Hemicolpus abdominalis Hustache, 1938
|
publication ID |
https://doi.org/10.11646/zootaxa.5227.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:FA079CCC-5878-40B5-AA87-6DD88FCD9925 |
|
DOI |
https://doi.org/10.5281/zenodo.7525355 |
|
persistent identifier |
https://treatment.plazi.org/id/104C87AC-FFB3-187B-36C4-FA01FAC1E044 |
|
treatment provided by |
Plazi |
|
scientific name |
Hemicolpus abdominalis Hustache, 1938 |
| status |
|
Hemicolpus abdominalis Hustache, 1938 View in CoL View at ENA
Figs. 31–61 View FIGURES 31–34 View FIGURES 35–38 View FIGURES 39–43 View FIGURES 44–47 View FIGURES 48–52 View FIGURES 53–57 View FIGURES 58–61
Hemicolpus abdominalis Hustache, 1938: 64–65 View in CoL [description, Brazil]; Wibmer & O’Brien 1986: 274 [catalog]; Hespenheide 2018: 120–121 [reconsideration]; Sanz-Veiga et al. 2017: 1–18 [host plant]; Sanz-Veiga et al. 2021: 1006–1018 [distribution, life cycle].
Hemicolpus abdominalis: Hespenheide 2018: 120 View in CoL [in part].
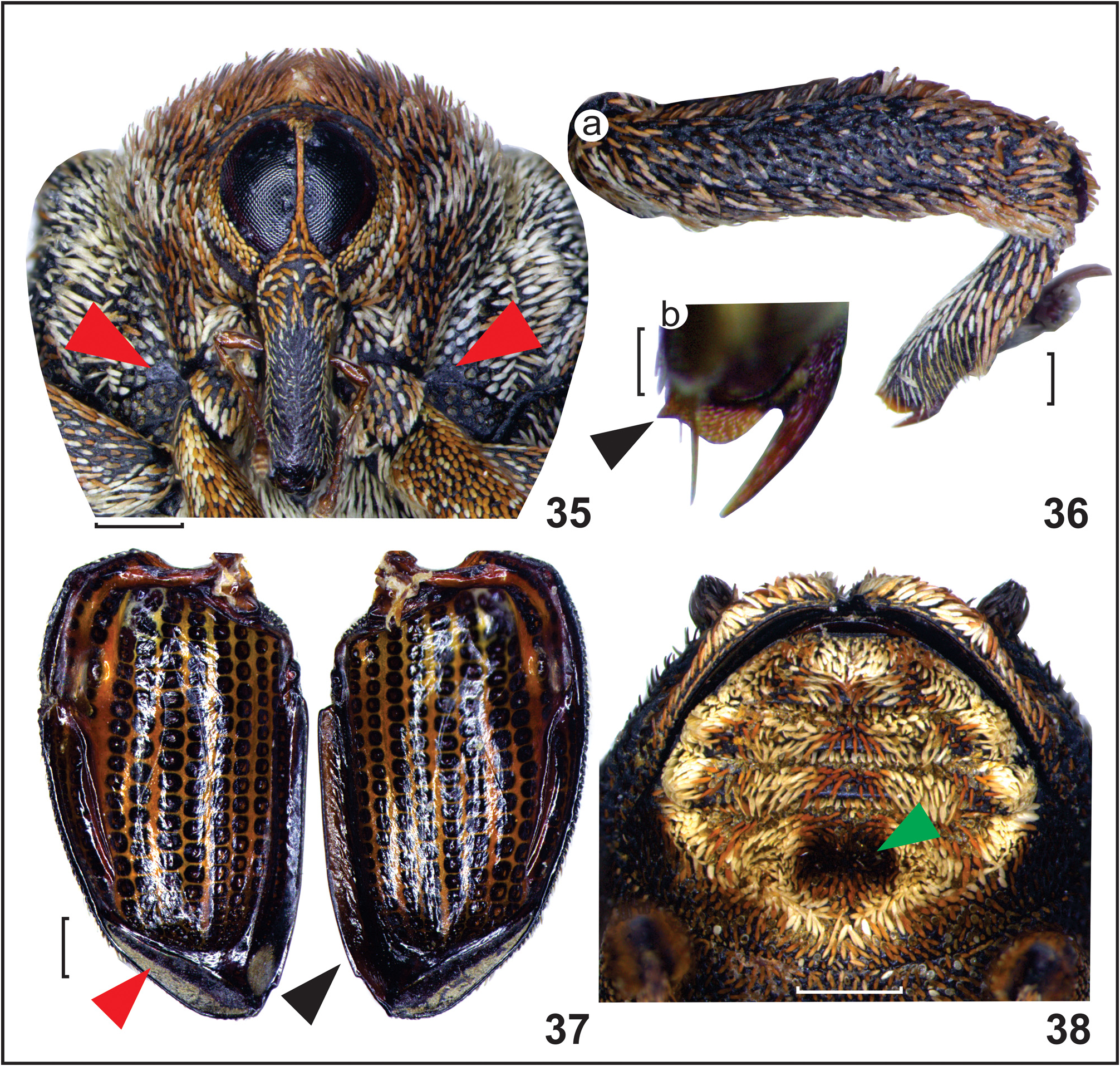
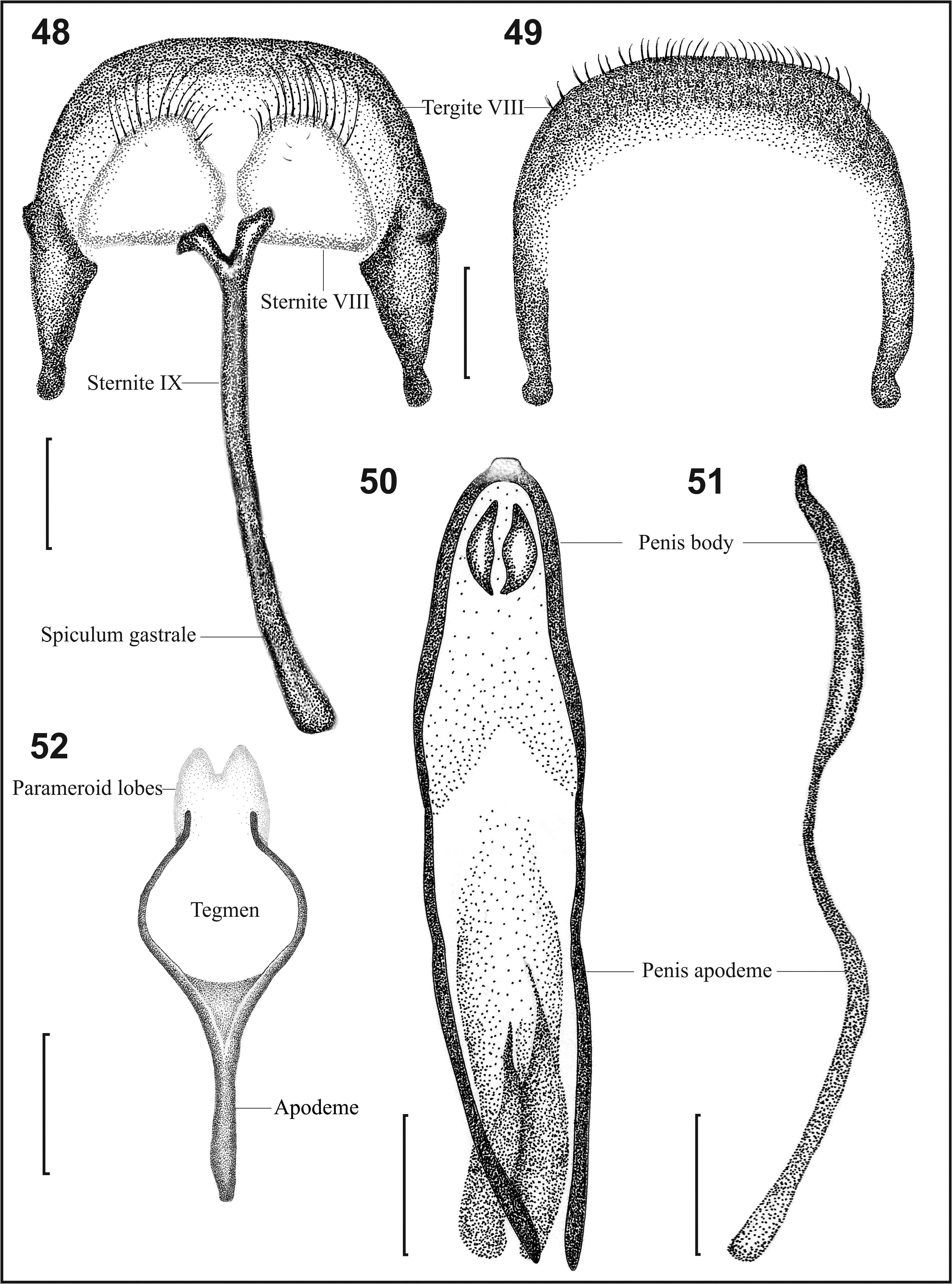
