Nesamblyops subcaecus (Sharp)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5375.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:F3D0E008-556C-4FAD-BF51-4F1A714325DA |
|
DOI |
https://doi.org/10.5281/zenodo.10248408 |
|
persistent identifier |
https://treatment.plazi.org/id/055987E2-8B2F-736D-FF7D-D2D6FB388F66 |
|
treatment provided by |
Plazi |
|
scientific name |
Nesamblyops subcaecus (Sharp) |
| status |
|
Nesamblyops subcaecus (Sharp) View in CoL (dissected 5 exx.)
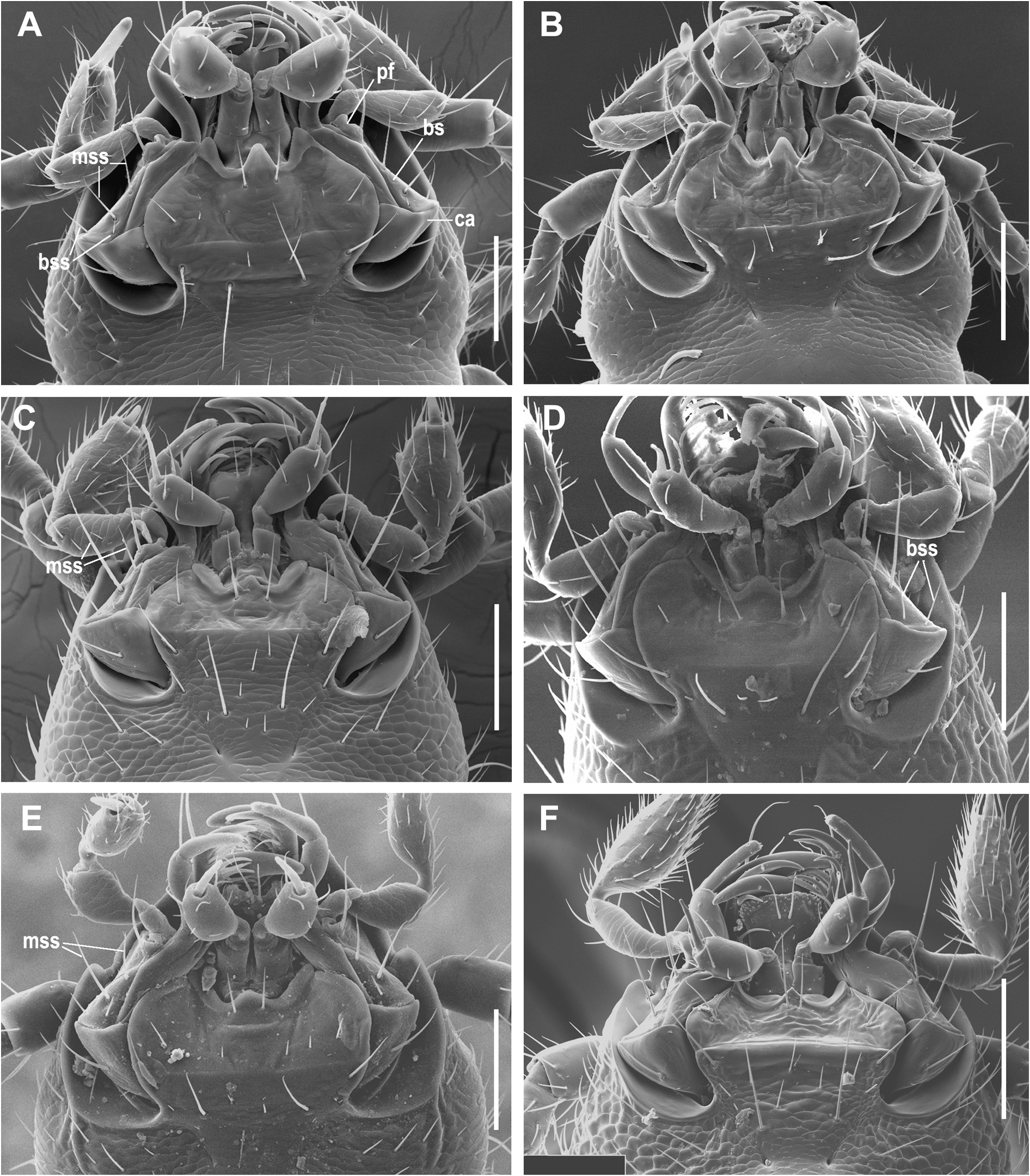
