzman, and S. Weitzman. GoogleMaps
Paratypes. -Following 2 lots of immatures to adults collected with holotype: MZUSP 28817 , spms. 42, SL 15.1-30.2 mm ; USNM 276557 , spms. 42, SL 14.7- 33.5 mm, 1 male SL 29.3 mm and l female SL 26.6 mm [both cleared and stained]. Following lots of immature to adult paratypes all collected 20 Mar 1985 by N. Menezes and party unless otherwise noted: MZUSP 28815 , spms. 77, SL l 1.0- 27.4 mm ; USNM 276547 , spms. 77, SL 14.4-27.4 mm, Brazil, Bahia, Município de Prado, first stream (locally called rio do Sul ) south of rio Cai , on road between Cumuruxatiba and Itamaraju , 17°00'S, 39° 12' W. GoogleMaps MZUSP 28816 , sprns. 28, SL 12.7-25.1 mm; 6, spms. 25, SL 13.2-24.0 mm, Brazil, Bahia, Municipio de Prado, small stream NW of Cumuruxatiba , about 17 °01' S, 39 ° 12 ' W. GoogleMaps USNM 300633 , spms. 5, SL 22.3-31.3 mm and USNM 300634 , spm. l, cleared and stained, SL 31.8 mm, Brazil, Bahia, Municipio de Porto Seguro, riacho Ronca Água , tributary to right margin of rio Camurugi , tributary to rio João de Tiba drainage, 15 km NW of Porto Seguro , approximately 16°20’ S, 30°07 ' W, 19 Feb 1986, I. Rosa and party. GoogleMaps
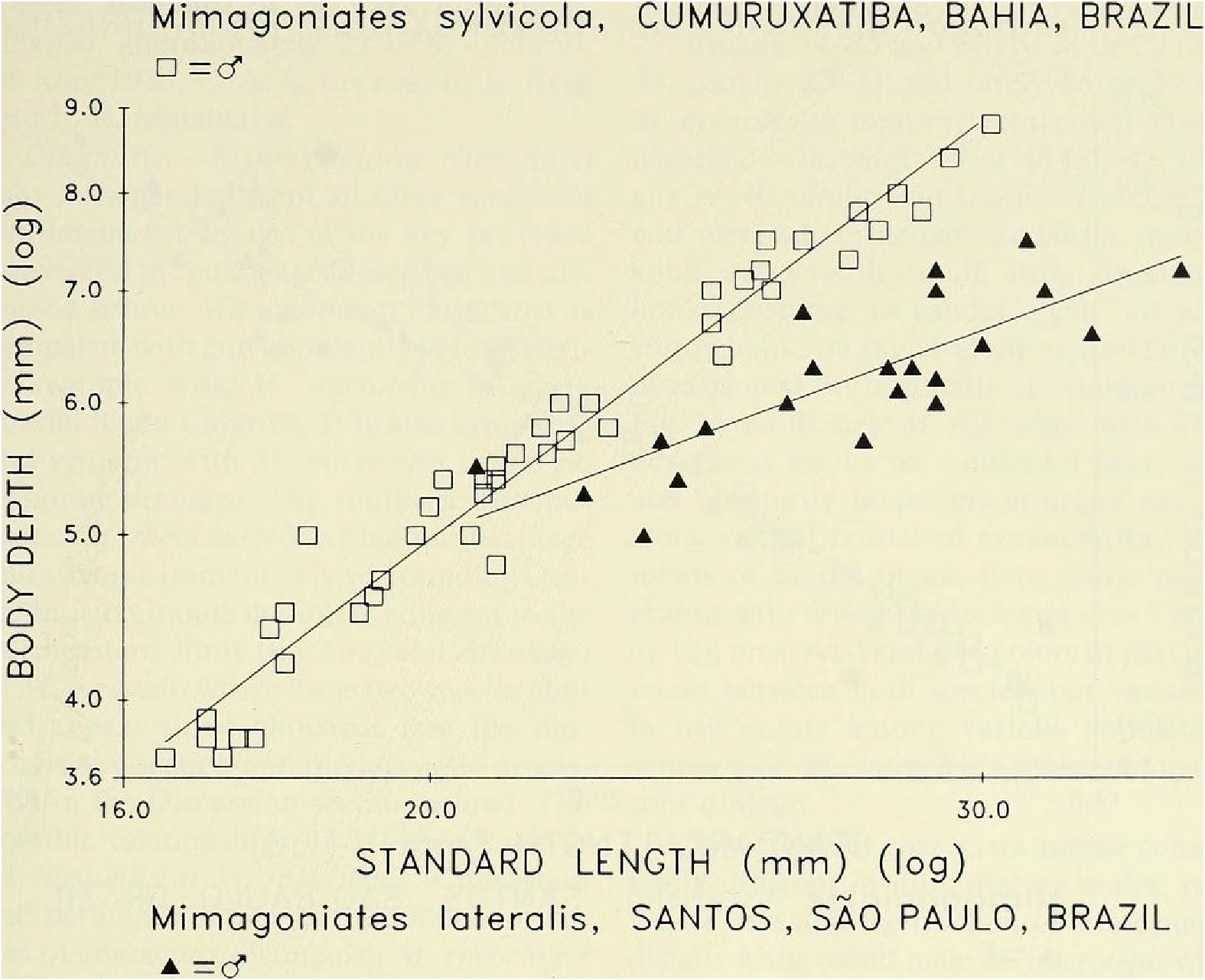
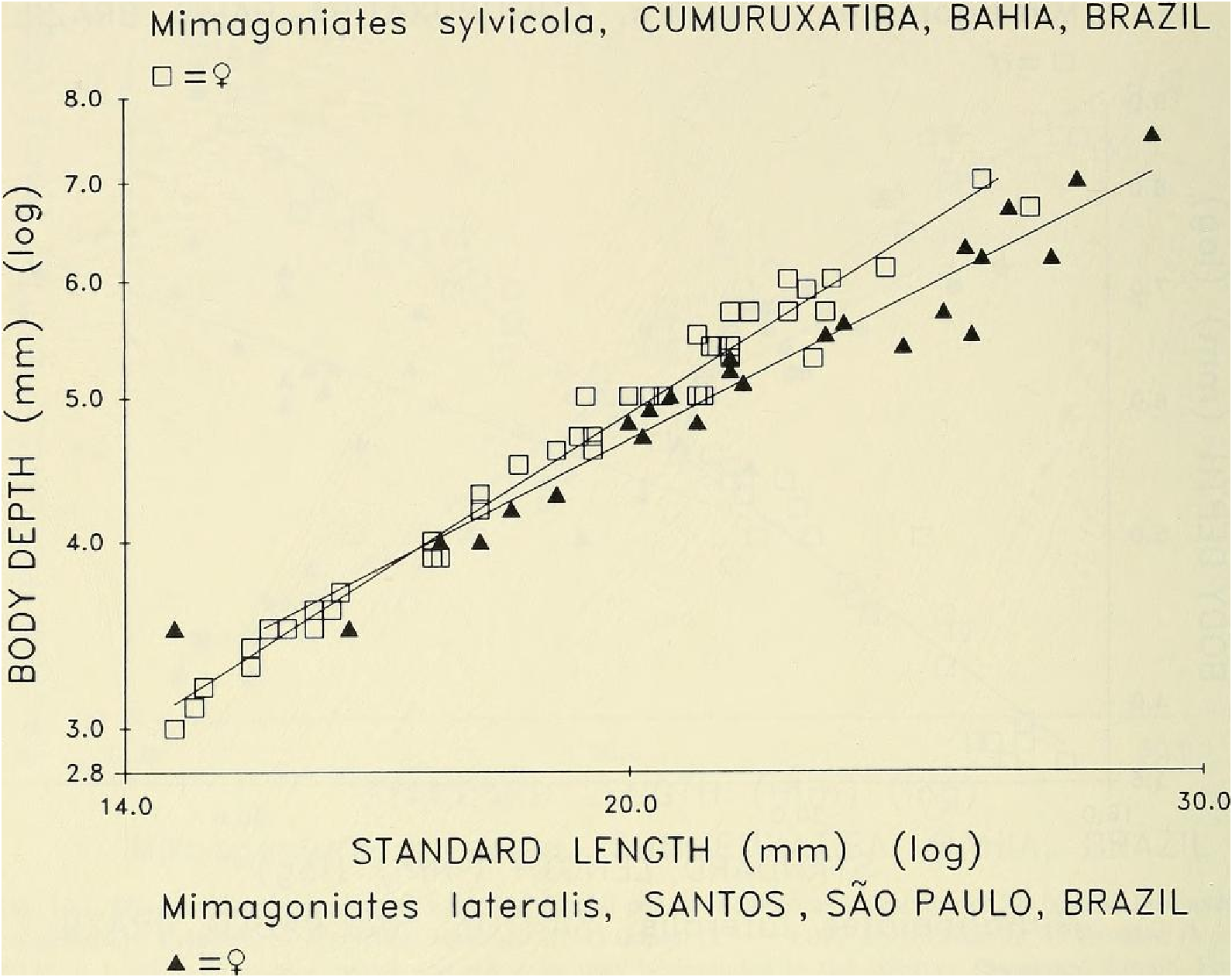
Diagnosis. - Mimagoniates sylvicola may be separated from all other species of Mimagoniates by use of the key to the species provided above. It is distinguished from its morphologically most similar relative, M. lateralis , by the following characters: lateral series scales 49-56 (37—44 for M. lateralis ), scale rows between dorsal-fin and anal-fin origins 16- 18 (12-15 for M. lateralis ). Certain body measurement ratios differ significantly between adults of these species but overlap broadly in young and juveniles. For example, body depths in adult males and females of M. sylvicola diverge considerably from those in adult males and females of M lateralis (body more elongate and slender). See “ Discussion ” below and Figs. 15 View Fig , 16 View Fig . Preserved and live colors differ between the species. Preserved males of M sylvicola with dark lateral body stripe relatively pale and diffuse, occurring mostly at and partly dorsal to mid-lateral body region. Approximately dorsal half of opercle dark. nearly black (relatively pale in M lateralis ). Mimagoniates lateralis with a dark, relatively narrow, clearly defined lateral body stripe that lies mostly ventral to mid-lateral body region. Dark stripe continues onto ventral onethird of opercle. Males of M sylvicola with distal one-fourth to one-fifth (less posteriorly) of anal-fin rays black (distal twothirds to one-half black in M. lateralis). Males of M. lateralis with distal one-fourth of most elongate anterior unbranched ray and branched portions