Diagnosis. This species differs from the remaining species (with the exception of H. maragatensis ) by the excavated mesoventrite ( Fig. 34 View FIGURES 31–34 ), and by the pair of tubercles on the second abdominal ventrite ( Figs. 46, 47 View FIGURES 44–47 ). From H. maragatensis it is distinguished by the darker integument ( Fig. 33 View FIGURES 31–34 ); the glabrous basal patch never extends over a third of the mesanepisternal area ( Fig. 35 View FIGURES 35–38 ); the lack of carina on metafemur ( Fig. 36a View FIGURES 35–38 ); the round inner flange of the tibial apex almost as wide as long ( Fig. 36b View FIGURES 35–38 ); the premucro oriented 45° ( Fig. 36b View FIGURES 35–38 ); and by the preapical constriction of the penis ( Fig. 50 View FIGURES 48–52 ).
Adult redescription. Body very robust, oval, widest at elytral humeri, 4.3–5.8 mm long in males, and 4.6–6.0 mm long in females. Body integument strongly to moderately punctate, black to dark reddish, with rostrum and tibia dark reddish, antenna and tarsi brownish. Body moderately to sparsely covered with pale or yellowish, brown, or reddish-brown scales ( Figs. 31, 32 View FIGURES 31–34 ). Scales slender, erect and recumbent on pronotum, slender, sparser and semierect on elytra, flat or oval on head and femur ( Figs. 31, 32 View FIGURES 31–34 ). Ventrally with dense, yellowish scales ( Figs. 46, 47 View FIGURES 44–47 ).
