Natalina cafra (Férussac, 1821)
|
publication ID |
https://doi.org/10.5733/afin.051.0101 |
|
persistent identifier |
https://treatment.plazi.org/id/110B87C2-FF98-FFC9-D7F0-FF12FC6EFB38 |
|
treatment provided by |
Felipe |
|
scientific name |
Natalina cafra (Férussac, 1821) |
| status |
|
Natalina cafra (Férussac, 1821) View in CoL View at ENA
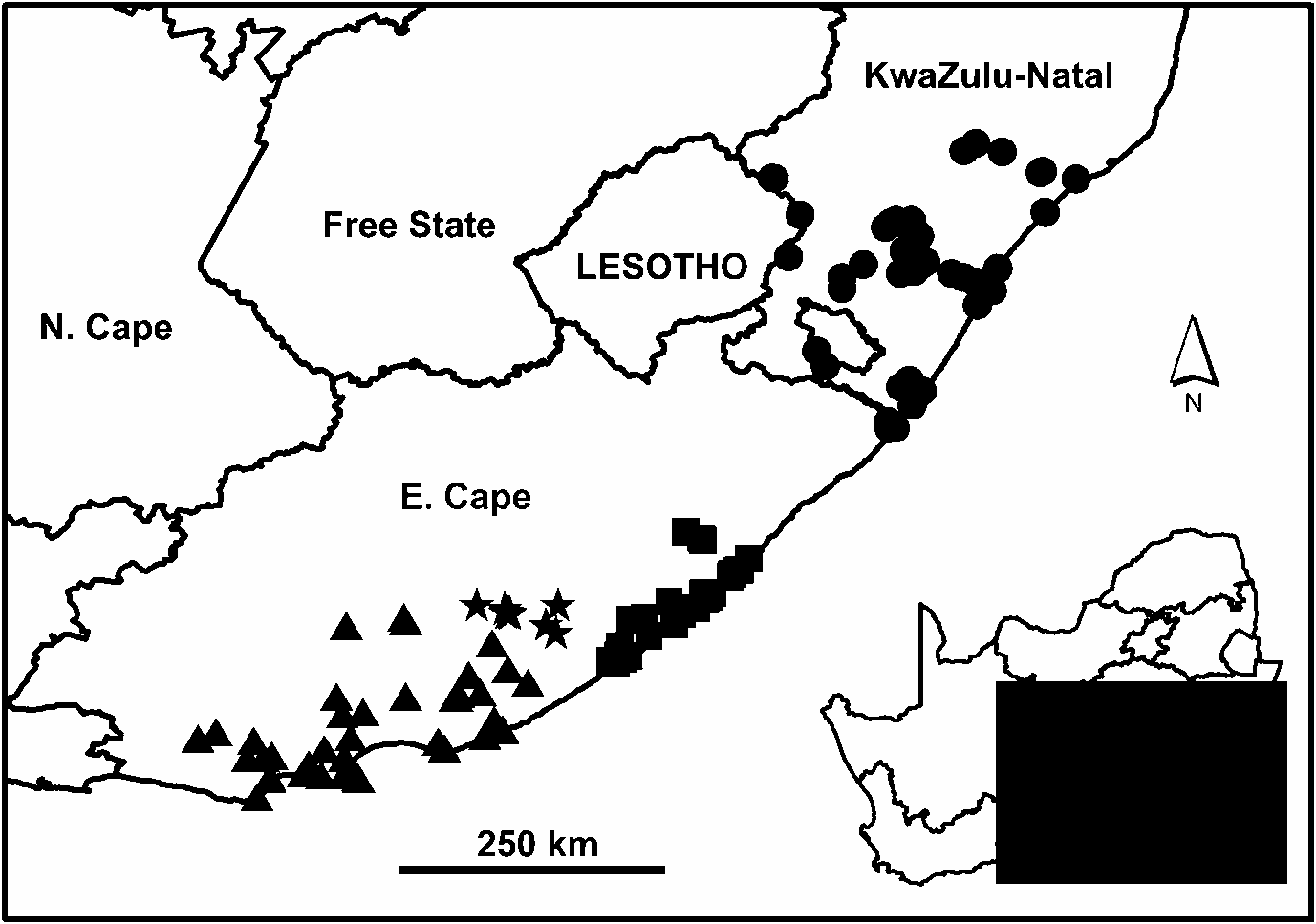
The conspicuous disjunction in the distribution of N. cafra ( Fig. 16 View Fig ) has remained an unexplained anomaly. Populations occur over a considerable extent in both KwaZulu-Natal and E. Cape, but there is a range hiatus of over 175 km in the north-eastern part of E. Cape (between Xora and Mtamvuna rivers), where no specimens have been recorded from either coastal or inland localities. Although this area cannot be described as well sampled, its malacofauna has been the focus of a number of recent surveys jointly undertaken by the Natal Museum and East London Museum. It is therefore likely that the lack of records from this region, particularly of a large and conspicuous animal such as this, reflects a genuine absence.A similar hiatus is evident in the distribution of the cerastid Gittenedouardia meridionalis (Pfeiffer, 1848) ( Herbert & Kilburn 2004) .
