Morphotype c
|
publication ID |
https://doi.org/ 10.5252/geodiversitas2020v42a18 |
|
publication LSID |
urn:lsid:zoobank.org:pub:030C3660-4FC5-4C68-BD56-226860C8FD1C |
|
DOI |
https://doi.org/10.5281/zenodo.4329651 |
|
persistent identifier |
https://treatment.plazi.org/id/17167930-FFA8-FFD6-FBA8-FA32C2669CAF |
|
treatment provided by |
Valdenar |
|
scientific name |
Morphotype c |
| status |
|
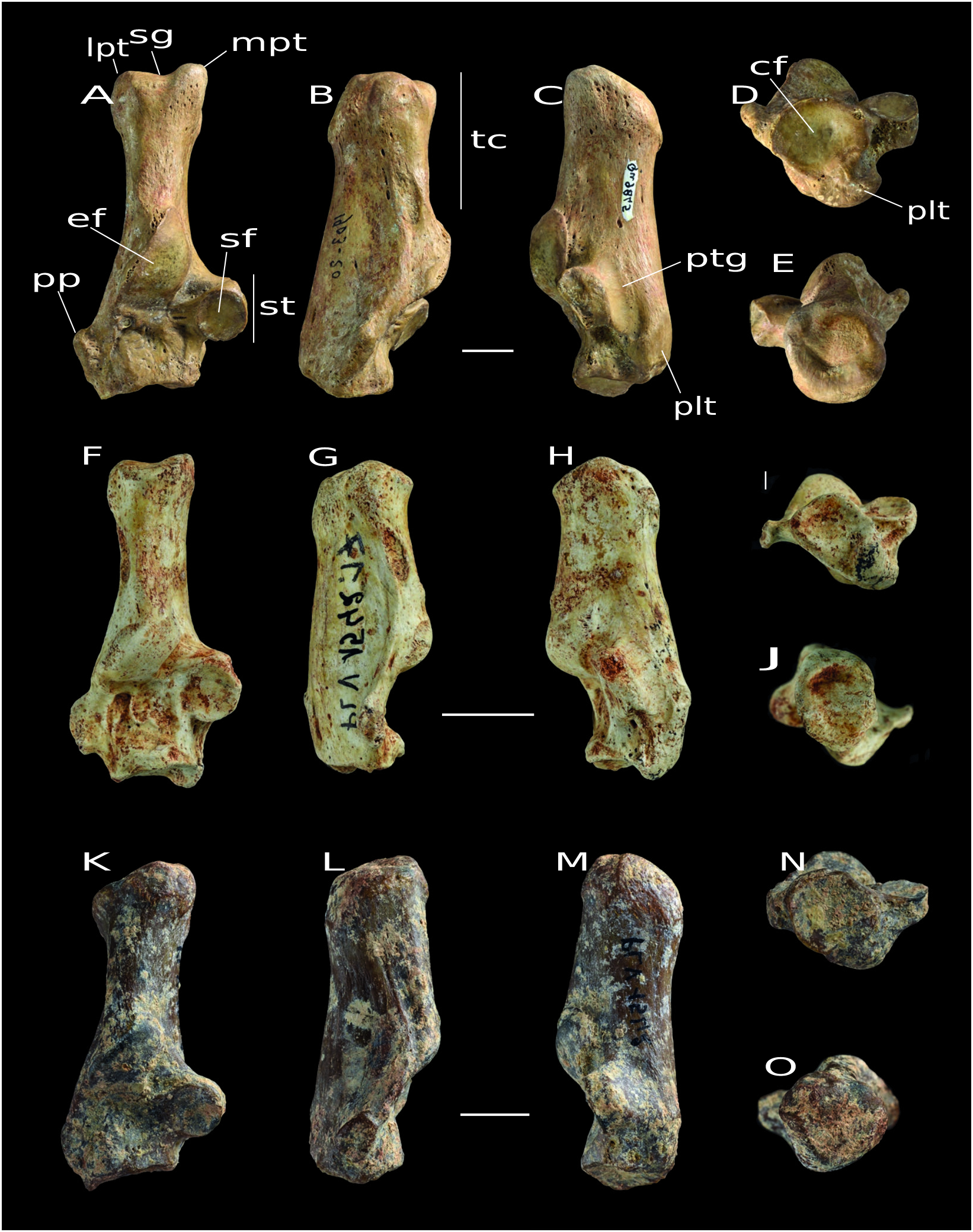
Morphotype C ( Fig. 4 View FIG K-O)
Dorsal view
The sagittal groove is poorly defined. The tuber calcanei is proximodistally quite elongated. The ectal facet is convex in the middle and very slightly concave in its proximal part. The edges of this facet are rather clear, except the distal edge, which merges with the body of the calcaneus (and is therefore difficult to delimit). The sustentaculum tali has a rounded articular surface, as in Morphotypes A and B, and extends slightly on the proximal edge. The peroneal process is not broad and merged with the ridge extending up on the tuber calcanei. The cuboid facet is concave and inclined with respect to the mediolateral axis, but its lateral edge does not extend up the body of the calcaneus as seen in Morphotypes A and B.