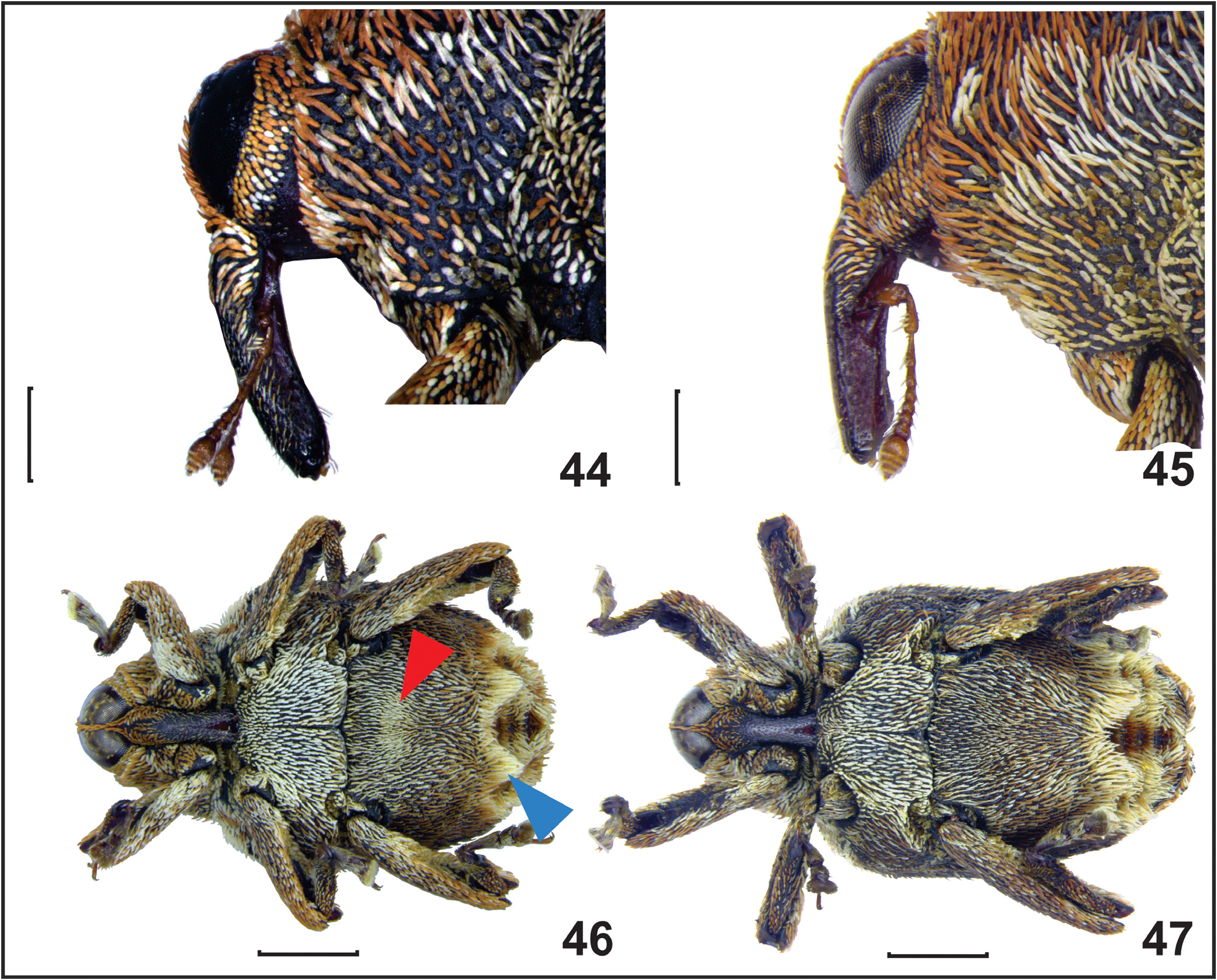
Head. Width 0.91–1.25, globose, vertex finely punctate, covered with small, flat, oval, yellowish and palebrown scales. Eyes emarginate dorsally, acuminate ventrally, separated by one or two rows of short, slender, palebrown scales at closest point near middle ( Fig. 35 View FIGURES 35–38 ). Rostrum. In males, 0.94–1.27 mm long; in females, 0.98–1.25 mm long; subcylindrical, ventrally flat, slightly wider at base, arcuate at basal third near antennal insertion, slightly curved toward apex, with weak lateral constriction in middle, feebly carinate at basal third along dorsal midline, with shallow punctures, smoother along apical ⅔ ( Figs. 44, 45 View FIGURES 44–47 ). Female rostrum weakly curved, almost straight at apical ⅔, slightly more slender than male ( Fig. 45 View FIGURES 44–47 ). Scrobe well defined, dorsally delimited by carina extending toward rostrum base. Basal third covered with pale-brown and yellowish scales, inconspicuous pale scales toward apex ( Fig. 44 View FIGURES 44–47 ), glabrous along midline and ventrally, in females glabrous along apical ⅔ ( Fig. 45 View FIGURES 44–47 ). Antenna inserted at basal third of rostrum in both sexes; scape short and clavate, 1.4 times as long as antennal segment I, segment II 0.8 times as long as I, and 1.6 times as long as III, IV 0.8 times III, and 1.2 times segment V, VI and VII subequal slightly smaller than V; club elongate oval, 1.6 times as long as wide, slightly longer than first segment.
Prothorax. Pronotum 1.68–2.13 mm long and 1.92–2.50 mm wide at the base in males, 1.62–2.22 mm long, and 1.92–2.53 mm wide in females; wider than long, widest posteriorly ( Fig. 32 View FIGURES 31–34 ). Dorsally convex ( Fig. 31 View FIGURES 31–34 ), sides slightly round, and gradually converging anteriad, anterior margin round, with weak anterior constriction, posterior margin bisinuate with midline lobe extending toward scutellum ( Fig. 32 View FIGURES 31–34 ). Integument with coarse broad punctures, carinate along dorsal midline. Dorsally mostly covered with erect or semierect, slender pale-brown scales interspaced with few pale or yellowish scales completely exposing integument ( Fig. 32 View FIGURES 31–34 ), laterally sides with dorsally oriented recumbent or semierect, pale, and pale-brown scales ( Fig. 31 View FIGURES 31–34 ). Prosternal canal with posterior margin of basisternum projecting over anterior margin of sternellum, covered by flat yellowish scales ( Fig. 34a View FIGURES 31–34 ).