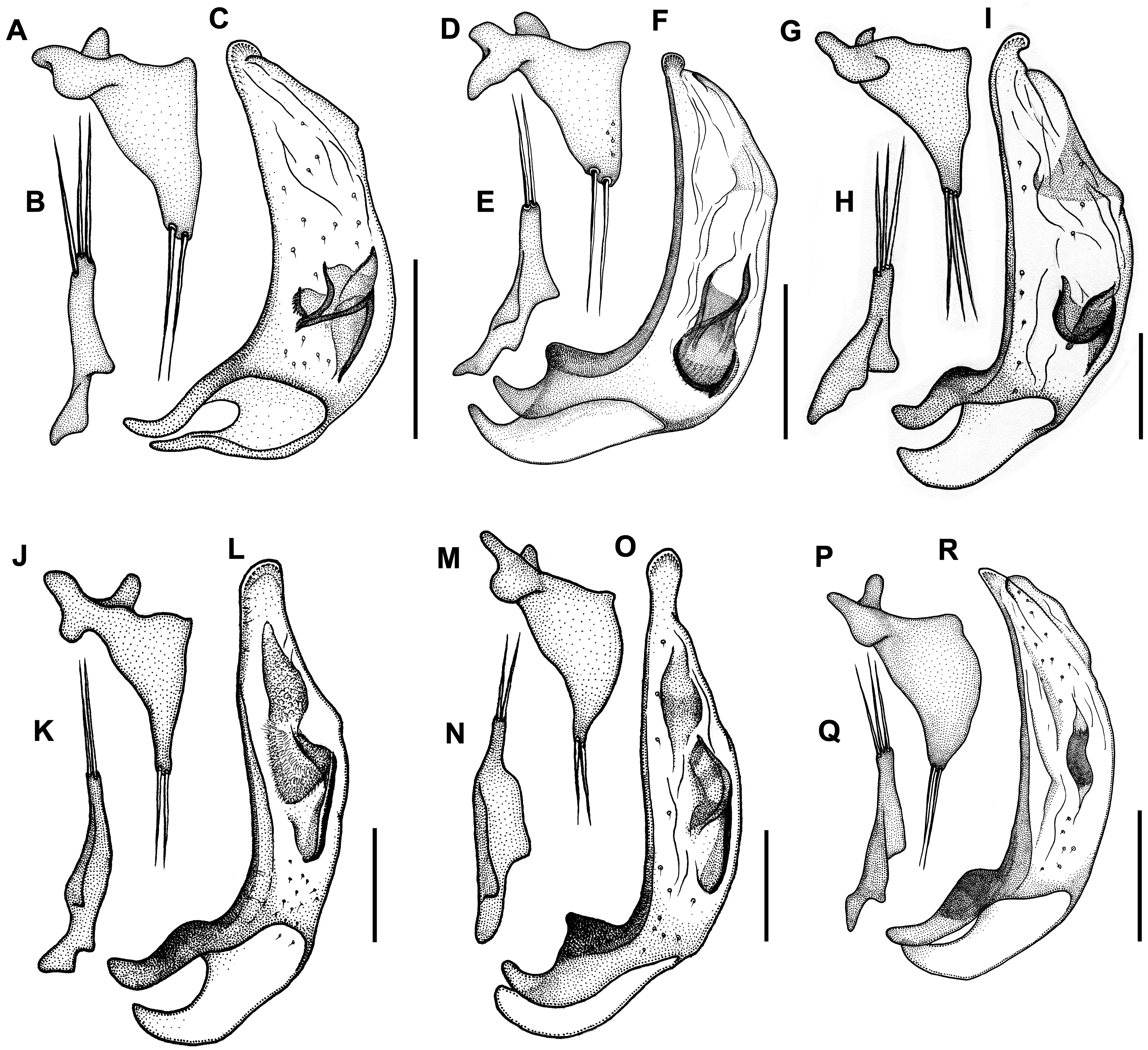
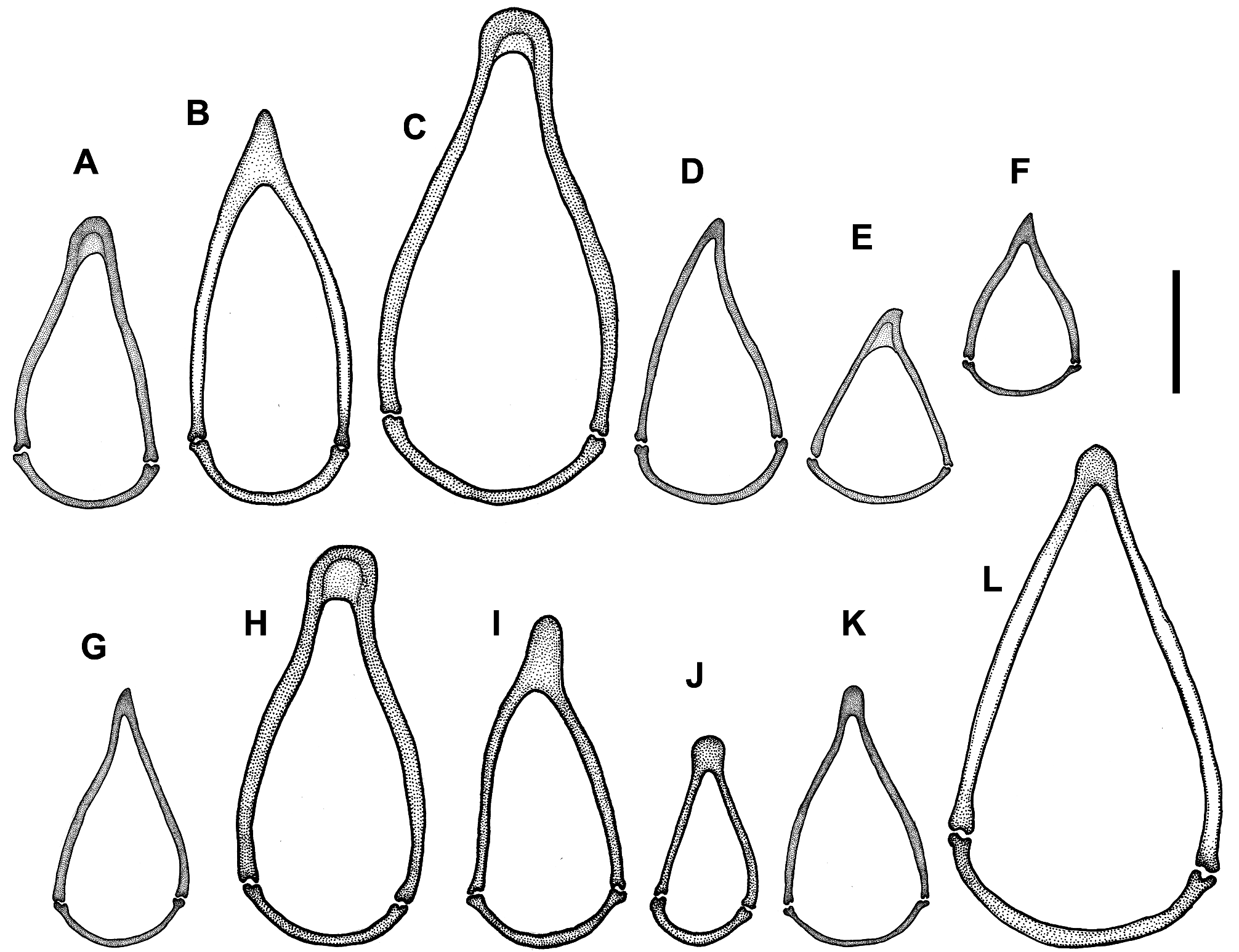
Figures. 10F View FIGURE 10 , 13C View FIGURE 13 , 15P–R View FIGURE 15 , 17J View FIGURE 17 , 18C View FIGURE 18 , 21 View FIGURE 21
= Cillenum subcaecum Sharp, 1886: 375 .