of anterior five to six branched rays hyaline or with a thin scattering of dark chromatophores, never black as in M. sylvicola . Numerous other, but less obvious, color differences occur in preserved males of both species. These best discerned by comparing respective color descriptions given below. Life color of these species quite different. Male M. sylvicola with dorsally located black lateral “ stripe ” obscured by silvery blue reflective color, especially anteriorly. Ventrally located black stripe of male M lateralis deep black except at its mid-length ventral to dorsal-fin origin where partly obscured by blue to silvery pigment in some population samples. Wild caught males of M lateralis with a yelloworange stripe just ventral to black lateral stripe, absent in M. sylvicola , although both species often with anal-fin base yellow to orange. Note, in aquaria at least, M. lateralis loses yellow or orange coloration but black stripe always present. See live color descriptions below for a more complete account of M. sylvicola .
Description. -Table 1 presents morphometrics of holotype and paratypes. Except where noted, entire description refers to lots from near Cumuruxatiba. These collections are treated statistically as one population sample since no statistical differences were found among them and all lots were collected from only a few kilometers apart. Counts for specimens from rio Camurugi are given only when they differ from those from near Cumuruxatiba.
Body compressed, moderately elongate; body deepest about midway between snout tip and dorsal-fin origin, near anal-fin origin. Predorsal body profile gently convex to snout tip. Body profile slightly elevated at dorsal-fin origin, straight along dorsal-fin base and nearly straight to origin of dorsal procurrent caudal-fin rays. Dorsal-fin origin nearer to caudal-fin base than to snout tip. Ventral body profile convex from anterior tip of lower jaw to point on abdomen about midway between pectoral- and pelvic-fin bases. Belly profile abruptly becomes concave and then straight to anal-fin origin. Body profile slightly convex along anal-fin base to anal-fin insertion. Ventral profile of caudal peduncle slightly convex, especially in adult males where profile formed by ventral procurrent caudal fin rays. In females and juveniles this profile nearly straight.
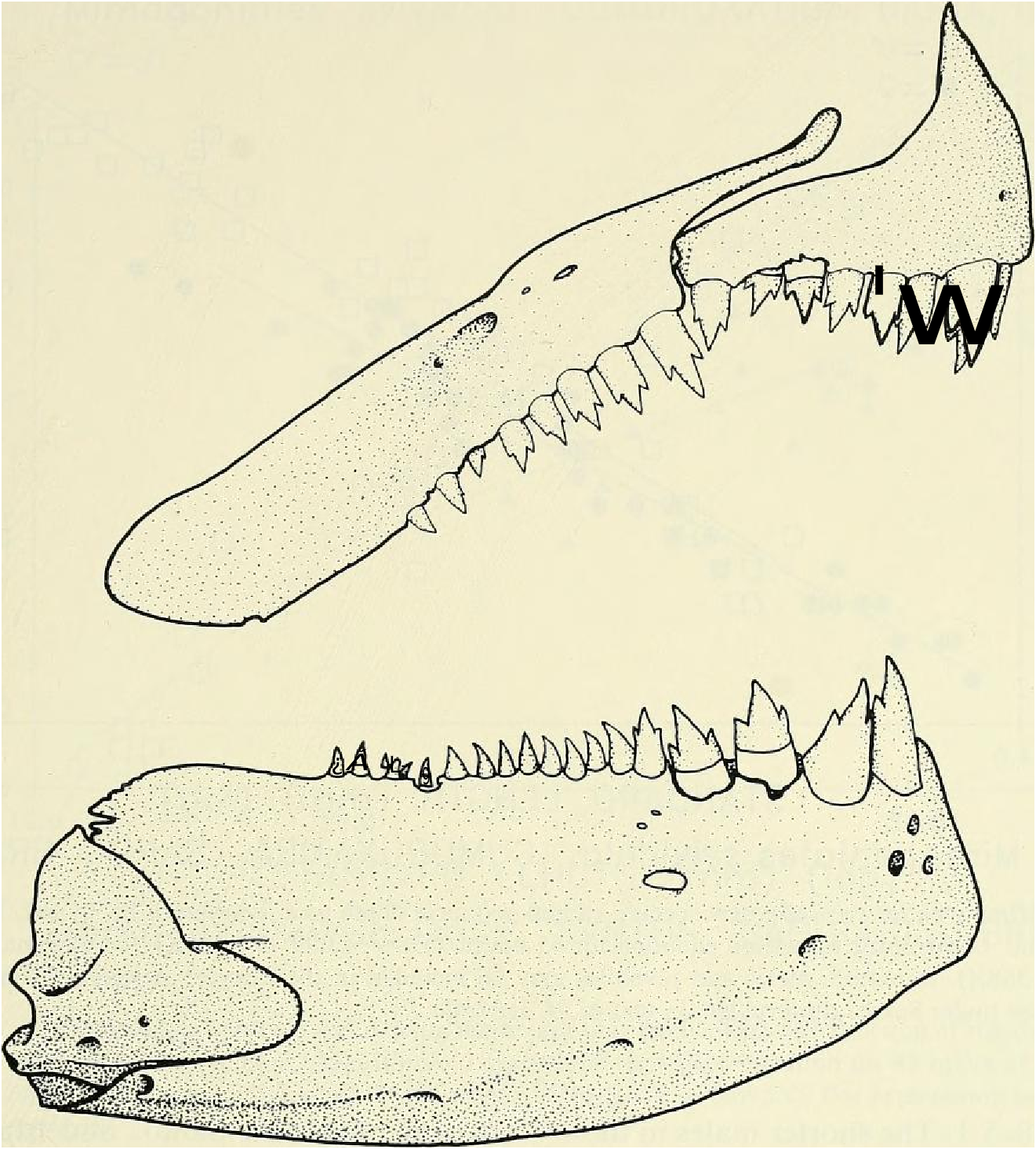
Head and snout of moderate size in proportion to body length. Lower jaw protruding, anterior to upper jaw. Lower jaw of males thick and heavy compared to that of females. Mouth angled posteroventrally from anterior tip of snout to posterior part of mandibular joint. Maxilla extending posteriorly to a point anterior of a vertical line drawn through anterior border of pupil of eye.