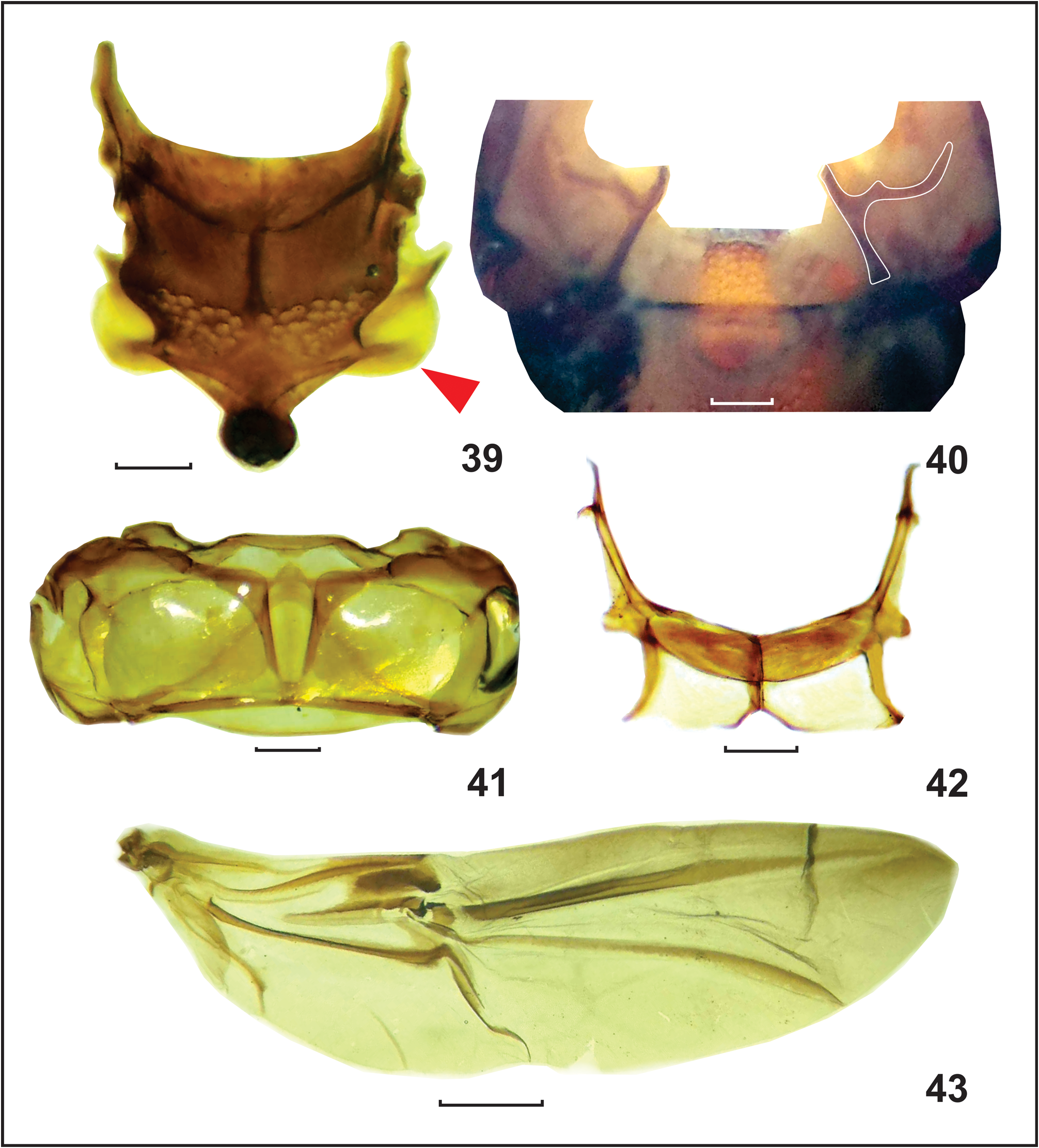
Mesothorax. Mesonotum with few punctures covered with setae at anterolateral margin; mesoscutummesoscutellum transition with broad punctures covered with pectinate scales; axillary cord round ( Fig. 39 View FIGURES 39–43 ); scutellar shield oval, covered with erect pale scales. Elytra, in males, 2.59–3.45 mm long, 2.78–3.68 mm wide at anterior extremity, 1.34–1.51 times as wide, and 1.40–1.82 times as long as pronotum; in females, 2.72–3.64 mm long, 2.74–3.73 mm wide at anterior extremity, 1.22–1.72 times as wide and 1.39–1.80 times as long as pronotum; widest at anterior third, with sides slightly round, gradually converging from middle toward posterior extremity, truncate at posterior margin ( Fig. 32 View FIGURES 31–34 ). Humeral callus round and prominent, covered by yellowish scales. Disc almost flat or slightly arcuate, slightly depressed behind scutellar shield and along elytral suture, with elytral declivity behind prominence of interstria 5 ( Figs. 31, 32 View FIGURES 31–34 ). Striae and interstriae width subequal, interstriae 9 and 10 slightly wider at basal half, striae with deep rectangular punctures, interstria with shallow punctures, interstria 5 with prominence near elytral declivity ( Fig. 31 View FIGURES 31–34 ). Sparsely covered with slender yellowish scales intermixed by brown and few longer dark-brown scales, prominence on interstria 5 with tuft of long brown or dark-brown scales, each strial puncture with only one pale or brown scale, pale scales densely arranged along posterior ¼ of elytral suture, posterior margin densely covered with pale, pale-brown or reddish-brown scales ( Fig. 32 View FIGURES 31–34 ). Ventrally, elytral apex with transverse stripe of yellowish microtrichia ( Fig. 37 View FIGURES 35–38 ); left elytron mesal flange abruptly produced from base to apex ( Fig. 37 View FIGURES 35–38 ). Mesanepisternum covered with yellowish and pale-brown scales, basal inner angle with small glabrous triangular patch over less than third of area of mesanepisternum ( Fig. 35 View FIGURES 35–38 ). Mesepimeron densely covered with semierect yellowish scales, dorsally with patch of long scales overlapping elytra. Mesendosternite with dorsally projecting, well-sclerotized, curved arm ( Fig. 40 View FIGURES 39–43 ). Mesoventrite strongly excavated, forming mesoventral canal ( Figs. 34a, b View FIGURES 31–34 ), laterally with two longitudinal carinae extending from anterior to posterior margin posteriorly meeting with metaventrite anterior carina ( Fig. 34b View FIGURES 31–34 ), channel covered with pectinate yellowish scales. Outer side of each lateral carinae with patch of dense yellowish scales behind mesocoxae ( Fig. 34b View FIGURES 31–34 ).