Lateral view
The dorsal and plantar edges of the tuber calcanei are very slightly concave. The cavity, which is proximal to the ectal facet, is not very deep.
Medial view
The tendinous plantar groove is not very marked. The sustentaculum tali is dorsoventrally quite thick, as in Morphotype A.
Distal view
The plantar tubercle is ventrally well broad.
Proximal View
The edges of the proximal part of the tuber calcanei are quite irregular.
COMPARISON
These morphotypes exhibit morphological differences from tarsal bones of Hyaenodonta , such as G. chronius ( Zack & Rose 2015) and Hyaenodon ( Bastl 2012). The neck of the astragalus is mediolaterally thinner and the medial edge of the head is more pronounced in these morphotypes. The plantar tendon groove is less pronounced, the proximal plantar tuberosity and the lateral processes are slenderer. The calcaneus of the morphotypes has a much less inclined cuboid facet than in I. raoi ( Rana et al. 2015) and G. chronius ( Zack & Rose 2015).
In comparison with Nimravidae , such as H. primaevus and N. brachyops ( Barrett 2016), all astragalus morphotypes have a longer neck and the head extends less on the medial side of this neck. The calcanei have a thinner sustentaculum tali and a shallower plantar tendon groove than in Nimravidae .
Compared to Miacidae such as Vulpavus ( Heinrich & Rose 1997), the tarsal bones of the morphotypes are smaller, and the four astragalar morphotypes have a shorter neck, which is less medially projected, and a less pronounced plantar tendon groove, which is less dorsally extended. Moreover, the sustentaculum tali is medially thinner and not as distally positioned as in Vulpavus.
These morphotypes are also very different from those of Ursoidea such as Ursus spelaeus ( Santi et al. 2005) and Ursus arctos ( Baryshnikov 2015) . They have less robust tarsal bones, the neck of the astragalus is much longer and the trochlea is mediolaterally thinner. The tuber calcanei is mediolaterally narrower, the sustentaculum tali is less distally positioned and its sustentacular facet is more rounded, not as elongated, and not inclined in the medioproximal-laterodistal direction as observed in ursoids.
Compared to the ailurid Simocyon batalleri ( Salesa et al. 2008), the morphotypes have a less flattened astragalar head, and a mediolaterally broader sustentacular facet. The sustentaculum tali is proximodistally thinner, the peroneal process is mediolaterally and proximodistally thinner and the plantar tendon groove is less marked than in S. batalleri.
Compared to mustelids such as M. sansaniensis, I. zibethoides and T. sansaniensis ( Peigné 2012), the astragalar neck of the morphotypes is shorter and less medially projected, and he sustentacular facet is mediolaterally broader. The calcaneus morphotypes are larger with a more rounded sustentaculum tali.
All these differences exclude an assignment of the bones to Hyaenodonta , Nimravidae , Miacidae , Ursidae , Ailuridae or Mustelidae and indicate a close relationship to amphicyonids. The astragalus of Morphotypes 1, 3, and 4 is very similar to that generally observed in Amphicyonidae such as Amphicyon major ( Argot 2010), Amphicyon galushai ( Hunt 2009), and the North American Ysengrinia americana Wortman, 1901 (Oligo- Miocene) ( Hunt 2002). The head is relatively projected on the medial side of the astragalus and quite broad; the trochlea is asymmetrical and its mediolateral width is greater than its proximodistal length. Futhermore, Morphotype 1 displays a relatively large trochlea, just as Morphotype 2, which also features a deep trochlear articulation. These characteristics are present in large amphicyonids such as Amphicyon giganteus (Miocene of Europe and Africa; Gagnaison et al. 2017). Despite these morphological similarities, these three morphotypes display some differences from these amphicyonids (i.e., A. major, A. galushai, and Y. americana). The neck of Morphotype 1 is mediolaterally thinner and proximodistally shorter, and the fibular facet is dorsoventrally thicker than in Morphotypes 3 and 4. Moreover, Morphotype 1 is taller than the two others. The neck of Morphotype 3 is proximodistally longer and the head edges are less marked than in the others. In Morphotype 4, the distal edge of the trochlea medial lip is more proximal than the lateral lip and the plantar tendon groove is more marked. Morphotype 2 is assigned to Amphicyonidae because of its strong resemblance to the amphicyonid Daphoenodon robustum (North America, Miocene) ( Hunt 2009). Indeed, the shape of the trochlea, relatively well excavated, the elongated neck, and the shape of the head, which is not mediolaterally wide in comparison to the width of the neck, are observed in Daphoenodon robustum.