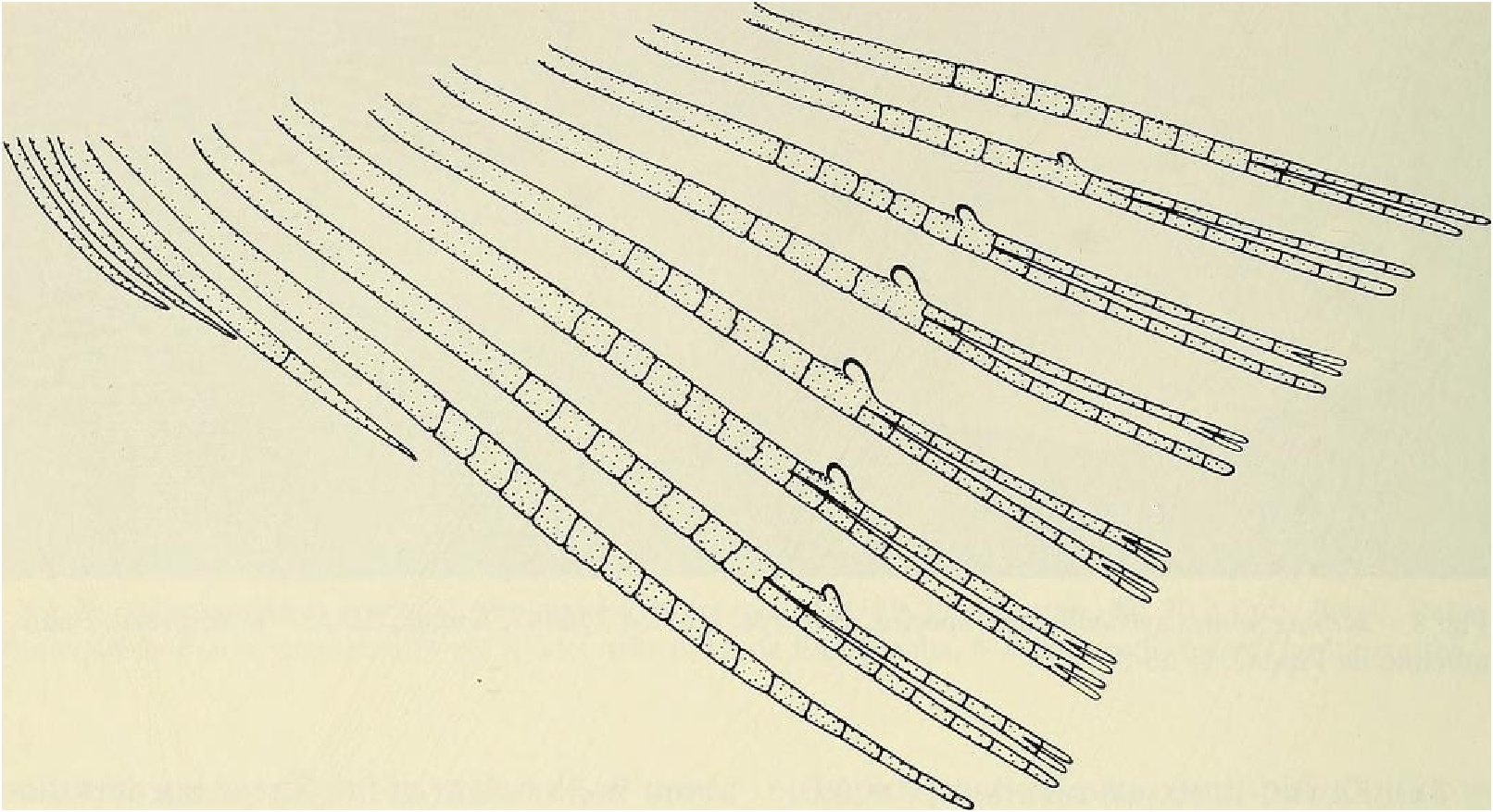
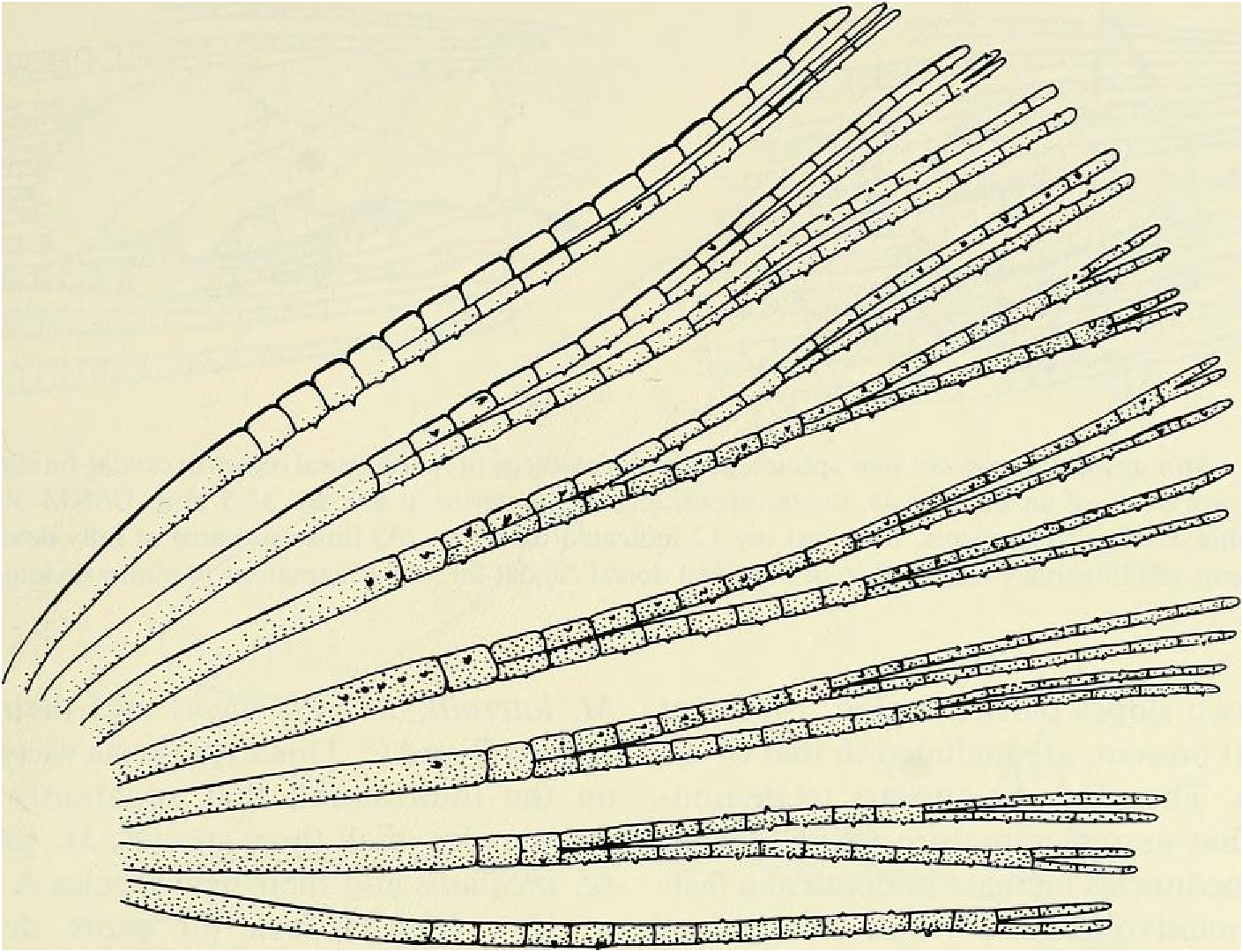
Dorsal-fin rays ii, 8 (unbranched rays ii in all specimens, branched rays x = 8.0 [3 spms. with 9], range = 8-9, n = 90); of6 specimens from rio Camunigi (not included in n = 90) 2 with 8 and 4 with 7 branched rays; posterior ray not split to its base and counted as 1. Adipose fin present, slender. Anal-fin rays iv, 25 (unbranched rays iv in all specimens, branched rays x = 24.8, range = 23- 26, n = 90); posterior ray split to its base and counted as 1. Anal fin with moderately developed lobed anterior portion including fourth unbranched ray and first 5-6 branched rays. Anal fin of sexually mature males with bilateral blunt hooks on anterior 6 branched fin rays, 1 set of hooks for each ray (see Fig. 9 View Fig ). Pectoral-fin rays i, lo (unbranched rays i in all specimens, branched rays x = 9.7, range = 9-11, n = 90); all 6 specimens from rio Camurugi with 10 branched rays. Posterior tips of longest pectoral-fin ray extend posteriorly beyond origin of pelvic fin; of about equal length in both sexes. Pectoral-fin rays without hooks. Pelvic-fin rays 8 (8 in all specimens except I with 9, n = 90). Pelvic fin with anterior most ray branched in all specimens (see Fig. 10 View Fig ). Adult males with total of over 100 small to tiny hooks present on rays of pelvic fin, distributed as in Fig. 10 View Fig . Each ray bears 9 to over 40 hooks, depending on the maturity of the specimen and/or the fin ray examined.