Metathorax. Metanotum with few long setae at anterolateral margin; scutellar groove with well-developed median longitudinal crest, anteriorly with well-developed transverse bridge; metascutum with convex angular posteromedial margin ( Fig. 41 View FIGURES 39–43 ). Metathoracic wing with well-developed 3A, unsclerotized R3 forming white line, with well-developed rm and mst, C-shaped 2rs, elongated rectangular 1rs smaller than 2rs, the 1A 2 defined near wing margin extending dorsally and merging with A through a1-a2 ( Fig. 43 View FIGURES 39–43 ). Metanepisternum mostly with pale and brown scales; metanepisternal suture with round yellowish type III sclerolepidia ( Fig. 33 View FIGURES 31–34 ). Metendosternite with wide stalk, slender furcal arm, and well-developed hemiductus ( Fig. 42 View FIGURES 39–43 ). Metaventrite excavated beneath anterior margin to receive rostrum apex, with anterior carina connected to mesoventrite lateral carinae ( Fig. 34b View FIGURES 31–34 ). Legs. Pro- and mesocoxae with inner tubercle. Ventrally, femora with longitudinal sulcus, slightly curved at apical third, both inner and outer apical margins with tiny tooth. All femora coarsely punctured; pro- and mesofemora mostly covered with densely arranged flat pale-brown scales interspaced with pale scales; metafemur with sparser darker scales at basal ¾ and paler scales at apical third ( Fig. 36a View FIGURES 35–38 ). Tibia covered with pale and brown scales, apically with longitudinal yellowish setal comb near outer margin ( Fig. 36a View FIGURES 35–38 ). Apex with large hook-like uncus, well-developed round inner flange almost as wide as long, and small premucro directed at 45° angle to longitudinal axis of tibia ( Fig. 36b View FIGURES 35–38 ), with few long setae at each side of premucro and inner flange ( Fig. 36b View FIGURES 35–38 ).
Abdomen. Abdominal ventrite I with shallow anteromedial depression in males, shallower or flat in females, weakly ascending at posterior half. Ventrite II strongly angulate, sharply ascending at posterior ¾, with somewhat deep posteromedial concavity ( Fig. 38 View FIGURES 35–38 ), anteriorly bordered by pair of tubercles ( Fig. 46 View FIGURES 44–47 ). Ventrites III–V strongly ascending, III and IV subequal, more slender than II and V. Ventrite V subtrapezoid ( Fig. 38 View FIGURES 35–38 ). Ventrite I covered with yellowish and pale-brown scales, males with oval patch of densely arranged yellow scales in anteromedial depression ( Fig. 46 View FIGURES 44–47 ), primarily absent in females ( Fig. 47 View FIGURES 44–47 ). Tubercles covered with tuft of long pale scales, concavity densely covered with dark brown or reddish-brown scales ( Fig. 38 View FIGURES 35–38 ). Ventrites III and IV with central patch of brown or reddish-brown scales, and smaller patches of pale and brown scales on each side, with pale scales in tufts on sides ( Fig. 38 View FIGURES 35–38 ).
Male terminalia ( Figs. 48–52 View FIGURES 48–52 ).Abdominal tergite VIII subrectangular, 1.2 times wider than long, slightly wider at basal half; sides emarginate at basal third, with small tooth projecting from outer margin near middle, slightly convex and gradually converging toward apex; apical margin slightly convex or almost flat, with sides round ( Figs. 48, 49 View FIGURES 48–52 ); sclerotized at apical third and along periphery, not sclerotized at basal ¾; apical third dorsally with broad punctures, densely covered by slender setae ( Fig. 49 View FIGURES 48–52 ). Sternite VIII composed of two separated subtriangular plates slightly arcuate apically, outer side of each plate slightly convex, and basal margin almost twice length of apex ( Fig. 48 View FIGURES 48–52 ); apical margin covered with long setae and few shorter setae. Sternite IX apically emarginate forming a V-shaped structure with short subequal arms; spiculum gastrale slender, weakly curved, 1.4 times length of penis body, slightly enlarged at base ( Fig. 48 View FIGURES 48–52 ). Penis body 2.1 times as long as wide; sides slightly arcuate, with weak lateral constriction at middle, then gradually converging toward apex, with preapical constriction producing short and truncate apical projection; two apical sclerites subtriangular elongate; endophallus almost as long as penis body, or slightly shorter, with lobular extremity; penis apodeme 1.4 times longer than body, slightly enlarged anteriorly ( Figs. 50, 51 View FIGURES 48–52 ). In lateral view, penis body arcuate and acuminate at apical end ( Fig. 51 View FIGURES 48–52 ). Tegmen parameroid lobes not sclerotized, connate at apical third, almost half length of tegminal apodeme ( Fig. 52 View FIGURES 48–52 ).
Female terminalia ( Figs. 53–57 View FIGURES 53–57 ). Tergite VIII subtriangular, 1.1 times wider than long, wider at basal third, sides subparallel at basal third, then concave and converging toward apex; apical margin slightly convex or almost flat; sclerotized at posterior third and along periphery, weakly sclerotized at basal half ( Figs. 53, 54 View FIGURES 53–57 ); apical third covered with sparse slender setae dorsally, denser and longer toward apex margin ( Fig. 54 View FIGURES 53–57 ). Sternite VIII with Ushaped lobe, arms 0.7 times shorter than spiculum ventrale, slightly diverging toward apex, with lateral constriction in middle, apex wider with round margin covered with long setae, spiculum ventrale emarginate at base ( Fig. 55 View FIGURES 53–57 ). Gonocoxite elongate, about three times longer than wide, wider at basal ¾, weakly sclerotized and glabrous; stylus cylindrical, almost twice longer than wide, apex slightly narrow with few small setae ( Fig. 53 View FIGURES 53–57 ). Bursa copulatrix membranous, elongate, three times longer than wide, round and lobular after insertion of common oviduct ( Fig. 56 View FIGURES 53–57 ). Spermatheca well sclerotized, C-shaped, cornu as wide as collum, with spermathecal duct near common oviduct. Spermathecal gland membranous, elliptical, 1.5 times larger than spermatheca, and positioned near spermathecal duct insertion ( Fig. 57 View FIGURES 53–57 ).
External sexual dimorphism. Weak sexual dimorphism is evidenced by differences in the rostrum and first abdominal ventrite. The male rostrum is weakly curved after antennal insertion ( Fig. 44 View FIGURES 44–47 ), almost straight, slightly more slender, and nearly glabrous after antennal insertion in females ( Fig. 45 View FIGURES 44–47 ). In males, ventrite I with anteromedial depression, including oval patch of yellowish scales ( Fig. 46 View FIGURES 44–47 ). In females, depression shallower or absent, usually lacking yellowish patch ( Fig. 47 View FIGURES 44–47 ).