The morphology of the calcaneus Morphotype A is closely similar to that of Amphicyon galushai ( Hunt 2009), which also has a broad tubercle on its proximal part that is much thinner on its distal part. Moreover, the sustentacular facet is rounded and distally placed. Morphotype B is similar to the specimen of Daphoenodon robustum illustrated in Hunt (2009). The sustentaculum tali is more proximally located than in the other morphotypes. The plantar tubercle is ventrally and distally long, forming a tip at the cuboid facet. Morphotype C is morphologically similar to the calcaneus of the European Amphicyon lathanicus Ginsburg, Cheneval, Janvier, Pouit & Sen, 2000 (Miocene) ( Ginsburg 2002). The sustentaculum tali is distally located, the cuboid facet is inclined with respect to the mediolateral axis and is distally strongly concave. Its morphology is also strongly similar to that of Cynelos lemanensis Pomel, 1846 ( Peigné & Heizmann 2003), Afrocyon ginsburgi Morales, Pickford, Soria & Fraile, 1998 ( Morales et al. 2016) and Amphicyon longiramus White, 1942 ( Olsen 1960). The four amphicyonids share the following features: the peroneal process is poorly broad, the sustentaculum tali is distally positioned and the cuboid facet is concave. Moreover, the sustentacular facet is rounded in both C. lemanensis and Morphotype C. At the opposite side, the sagittal groove is more defined and the tuber calcanei is distally a little thinner in C. lemanensis. Relative abundance and body mass (see below) support the attribution of these morphotypes to amphicyonids, but they cannot be further assigned to infra-familial ranks.
RELATIVE ABUNDANCES
In almost all collections studied here, amphicyonids are by far the most abundant group ( Table 2). This is also true for the dental specimens of this carnivoran group in the ULiege collection, with 30.73%. Amphicyonids are not the most abundant carnivorans in the KUL collection, but still represent 31.03% of the assemblage, making it the second most abundant group in this collection after Ursoidea (39.66%). The high relative abundance of amphicyonid tarsal bones is thus congruent with the high relative abundance of amphicyonids based on dental remains.
BODY MASS ESTIMATION
The body mass estimated for Cynodictis lacustris based on its astragalus (1 to 2 kg; Table 4) is lower than the values obtained from dental material (c. 5 kg; Table 3). For the European amphicyonids, the body mass estimated from the lower first molar varies between 4 and 134 kg ( Table 3). This range is very broad and is greater than the values obtained for the four astragalar morphotypes. Morphotypes 1 and 2 fall within this range, but Morphotypes 3 and 4 are just outside this range with values below 3 kg; one can note that the latter case is similar to that of C. lacustris ( Table 4).
Among the amphicyonids recorded in the Paleogene of Europe ( Table 3), body masses between 5 and 10 kg correspond to the genera Cynodictis, Symplectocyon Springhorn, 1979 , and “ Cynodictis ” (C. exilis Teilhard de Chardin, 1915 and “ C. ” palmidens Teilhard de Chardin, 1915). Therefore, Morphotypes 3 and 4 might correspond to these genera.
Morphotype 1 groups heavier specimens (between 8 and 17 kg based on astragalus; Table 4). Because it seems that the body masses estimated from the astragalus are lower than those estimated from the m1, Morphotype 1 could correspond to the smallest species of Cynelos, Cynelos rugosidens Schlosser, 1899 or Cynelos crassidens Filhol, 1876 (≈ 23-24 kg). Some specimens could also correspond to the Oligocene amphicyonid Goupilictis Ginsburg, 1969 (13 kg).
Finally, Morphotype 2 could also include specimens that correspond to the genus Cynelos Jourdan, 1862. It may also include representatives of the genera Pseudocyonopsis Kuss, 1965 and Haplocyon Schlosser, 1901 ( Table 3).
Interestingly these new tarsal bones indicate significant differences in body mass within the same family ( Table 4), as does the dental material ( Table 3). Furthermore, there are no specimens that may correspond to the largest European amphicyonids Brachycyon Filhol, 1872, Harpagocyon Springhorn, 1977, Ysengrinia Ginsburg, 1965, Haplocyonopsis Bonis, 1973, and Crassidia Heizmann & Kordikova, 2000. It is worth noting that these genera are only known in the Chattian (MP26-MP30). Thus, the astragali studied here might come from Priabonian and Rupelian localities, except if some belong to Goupilictis.