Material examined: \ NEW ZEALAND, BR Greymouth King Domain 122m 10 Jun 1983 H.P. McColl \ Litter 15/83 \ NZ PB\ (1 female); \ Kings Domain Greymouth Westland \ Coll. E.Fairburn 8-1-1945 \ Anillus sp. \ Leafmould \ A.E. Brookes Collection \ (1 female); \ Moa Basin \ T.Broun Collection \ A.E. Brookes Collection \ DSIR \ Ns \ NZ PB \ (1 male); \ Arthurs Pass 2500’ 24.3.65 \ N.A. Walker \ DSIR \ Ns \ NZ PB\ (1 female); \ NEW ZEALAND MC Arthurs Pass Bealy Valley Margarets Tarn 883m \ 8 Feb 1982 J.S. Dugdale Litter 82/21 \ NZ PB \ (4 males and females); \ NEW ZEALAND MC Arthurs Pass Dobson Nature Walk 8 Feb 1982 \ C.F.Butcher sifted litter 82/26 \ NZ PB\ (1 male); \ in moss Temple Basin Arthurs Pass 3600’ 12 Nov 1966 B.M. May \ (1 female); \ NEW ZEALAND WD Mt Tuhua, 1067m E.side of L. Kaniere 20 Nov 1984 \ C.F. Butcher Litter and mats 84/74 \ NZ PB\ (7 males and females).
Taxonomical notes. My interpretation of N. subcaecus is based on the specimen (see Material examined above) originated from the type locality of the species (“ Greymouth. Helms, ex. Reitter”, p. 375, Sharp 1886). Unfortunately, this specimen is a female, so, its association with a certain group of specimens is based exclusively on external morphological data. Another issue needs to be mentioned here is that the type locality of Tachys coriaceus Broun (synonym of N. oreobius ( Broun, 1893) , established by Moore, 1980) located within the range of the species I consider to be named as N. subcaecus . If the geographical label is correct, then the type specimen of T. coriaceus cannot be a synonym of N. oreobius , which range is limited to the central parts of the North Island ( Sokolov 2023). At the same time, it cannot be a synonym of N. subcaecus also, because Thomas Broun writes in his description of T. coriaceus that the specimen has “broader form, evidently more transverse thorax” than N. oreobius , described by him 15 years earlier. According to our data N. subcaecus is distinguished from N. oreobius by having narrower proportions (cf. pronotal proportions WPm/ LP 1.22±0.025 vs 1.26±0.021, and elytral proportions WE/ LE 0.65±0.020 vs 0.69±0.012, for N subcaecus and N. oreobius subsequently). In addition, in the description the size of the type specimen of T. coriaceus is mentioned as 1.85 mm (“7/8 line”, Broun 1908); this unequivocally points to affinity of the type to the group of large species of Nesamblyops . In examined material none of the large species of Nesamblyops has been collected in this area, hence at present the name T. coriaceus cannot be attributed to a particular local species with certainty.
The original description of N. subcaecus contains little diagnostic information that would allow correct identification. Below, I redescribe the species to make comparison of N. subcaecus with other species easier.
Type locality. New Zealand, South Island , West Coast, Greymouth area .