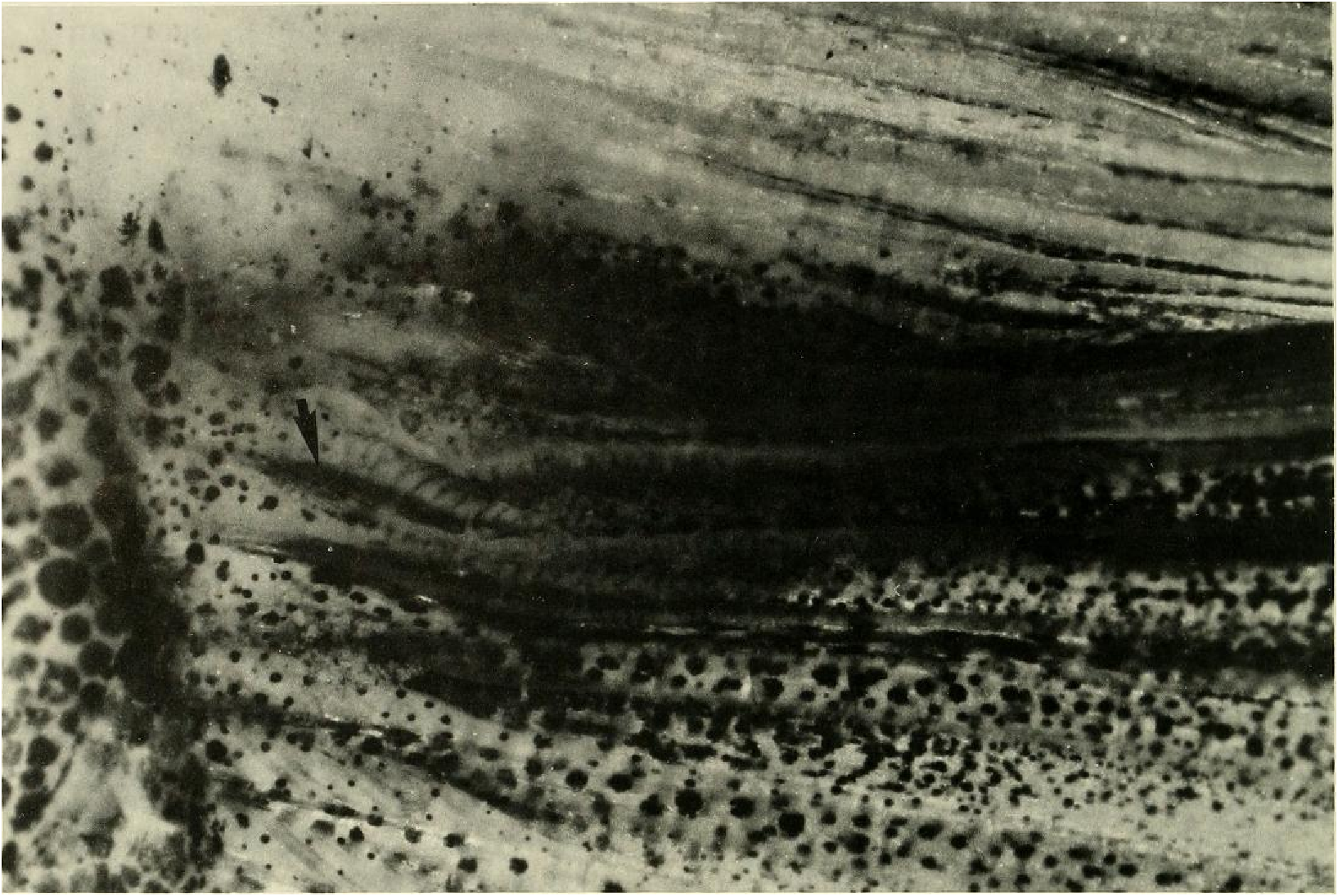
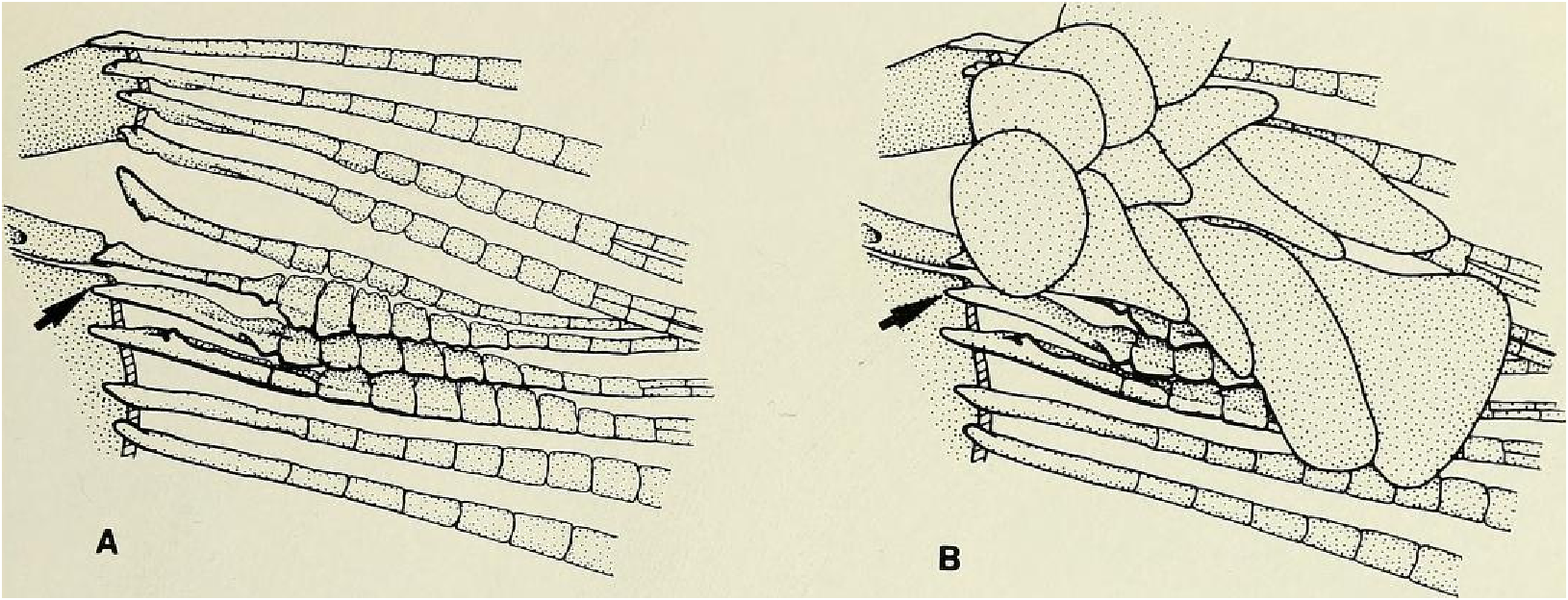
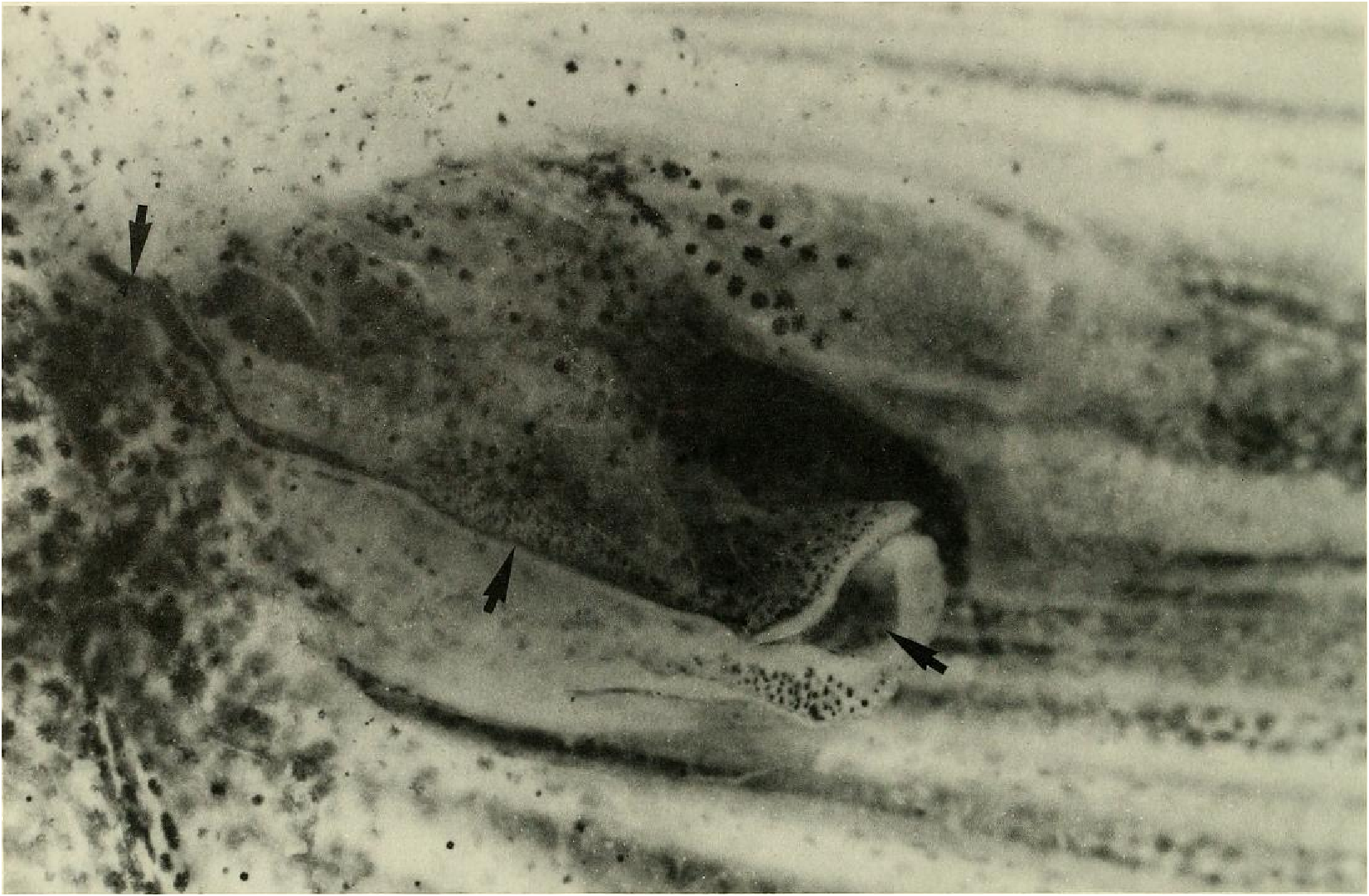
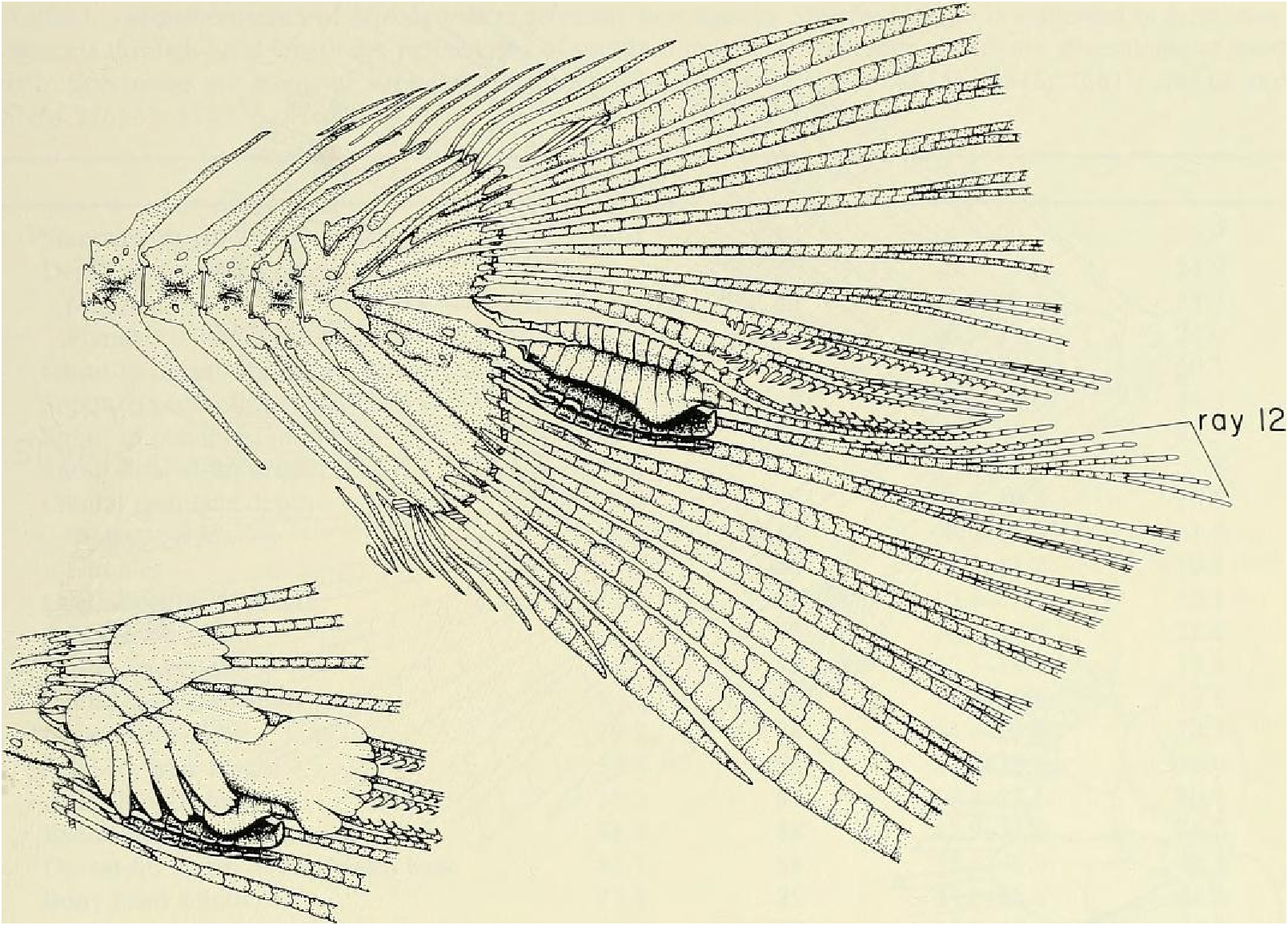
Principal caudal-fin ray count 10/9 in all specimens ( n = 90). Fin rays modified in association with caudal pheromone pump as in Figs. ll and 12. Fig. ll illustrates a relatively immature pump. while Fig. 12 View Fig shows a presumably mature pump in which pump chamber has well-developed water entrances and exit. Fin rays modified very much like those of M lateralis . Caudal-fin rays without bony hooks. See also Weitzman & Fink (l985:98-99), Weitzman et al. (1988:384-413) and “ Discussion ” below, regarding phylogeny of M sylvicola for hypothetical function of secondary sexual characters and phylogeny for glandulocaudins as indicated by their caudal pump morphology.
Scales cycloid, almost deciduous, with few radii along posterior border; smallest scales often nearly without or without radii. Terminal scale of modified caudal series with exaggerated radii appearing as incisions of posterior scale borders (see Figs. 11 b, 12b).