LOCOMOTION
Posture and locomotion significantly affect the morphology of the tarsal bones ( Szalay & Decker 1974; Jenkins & McClearn 1984; Taylor 1989). The crurotarsal joint, where the tibia and astragalus are in contact, is the main axis of flexion of the foot. The posture is therefore related to the morphology of this articulation ( Wang 1993). The calcaneus, which articulates with the astragalus, also has an important role in the movement of the hindlimb because of the insertion of the m. gastrocnemius and m. soleus. They attach to its distal end via the achilles tendon, which constitutes the main lever of plantar flexion ( Barone 2000). Among the Carnivora , two postures are recognized: plantigrady and digitigrady. Some authors define an intermediate state present in many extant Mustelidae and Viverridae : semi-digitigrady ( Wang 1993; Polly 2008).
It appears that the absence of the trochlear foramen and a tendinous groove in digitigrade predators allows the tibia to rotate over the entire surface of the trochlea and thus to have a greater amplitude of flexion-extension ( Wang 1993). According to Ginsburg (1961), an elongated astragalus is associated with digitigrade locomotion. Carrano (1997) remarked that the orientation of the astragalus head is a distinctive element between digitigrade and plantigrade postures: the head is oriented in the same direction as the body in dorsal view in a plantigrade mammal, whereas in a digitigrade mammal, it is inclined relative to the direction of the body of the astragalus. According to Polly (2008), in a digitigrade animal the calcaneal ectal facet is sharply convex, the peroneal process is small, the sustentaculum tali is more proximally and posteriorly positioned, and the astragalar ectal facet is sharply curved. In plantigrade animals: the calcaneal ectal facet is rounded, the peroneal process is long, the sutentaculum tali is larger and distally positioned, the astragalar neck is narrow, and the astragalar ectal facet is shallow. Finally, the presence of large and flat facets on these two bones causes a reduction of intertarsal mobility (Polly 2008).
All the postures and locomotion hypothesized for the sample presented herein are listed in Table 6. Morphotype 1 has a plantar tendinous groove, and the head is not inclined towards the mediolateral axis. The ectal facet is shallow, as for Ailurus fulgens Cuvier, 1825 (Ailuridae) and Bassaricyon gabbii Allen, 1876 (Procyonidae) , two plantigrade species (Polly 2008). It has a narrow neck and an ectal facet that is shallow. These characters suggest that Morphotype 1 corresponds to a plantigrade animal. Morphotype 2 has a longer neck than in Morphotype 1, and a head that is slightly inclined towards the mediolateral axis. The neck is also larger and the ectal facet is sharply curved, as observed in Canis lupus Linnaeus, 1758 (Canidae) , Felis catus Linnaeus, 1758 , Leptailurus serval ( Schreber, 1776) , and Lynx rufus ( Schreber, 1777) (Felidae) , which are all digitigrade (Polly 2008). Morphotype 2 therefore better corresponds to a digitigrade animal. Moreover, it strongly resembles the astragalus of the North American amphicyonid Daphoenodon robustum, which was considered a digitigrade animal capable of powerful propulsive force ( Hunt 2009). However, Morphotype 2 has a tendinous groove, which is placed fairly ventrally on the trochlea and weakly excavated. Flexion-extension should therefore have moderate amplitude, but more than a plantigrade animal with a strongly defined tendinous groove. Daphoenodon robustum does not appear to have a tendinous groove ( Hunt 2009 does not mention this structure, which is also not visible on the illustrations). Because of this morphology, Morphotype 2 is probably better characterized as a semi-digitigrade animal. Morphotypes 3 and 4 have a head that is oriented on the mediolateral axis as well as a plantar tendon groove, like in the plantigrade species used in Carrano’s (1997) analyses and Procyon lotor Linnaeus, 1758 (Procyonidae) ( Wang 1993). The neck is long and narrow and the ectal facet is well curved (deeper in Morphotype 3 than in Morphotype 4), as in B. gabbii and A. fulgens , which are plantigrade mammals (Polly 2008). Morphotypes 3 and 4 are therefore interpreted as plantigrade, but the latter displays a larger range of movements enabled by a less deep trochlear surface.