Remarks. The anteromedial depression on the first ventrite can be shallower in some males or extend toward the posterior margin, and the oval patch of yellowish scales is sometimes less distinct. Scale color may vary between individuals from a more yellowish or brownish general color to reddish-brown in some specimens. Metafemur scales vary from dark brown to pale or yellowish. The tooth on the lateral margin of the male tergite VIII is strongly produced or feeble. The male sternite VIII may vary from subtriangular to subtrapezoidal, and the apex of sternite IX from somewhat angular to curved. A few individuals also showed a slightly narrower apical projection of the aedeagus, with the two apical sclerites relatively longer. The specimens can be further differentiated from H. maragatensis by presenting a longer tuft of scales on interstria 5; by the lack of carina on metafemur ( Fig. 35 View FIGURES 35–38 ); the scales on pro- and mesofemur somewhat denser, mainly flat, or oval (sparser and slender scales in H. maragatensis ); and the premucro is oriented 45° ( Fig. 36b View FIGURES 35–38 ).
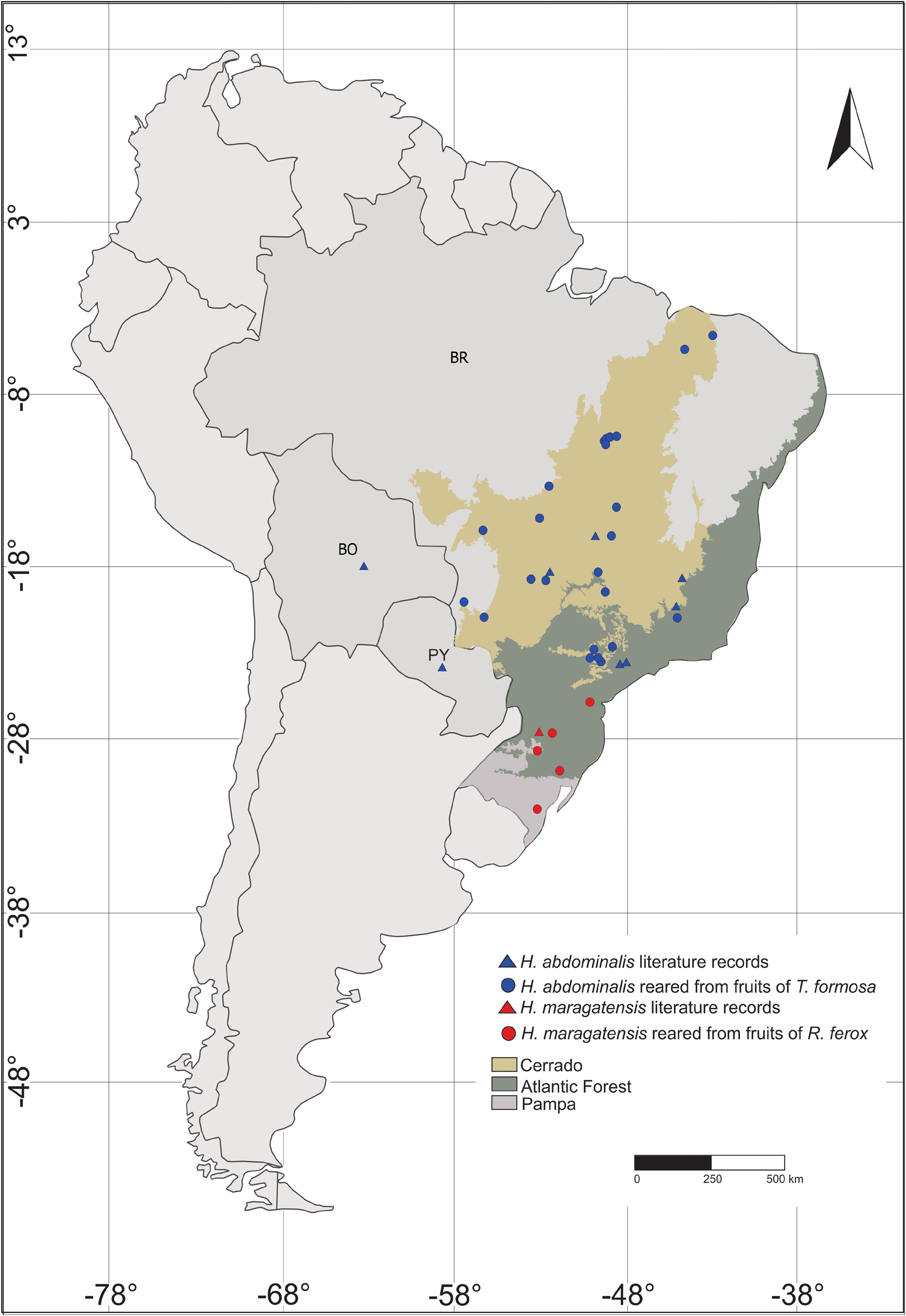
Distribution. This species is widespread in Brazil ( Fig. 1 View FIGURE 1 ): S„o Paulo (Itirapina, Jundiaí, Itu, Botucatu, Itatinga, Águas de Santa Bárbara, Pratânia, and Bauru), Minas Gerais (Uberlândia, Congonhas, Diamantina, and Belo Horizonte), Mato Grosso (Chapada dos Guimar„es, Nova Xavantina, and Ribeir„o Cascalheira), Mato Grosso do Sul (Miranda and Aquidauana), Goiás (Chapad„o do Céu, Serranópolis, Caldas Novas, Jataí, Pirineus and Chapada dos Veadeiros), Distrito Federal (Brasília), Tocantins (Palmas, Rio do Sono and Aparecida do Rio Negro), Piauí (Piripiri and Cocal); Bolivia and Paraguay ( Wibmer & O’Brien 1986; Hespenheide 2018; Sanz-Veiga et al. 2021). Its occurrence in the French Guiana is uncertain ( Hustache 1938).
Biology. Larvae are predispersal seed predators on fruits of Tocoyena formosa (Rubiaceae) , within which they develop by feeding on the pulp and seeds until it pupates, and from which the adults emerge at the end of the fruiting period (see details in Sanz-Veiga et al. 2017; 2021).