Lateral line incomplete, perforated scales 8 ( x = 7, range = 6-8, n = 34); 2 specimens from rio Camurugi with 9 perforated scales. Lateral series scales 53 ( x = 52.7, range = 49-56, n = 34). Predorsal scales 25 ( ic = 25.9, range = 24-28, n = 39). Scale rows between dorsal-fin origin and anal-fin origin 17 ( x = 16.7, range = 16- 18, n = 64). Scale rows around caudal peduncle 20 ( x = 19.7, range = 19-20, n = 21); 1 specimen from rio Camurugi with 22 scale rows around caudal peduncle.
Premaxillary teeth in 2 distinct rows although this is not clear in Fig. 13 View Fig . Larger teeth tricuspid, smaller teeth tricuspid or bicuspid, smallest ones unicuspid. Outer row teeth 6 ( x = 5.4, range = 3-7, n = 90). Inner row teeth 3 ( x = 3.0, range 2-5, n = 90). Outer and inner row premaxillary teeth somewhat compressed compared to most “ tetragonopterine ” characid teeth which often appear almost circular in cross section. Maxillary teeth 6 ( x = 6.8, range = 5- lo, larger specimens usually with highest counts, n = 90); two specimens from rio Camurugi with ll maxillary teeth. Maxillary teeth show an increase in number with increasing SL from a mean of 5.9 in 9 specimens between 15.5 and 16.5 mm SL to a mean of 7.6 in 14 specimens between 25.0 and 30.5 mm SL. Anterior 4-5 maxillary teeth tricuspid and larger than remaining teeth with 2 or l cusps. Dentary with 4 large tricuspid teeth in all specimens, n = 90; smaller posterior dentary series unicuspid except anterior tooth which is tricuspid, 10 ( x = 8.9. range = 6—12, n = 90); 1 specimen from rio Camurugi had 13 dentary teeth. Maxillary and dentary teeth shaped much like premaxillary teeth as described above. At any given SL considerable variation in tooth count occurs and nearly any tooth count within ranges given may be expected. No significant dilferences in tooth number found between males and females.
Vertebrae 40 ( x = 39.9, range = 39-41, n = 88). Dorsal limb gill-rakers 6 ( x = 6.0, range 6-7, n = 90, two specimens from rio Camurugi with 5 dorsal limb gill-rakers); ventral limb gill-rakers 12 ( ic = l 1.7, range = 11- 13, n = 90). Branchiostegal rays 4 in 3 cleared and stained specimens, 3 rays originating from anterior ceratohyal and 1 ray from posterior ceratohyal.
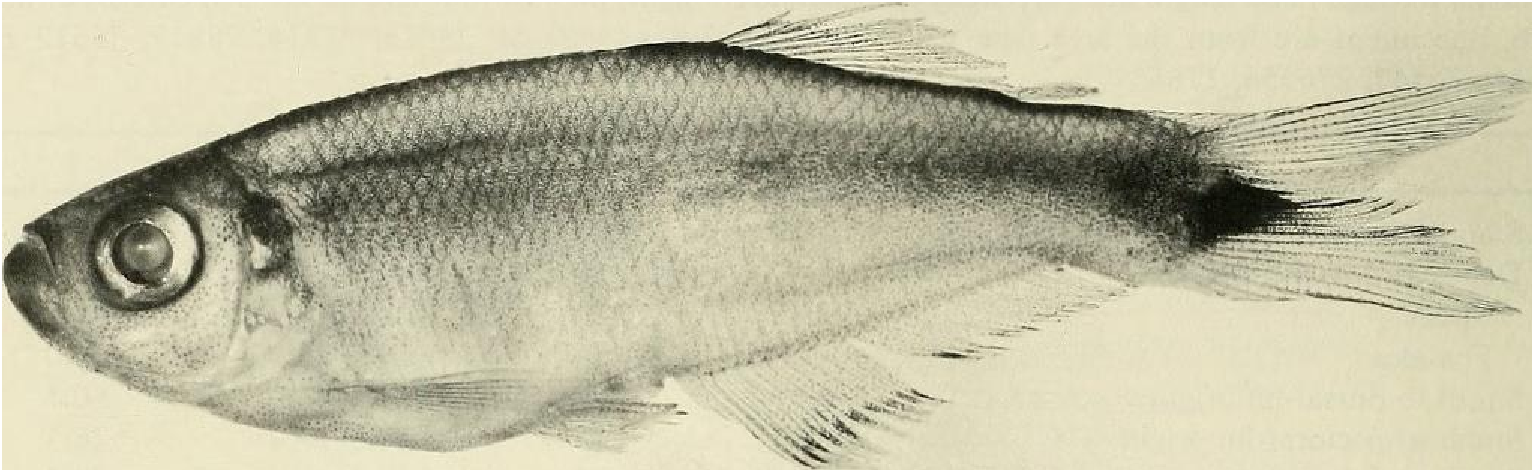
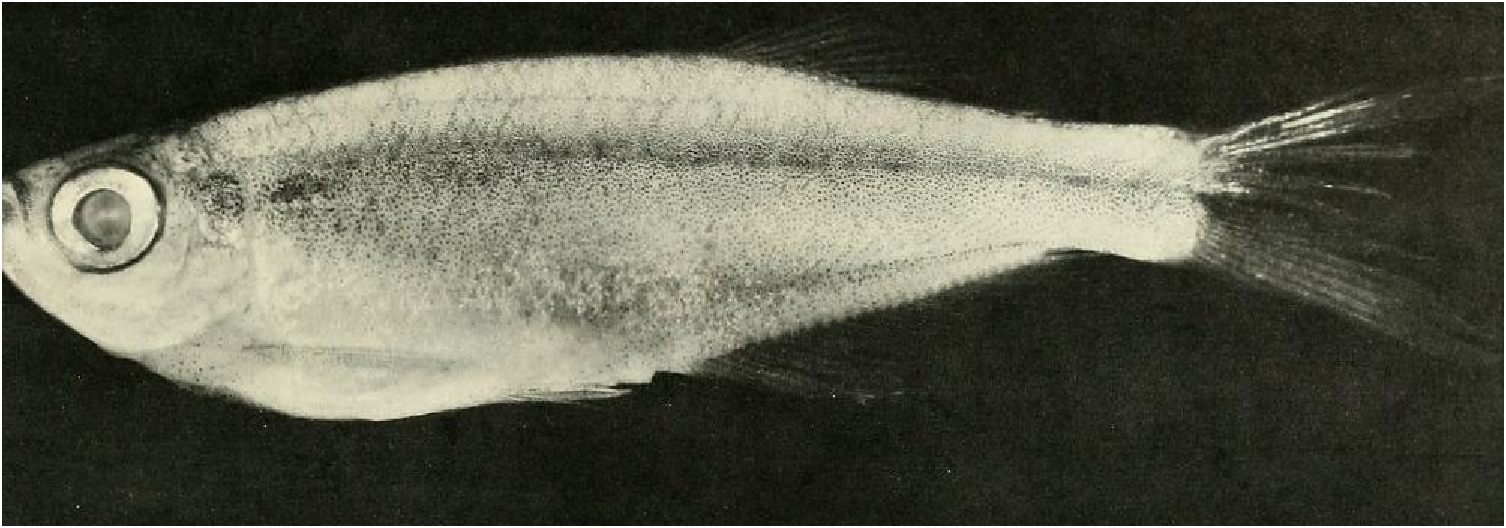
Color in alcohol.- See Figs. 7 View Fig and 8 View Fig for preserved color pattern in males and females. Body pale to medium brown, almost white ventrally, darkest dorsally. Lateral body stripe pale, diffuse, best developed in males. Stripe extending from darker opercular spot on dorsal half of opercle posteriorly to a dark, spot-like region on caudal peduncle. Immediately posterior to this spot, caudal gland region enveloped in black pigment forming triangular-shaped area with its posterior apex continuous onto ray 11 and to a certain extent ray 12. Remainder of caudal fin dusky due to scattering of dark chromatophores, especially along ventral border of 19 th principal caudal ray. Dorsal body surface dark dusky, especially in area of predorsal scales.