Morphotypes A and C have a rounded sutentaculum tali that is rather distal; this structure is even more distal in Morphotype C, as observed in A. fulgens and B. gabbii , which are plantigrade (Polly 2008). This would therefore be characteristic of a plantigrade animal. However, in Morphotype C, the peroneal process is poorly broad, which seems to be a digitigrade feature (e.g., L. serval ; Polly 2008). The gear ratio for morphotype A is 1.23 ( Table 5), which is similar to that of Vulpes velox ( Say, 1823) ( Canidae ; digitigrade, terrestrial), Spilogale gracilis ( Linnaeus, 1758) ( Mephitidae ; plantigrade, terrestrial), Taxidea taxus ( Schreber, 1777) ( Mustelidae ; plantigrade, semifossorial), and Nasua narica ( Linnaeus, 1766) ( Procyonidae ; plantigrade, scansorial) ( Polly 2010; Polly et al. 2017). The gear ratio for Morphotype C (1.18; Table 5) is similar to that of Tremarctos ornatus ( Cuvier, 1825) ( Ursidae ; Plantigrade, scansorial), Potos flavus ( Schreber, 1774) ( Procyonidae ; plantigrade, arboreal) and Galictis vittata ( Schreber, 1776) ( Mustelidae ; plantigrade, semi-fossorial) ( Yensen & Tarifa 2003; Polly 2010; Polly et al. 2017). Morphotypes A and C may therefore correspond to plantigrade amphicyonids. Morphotype B has a more proximal sustentaculum tali, as in F. catus and L. rufus (Polly 2008) . In addition, its morphology is close to that of the calcaneus of Daphoenodon robustum, which is digitigrade as indicated above ( Hunt 2009). It also resembles the specimen of the Miocene digitigrade Plithocyon ursinus Cope, 1875 in North America (Paleontological Collection of MNHN), which also has a proximal sustentaculum tali and a distally long plantar tubercle. On the other hand, the peroneal process is well broad and the sustentaculum tali is located a little more dorsally, which seem to be plantigrade features (e.g., A. fulgens ; Polly 2008). Even if the sustentaculum tali is more proximal than the other morphotypes, it is still far distal relative to extant digitigrade carnivorans (e.g., F. catus ; Polly 2008). The gear ratio for Morphotype B is 1.25 ( Table 5), which is close to P. lotor (semi-digitigrade, scansorial), Martes americana ( Turton, 1806) ( Mustelidae ; plantigrade, scansorial) and Conepatus chinga ( Molina, 1782) ( Mephitidae ; semi-digitigrade, terrestrial) ( Polly 2010; Polly et al. 2017). Morphotype B would therefore rather be a semidigitigrade and terrestrial or scansorial animal, but probably not cursorial (gear ratio less than 1.22).
The head of the astragalus of Cynodictis lacustris is inclined with respect to the mediolateral axis and the tendinous groove seems to merge with the trochlea, as in the digitigrade Urocyon cinereoargenteus ( Schreber, 1775) ( Wang 1993) . However, the neck is quite short and the astragalar ectal facet is well curved, as in F. catus and L. rufus which are digitigrade and scansorial. In addition, the sustentaculum tali of the calcaneus is not placed very distally, as can be observed in a digitigrade animal (e.g., C. lupus ; Polly et al. 2017). The gear ratio is 1.27 for C. lacustris ( Table 5), which is similar to Vulpes vulpes ( Linnaeus, 1758) ( Canidae ; digitigrade, terrestrial), Canis latrans Say, 1823 ( Canidae ; digitigrade, terrestrial), Canis rufus Audubon & Bachman, 1851 ( Canidae ; digitigrade, terrestrial), and Panthera onca Linnaeus, 1758 ( Felidae ; digitigrade, scansorial) ( Polly et al. 2017). The canid species (i.e., Canis Linnaeus, 1758 and Vulpes Frisch, 1775 ) are all cursorial ( Polly et al. 2017). Therefore, Cynodictis lacustris can be considered as digitigrade and cursorial.
Interpretations are more contentious regarding locomotion. Based on the model of Polly & MacLeod (2008), the calcaneus of Cynodictis morphology is intermediate between the terrestrial and the scansorial morphologies. Morphotypes A and C are between the arboreal and terrestrial morphologies. But the specimens used for the implementation of this model are all extant animals and, in the case of plantigrady, the majority of the specimens are arboreal. Therefore, Polly & MacLeod’s (2008) model links plantigrady and arborality. While it is true that arboreal animals are often plantigrades ( Taylor 1989; Panciroli et al. 2017), plantigrades are not necessarily arboreal ( Wang 1993). Morphotypes A and C do not exhibit a large sustentaculum tali, in contrast to arboreal animals. Therefore, Morphotypes A and C may represent terrestrial predators.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.