Molecular data has now shed further light on this issue, indicating that the KwaZulu-Natal population constitutes a distinct genetic lineage standing apart from the E. Cape population ( Moussalli et al. 2009). Furthermore, the E. Cape population itself comprises three distinct lineages with strong geographic structure. Although these findings add to the complexity of the problem, they also illuminate some of the associated taxonomic uncertainties surrounding the use of the available names in identifying specimens.
In the light of this genetic data, it has been necessary to completely re-evaluate the taxonomy of N. cafra . Accordingly, we recognise each of the lineages identified genetically as separate subspecies. This decision is strongly supported by the fact that the lineages are geographically allopatric ( Fig. 16 View Fig ), acknowledging that sampling of the Amathole lineage is limited. Nevertheless, in terms of shell characters and soft part anatomy there is little, and sometimes nothing which serves to reliably differentiate the subspecies morphologically. Variation in shell proportions and colour is considerable, while the anatomy of the distal reproductive tract is highly conservative. Some of the subspecies, however, show consistent (though not diagnostic) features in shell proportions which are discussed below. There also appear to be consistent differences in the number of lateral teeth per transverse row of the radula, but in some cases this data is based on a limited sample.
Connolly (1939) described globose, cafra -like specimens as a new species, Natalina compacta , but in practice it has in the past been difficult to apply this name with confidence due to intergrading variability in shell proportions. However, sequence data from a globose Natalina specimen from the type locality of N. compacta (and thus almost certainly representative of that taxon) indicates that it clusters, together with less elevated southern Eastern Cape specimens, in a well-supported N. cafra cafra clade ( Moussalli et al. 2009). We thus consider Natalina compacta to be a synonym of N. cafra cafra .
A further complication emerging from the analysis of molecular data is the fact that Natalina cafra appears paraphyletic ( Moussalli et al. 2009). N. beyrichi , itself comprising a well-supported clade showing little genetic diversity, nests with strong support within the N. cafra complex, as sister taxon to the E. Cape N. cafra lineage. N. beyrichi , however, is a morphologically distinctive taxon which overlaps in distribution with N. cafra between the Kei and Mbashe rivers, yet the two remain clearly separable in the overlap zone. We thus have a situation where the gene tree does not match the species tree. Funk and Omland (2003) have outlined a number of genetically legitimate reasons why gene trees may render species paraphyletic, and suggest that speciation by peripheral isolation (‘budding’) may commonly result in the nesting of a geographically restricted daughter species within a more widely distributed parental species. Similarly, Avise and Robinson (2008) have drawn attention to gene tree vs species tree discordance due to the phenomenon of hemiplasy resulting from the sorting of ancestrally polymorphic lineages retained across successive nodes in a species tree. Given the absence of morphological differentiation between the E. Cape and KZN N. cafra lineages, and the fact that resolution at this level in our phylogenetic tree is largely provided only by mtDNA ( Moussalli et al. 2009), we consider that the evidence currently available is insufficient to justify forcibly removing this paraphyly by recognising the KwaZulu-Natal lineage as a distinct species. Of course, this may ultimately need to be considered if additional morphological and molecular evidence is obtained.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.