Pectoral, pelvic, dorsal and anal fins dusky from scattered dark chromatophores along fin rays. Pelvic fins considerably darker than pectoral fins. Anal fin with a dark, elongate stripe running length of fin. Width of stripe about ¼ - ⅕ height of fin. Stripe borders distal ends of fin rays posteriorly; anterior portion of dark stripe separated from distal ends of first five or six fin rays by relatively hyaline area on anterior lobe of fin. Dorsal fin with horizontal dark stripe extending posteriorly from about mid-length of anterior elongate undivided ray to posterior tips of two terminal dorsal-fin rays. Adipose fin dusky with scattered dark chromatophores. Head dark brown around mouth and on dorsal surface of snout, between eyes, dorsum of cranium and nape. Iris dorsal to pupil dark brown to black, most of remainder of iris silvery with some dark brown or black areas ventrally. Circumorbitals pale brown or silvery with evenly scattered dark chromatophores. Ventral area of opercle, preopercle and posterior region of branchiostegal rays silvery, without much dark brown pigment.
Color in life.-Life color patterns taken from color slides and color notes made while collecting specimens listed above from clear and black waters near Cumuruxatiba. Sides of body silvery deep blue with back dark brown and abdominal area silvery white. All fins translucent, lemon yellow with dark brown pigment described above under preserved color description appearing brown to black. Females with similar color pattern but blue, yellow, and dark pigment patterns much paler. In life caudal-fin rays 13 and 14 considerably darkened with black pigment. Some male specimens display a considerable but rather diffuse lateral dark brown stripe below lateral mid-region of body, suggesting elongate lateral stripe of M lateralis . Specimens from rio do Sul, Cumuruxatiba area (USNM 276547), gold silvery in color and without blue coloration. Some of these specimens with black pigment considerably reduced, absent or covered in patches by guanine, especially on body sides. These specimens may have been infested by metacercaria of a trematode as noted for other similar appearing characids by Géry & Delage (1963).
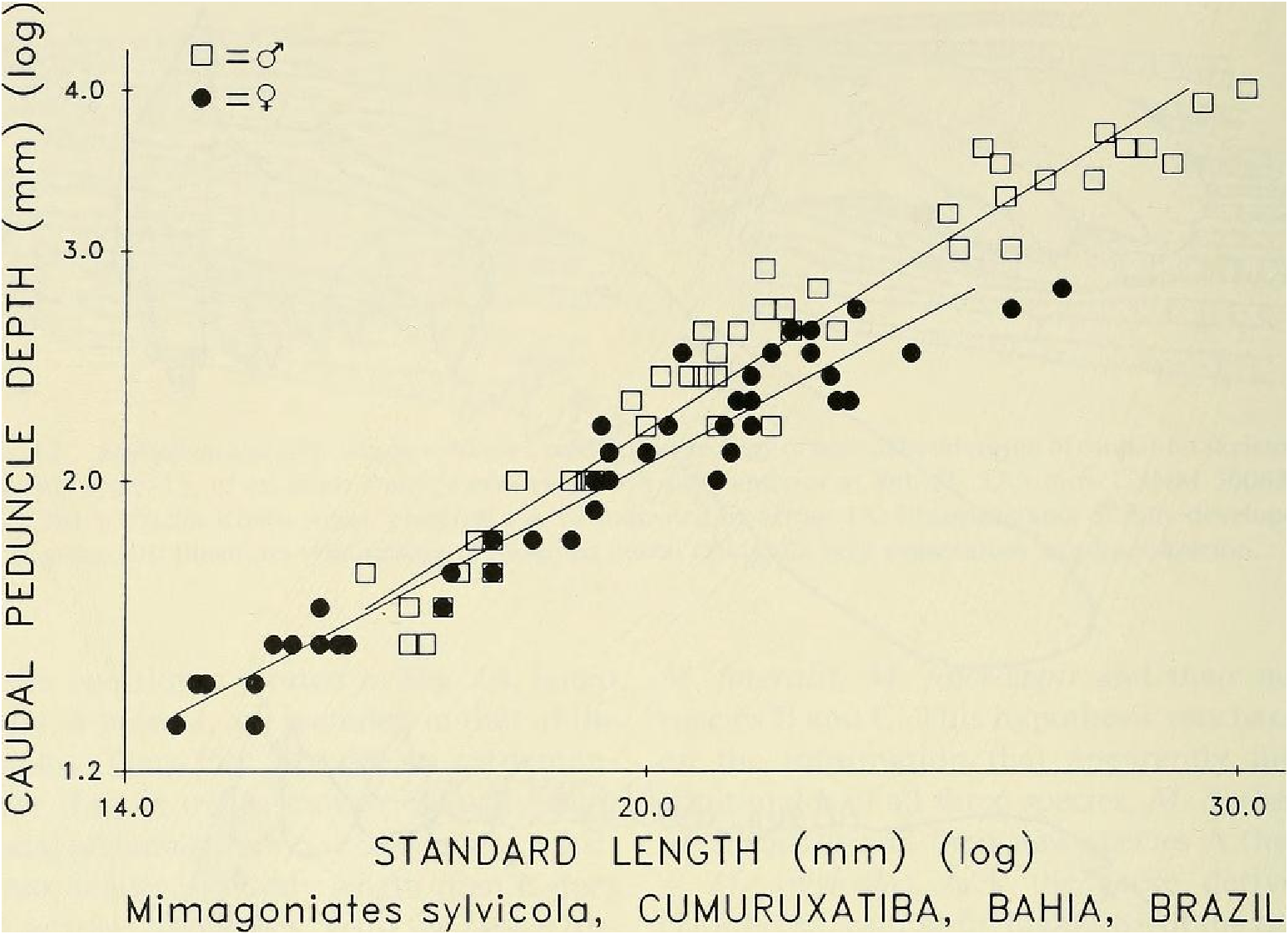
Sexual dimorphism.- Females lack the caudal pheromone pump organ, anal-fin and pelvic-fin hooks of males ( Figs. 9 View Fig , 10 View Fig ) and display a more subdued live body coloration as noted above. Figure 14 View Fig presents graphic evidence that the caudal peduncle depth is usually deeper in adult males than in adult females and that males reach a greater adtflt length than females, so far as known. Below in covariance analyses we compare males and females of unequal length ranges, although the range of the females is included within that of the males. We do this because we believe these ranges are expressions of their natural differences. In our population samples of various species of Mimagoniates the length of the largest males always exceeds that of the largest females. If in nature the females reached the same lengths as the males our results could be biased, but longer females were not represented in our samples. We hypothesize that most of our samples fairly represent the adult lengths of both sexes. Figure 14 View Fig indicates that caudal peduncle depth divergence between males and females begins around 18 mm in SL. Even though we have few female specimens in the size range between 23.0 to nearly 28.0 or longer, we are inclined to accept that 28.0 mm SL may be the approximate adult size limit for females. Also. at least some glandulocaudine species undergo delayed sexual maturation in males, Weitzman & Fink (1985: 38, 42). If present in species of Mimagoniates , this kind of growth pattern might affect the regression slope shown for sexually maturing and sexually mature males towards that of the females in Fig. 14 View Fig , if the large males of latent sexual maturity were included in the male population sample graphed and analyzed. In the two slopes plotted in Fig. 14 View Fig , latent males, if present, are included in that of the females. Thus Fig. 14 View Fig appears to demonstrate that as males mature sexually their caudal peduncles increase in depth at a faster rate relative to body length than it does in juveniles or females. With the above reservations and explanations in mind, we find that divergence in caudal peduncle depth on SL by apparent sex in an F -ratio test for homogeneity of slopes in an analysis of covariance were significantly different ( F ₀.₀₅,(₁, ₈₆) = 9.42, P <0.002) between 45 males and 45 females from the area near Cumuruxatiba (MZUSP 28815, 28816, 28817, 36612 and USNM 276547, 276556, 276557).
Etymology. —The name sylvicola is from the Latin silva (forest) and colo (dwell or inhabit) and is in reference to the forested nature of the streams in which this fish is found.