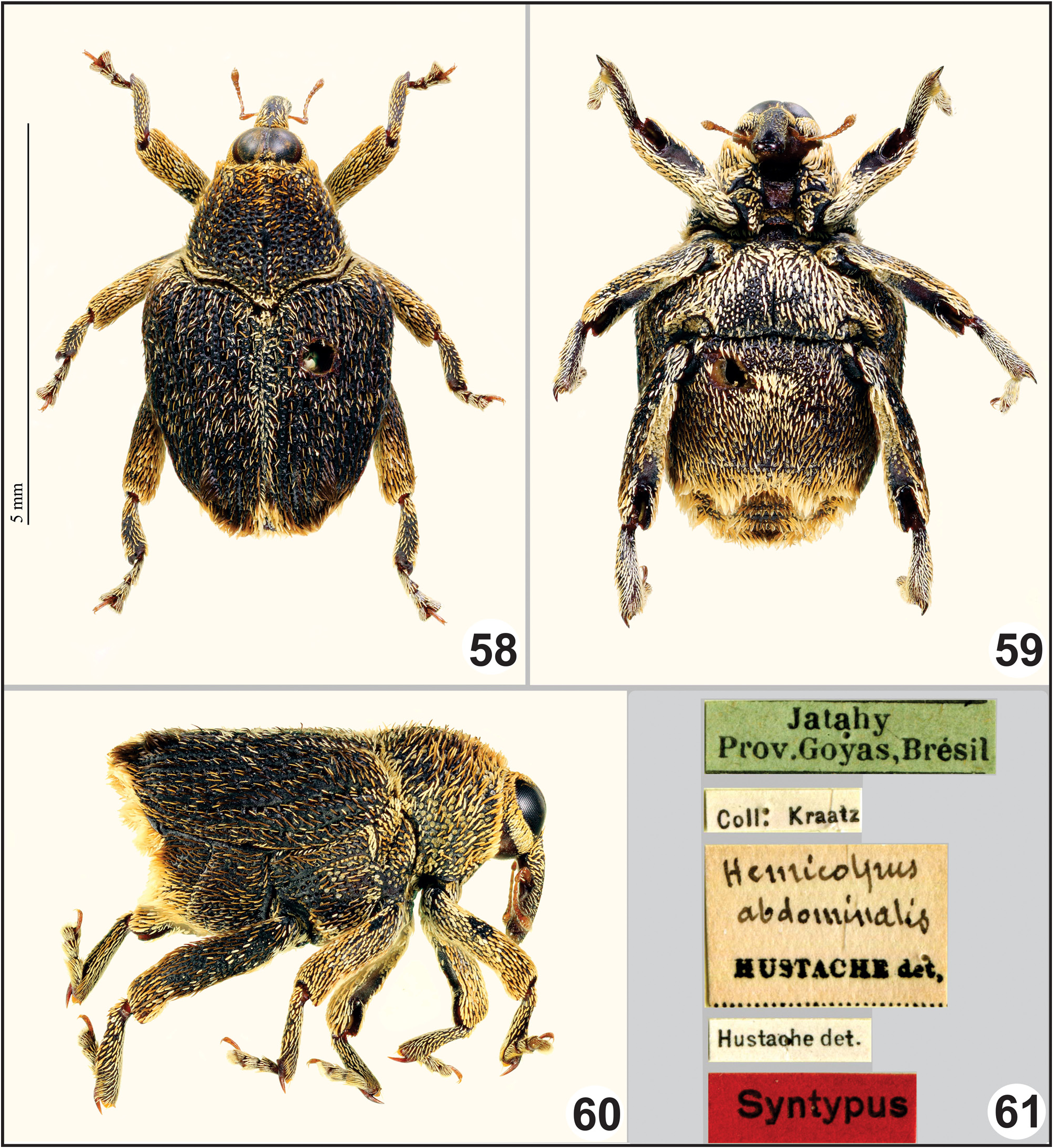
Type data. This species was originally described by Hustache (1938) from a series of 10 specimens from Jataí in the state of Goiás, and from Diamantina, Belo Horizonte , in the state of Minas Gerais, in Brazil. In the original description its occurrence in French Guiana was pointed as uncertain by the author ( Hustache 1938). Hespenheide (2018) first published dorsal and lateral images of a male syntype from Jataí located at the Deutsches Entomolologisches Institut, Senckenberg, M̧ncheberg, Germany ( SDEI). The male ( Figs. 58–60 View FIGURES 58–61 ) is here designated as the lectotype of Hemicolpus abdominalis Hustache to stabilize the usage of this name. It is labeled ( Fig. 61 View FIGURES 58–61 ) “Jatahy Prov. Goyas, Brèsil ” [green label with fine black border] / “Coll: Kraatz” [white label] / “ Hemicolpus abdominalis [handwritten] HUSTACHE det.” [yellow label] / “Hustache det.” [white label] / “ Syntypus ” [red label] / “ LECTOTYPE ♁ Hemicolpus abdominalis Hustache by Sanz-Veiga, Savaris & Leivas [red-bordered label added by us]; and “ Hemicolpus abdominalis Hustache det. Sanz-Veiga, Savaris & Leivas IV 2022 ”. The lectotype is pin mounted and is in good conditions, and is deposited in the Deutsches Entomolologisches Institut, Senckenberg, M̧ncheberg, Germany.