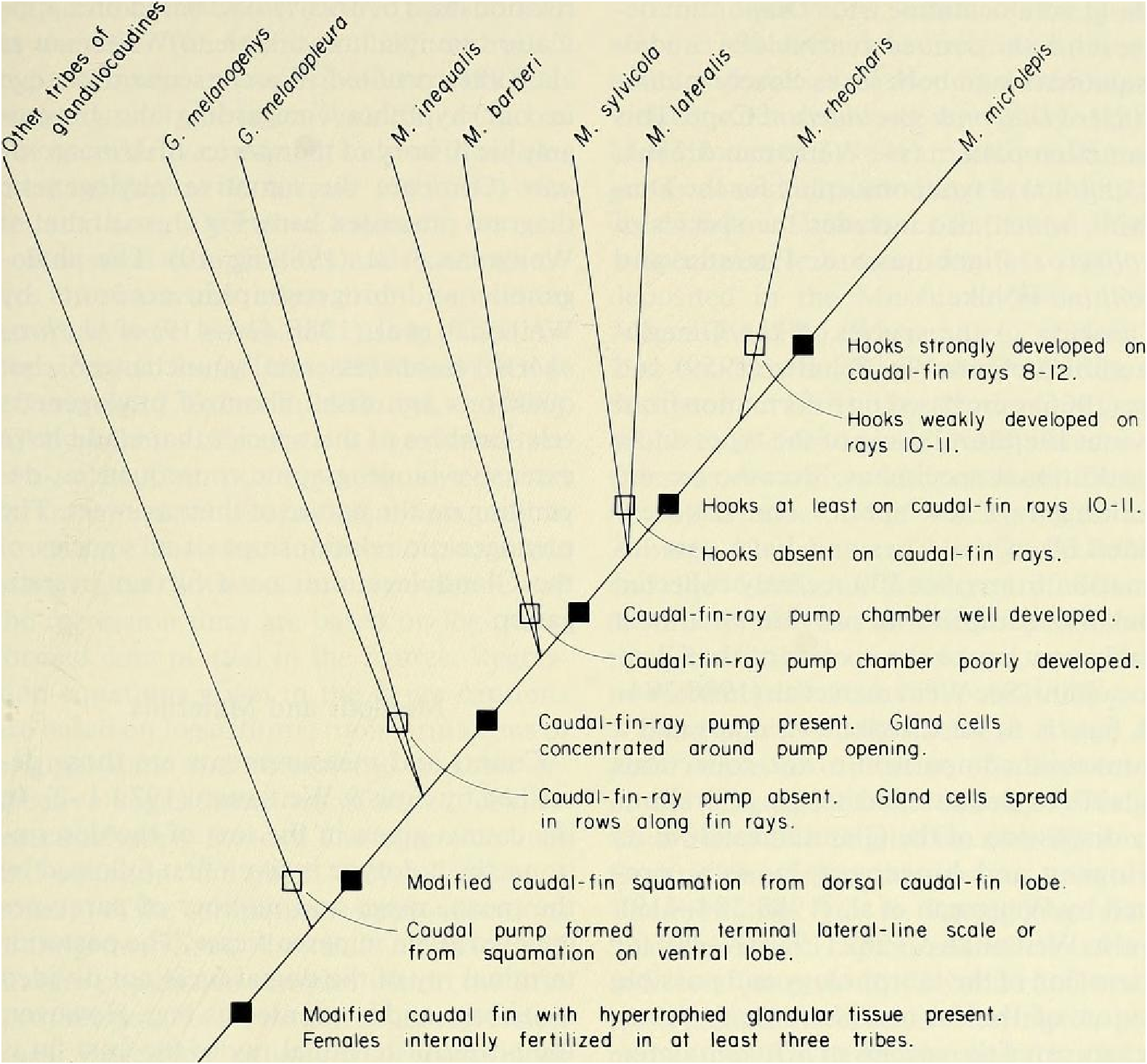
Discussion. — Weitzman et al. (1988:404, 414-419, fig. 10), as new species A, tentatively hypothesized that M sylvicola was a relatively primitive species of Mimagoniates with respect to caudal-fin pump evolution. They placed M sylvicola in an unresolved polytomy in their phylogenetic diagram along with M. barberi and M. inequalis and a monophyletie line leading to M. lateralis , M microlepis and their new species B and C. This hypothesis was based on the information that apparently fully adult males of all three species, M barberi , M. inequalis and their new species A (here = M sylvicola ), lack the more derived chambered, caudal-fin ray pump of the other four species. Compare caudal pump structures in Figs. 2 View Fig and 3 View Fig with those in Figs. 4 View Fig and 5 View Fig .
Evidence taken from a specimen of M sylvicola , USNM 300633, SL 32.5 mm, subsequently available to us from near Porto Seguro, Bahia, showed that the caudal gland matures with a well-developed pump chamber, (compare Figs. ll and 12). However, this species lacks the caudal-fin hooks found in the more derived species, M. rlıeocharis , described below, and in M. microlepis (compare Figs. 5 View Fig , ll, 12, and 24). This information would place M sylvicola in a trichotomy, Fig. l, with M lateralis and a monophyletic line leading to the two species with caudal-fin hooks. This hypothesis would leave M. inequalis , M barberi and a monophyletic line leading to the species of Mimagoniates With more derived caudal pumps in a trichotomy at a lower level in the phylogenetic diagram (Fig. l). If this relationship can be supported by further phylogenetic evidence, then the biogeographic comments of Weitzman et al. (19 88: 412, 413) need some alteration (see section on biogeography below).
₍ ₁,
)
It was noted in the diagnosis above that adult males and females of M. sylvicola from Cumuruxatiba (MZUSP 28815, 28816, 28817, 36612 and USNM 276547, 276556, 276557), difi`er in body depth from adult males and females of M lateralis from the Santos region (CAS 36634, MZUSP 40276, 40277, USNM 226468, 254268, 257200, and 257202). Forty five males of M sylvicola and 22 males of M. lateralis were significantly different ( F ₀.₀₅, =49.75, P <0.000) in an analysis of covariance for body depth on SL in an F -ratio test for homogeneity of slopes, Fig. 15 View Fig . Body depth ratios of SL useful for identification of fully or near fully mature males (at or above 23.0 mm SL) are as follows: n = 12 specimens of M sylvicola from Cumuruxatiba and near Porto Seguro, x = 3.6, range = 3.4-3.8 and n = 23 specimens of M lateralis from near Santos and Município de Cananéia, x = 4.3, range = 3.8-5.1. The shorter males in these size ranges accounted for most of the overlap.
In a similar covariance analysis for slopes, 45 adult females of M. sylvicola and 26 females of M. lateralis from the same population samples as the males discussed above, also displayed significant difference in body depth ( F ₀.₀₅, (₁, ₆₇) = 16.3, P <0.001) ( Fig. 1 6 View Fig View Fig View Fig View Fig View Fig View Fig ).
The number of branched anal-fin rays in the two species was significantly different ( t = 22.012, P <0.00 in a two-sample, two-tailed t-test), although there was some overlap in counts: n = 90 specimens of M. sylvicola from near Cumuruxatiba (same lots as listed above), range = 23-26, x = 24.8, SD = 0.7728 and n = 91 specimens of M. lateralis from near Santos and Municipio Cananéia (same lots as listed above), x = 27.9, range = 25-31, SD = 1.0899.
Ecology. -Little is known about the ecology of this species. Most of the strwms in which it was captured were relatively slow moving, with little gradient. They were approximately 4 to 6 meters wide, to 1.5 meters deep and surrounded by vegetation, usually trees of a few to many meters high. The water varied from clear to black (tea color). The fish occurred in depths of 0.1 to about 0.5 meters usually in areas of little current over white sandy, rocky or dark mud bottoms. They occurred in both sunlight or shaded areas, most often near shore, especially near emergent or submerged vegetation where almost immediate cover could be taken from predators such as large cichlids or the characid Oligosarcus .
Specimens of M. sylvicola , now MZUSP 28817 and USNM 276557, were collected 22 March 1985 in a well-shaded forest rivulet less than 1 meter wide and about 20-30 cm deep in most places. This creek was in a ravine of a mostly uncut. undisturbed tall forest, 1 or 2 km from the Atlantic coast, about 17 km from Cumuruxatiba, Bahia. The stream bottom consisted of forest litter, rocks, soil and sand with a mild nearly 0° to 30° gradient, well covered by riparian vegetation in many places. The water was tea colored. Other fish species taken at this site were Rachoviscus graciliceps , species of Astyanax, Characidium, Aspidoras, Heptapıerus , a gobiid and a hypoptopomine loricariid catfish.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
Genus |