Specimens examined. BRAZIL. Goiás: Pirineus [ Parque Estadual da Serra dos Pirineus], GO [Goiás] 2.II.1962 J. Bechyné col., 2 specimens ( MZUSP). GoogleMaps Chapad „o do Céu, P.N. [Parque Nacional das Emas] EMAS, 15 Apr 2019, 18º17′3.6″S 52º51′56″W, 811 m, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., 5 specimens ( MELQ ESALQENT000603–607 ); GoogleMaps same, 2 specimens ( CESP); same, May 2019, 2 specimens ( CESP). Chapada dos Veadeiros, 14°04′5.4″S 47°37′20.4″W, 1222 m, Jan 2019, on fruit of Tocoyena formosa, F.W. Amorim, A.P. Moraes cols., 5 specimens ( MELQ ESALQENT000613–617 ); GoogleMaps same, 2 specimens ( CESP). Distrito Federal, Brasília, P. N. [Parque Nacional] de Brasília, 15°44′18.5″S 47°55′23.4″W, 1032 m, Jan 2019, reared from fruit of Tocoyena formosa, F.W.Amorim, A.P. Moraes cols., 5 specimens ( MELQ ESALQENT000623–627 ). GoogleMaps Mato Grosso: Chapada dos Guimarães, 15°24′43.7″S 55°50′15.4″W, 626 m, Mar 2019, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., 5 specimens ( MELQ ESALQENT000593–597 ). GoogleMaps Nova Xavantina, Bacaba Park, 14°42′40.7″S 52°21′07.1″W, 304 m, Mar 2019, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., 1 specimen ( MELQ ESALQENT000631 ); GoogleMaps same, May 2019, 1 specimen ( CESP). Ribeirão Cascalheira , 12°50′59.9″S 51°46′27.2″W, 343 m, Mar 2019, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., 1 specimen ( MELQ ESALQENT000632 ). GoogleMaps Mato Grosso do Sul: Aquidauana, 20°27′50″S 55°46′25″W, 160 m, May 2020, reared from fruits of Tocoyena formosa, C. Aoki, col., 3 specimens ( MELQ ESALQENT000628–630 ); GoogleMaps Minas Gerais: Uberlândia, Caça e Pesca, May 2018, 18°59′43.61″S 48°18′18.43″W, 842 m, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., 5 specimens ( MELQ ESALQENT000583–587 ); GoogleMaps same, May 2018, 4 specimens ( CESP); same, Jun 2018, 2 specimens ( CESP); GoogleMaps São Paulo: Águas de Santa Bárbara, May 2018, 22°49′36.21″S 49°14′42.18″W, 621 m, reared from fruit of Tocoyena formosa, P.A.S Veiga, col., 4 specimens ( CESP); GoogleMaps same, 2 specimens ( USNM). Jundiaí, SP [S„o Paulo ] XI. 1961, W. Bokermann col., GoogleMaps 2 specimens ( MZUSP). Itatinga, May 2018, 23°02′48.93″S 48°34′45.76″W, 836 m, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., GoogleMaps 3 specimens ( CESP); same, Jun 2018, 1 specimen ( CESP); GoogleMaps same, Jul 2018 ( CESP). Itu, Faz. [Fazenda] Pau d´Alho, 1.XI.1961, U. Martins col., GoogleMaps 1 specimen ( MZUSP). Pratânia, Faz. [ Fazenda] Palmeira Serra, 20 Apr 2017, 22°48′56.64″S 48°44′39.10″W, 722 m, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., 5 specimens ( MELQ ESALQENT000588–592 ); GoogleMaps same, 2 specimens ( CESP); same, May 2017, 2 specimens ( CESP); same, May 2015, 2 specimens ( CESP); same, May 2018, 1 specimen ( CESP). Bauru, Jardim Botânico, 22°20′18.39″S 49°00′38.64″W, 547 m, 07 May 2018, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., 5 specimens ( MELQ ESALQENT000618–622 ); GoogleMaps same, 2 specimens ( CESP); same, Apr 2018, 2 specimens ( CESP); same, Dec 2018, hand collected on flower of Tocoyena formosa, 1 specimen ( CESP); same, Jan 2019, 1 specimen ( CESP) GoogleMaps . Tocantins: Gererê, 10°25′25″S 48°16′38″W, 260 m, Jan 2019, reared from fruit of Tocoyena formosa, F.W. Amorim, A.P. Moraes cols., 5 specimens ( MELQ ESALQENT000608–612 ); GoogleMaps same, Jan 2019, 1 specimen ( CESP). Lajeado, 10°05′37″S 48°14′39″W, 700 m, Jan 2019, on fruit of Tocoyena formosa, F.W. Amorim, A.P. Moraes cols., 5 specimens ( MELQ ESALQENT000603–607 ). GoogleMaps Palmas, 10°13′41″S 48°22′03″W, 273 m, Apr 2019, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., 5 specimens ( MELQ ESALQENT000598– 602 ); GoogleMaps same, 1 specimen ( CESP); same, May 2019, 1 specimen ( CESP). Rio do Sono , 9º55′25″S 47º37′42″W, 310 m, May 2019, reared from fruit of Tocoyena formosa, P.A.S. Veiga col., GoogleMaps 2 specimens ( CESP); same, 2 specimens ( USNM); same, Jun 2019, 2 specimens ( CESP) GoogleMaps . Piauí: Cocal, 3º27′12″S 41º33′33″W, 130 m, 29 Jul 2022 reared from fruits of Tocoyena formosa, J. Siqueira col., 2 specimens ( MELQ ESALQENT0001734–735 ) GoogleMaps .
DNA Barcoding. Edited and aligned COI barcode region yielded fragments of 603 pb. Sequences of H. abdominalis and H. maragatensis were deposited at NCBI-GenBank database under the accession numbers: OP837054 View Materials –63 and OP837064 View Materials –73, respectively.The intraspecific genetic distance of COI barcode region ranged from 0.0017 to 0.021 (mean = 0.008, SD = 0.004) within H. abdominalis , and from 0.00 to 0.008 within H. maragatensis (mean = 0.003, SD = 0.002). The interspecific distance between the two species ranged from 0.165 to 0.187 (mean = 0.175, SD = 0.006), representing a genetic divergence of 17% between the two Hemicolpus species (Table 1).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hemicolpus abdominalis Hustache, 1938
| Sanz-Veiga, Priscila A., Savaris, Marcoandre, Leivas, Fernando W. T., Medeiros, Alexandre Da Silva & Amorim, Felipe W. 2023 |
Hemicolpus abdominalis: Hespenheide 2018: 120
| Hespenheide, H. A. 2018: 120 |
Hemicolpus abdominalis
| Sanz-Veiga, P. A. & Polizello, D. S. & Silva, D. P. & Savaris, M. & Amorim, F. W. 2021: 1006 |
| Hespenheide, H. A. 2018: 120 |
| Sanz-Veiga, P. A. & Jorge, L. R. & Benitez-Vieyra, S. & Amorim, F. W. 2017: 1 |
| Wibmer, G. J. & O'Brien, C. W. 1986: 274 |
| Hustache, A. 1938: 65 |