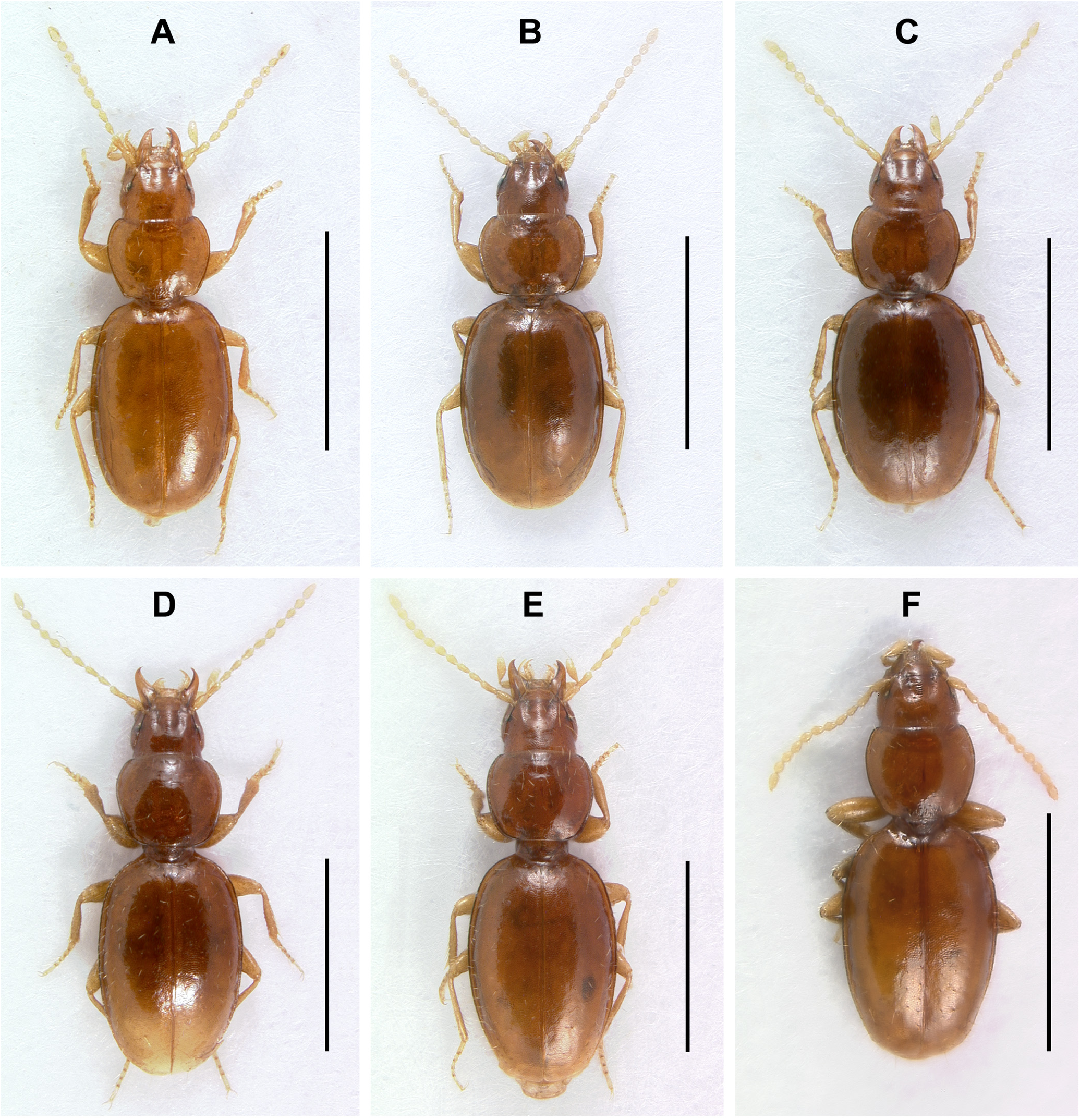
Recognition. Adults of this species ( Fig. 10F View FIGURE 10 ) are practically indistinguishable from the adults of many Nesamblyops species based on external characters (e.g., Figs 10D–E View FIGURE 10 ) and are distinguished from them by the structures of the male genitalia.
Description. Small for genus (SBL range 1.51–1.70 mm, mean 1.62± 0.062 mm, n=14).
Habitus. Body form ( Fig. 2F View FIGURE 2 ) moderately convex, elongate ovoid, general proportions wide (WE/SBL 0.39±0.009), head wide relative to pronotum (WH/WPm 0.76±0.023), proportions of pronotum in comparison to elytra average for genus (WPm/WE 0.73±0.015).
Color. Body color rufotestaceous, appendages testaceous.
Prothorax. Pronotum ( Fig. 13C View FIGURE 13 ) moderately long in comparison to elytra (LP/LE 0.39±0.010) and moderately transverse (WPm/LP 1.22±0.025), with lateral margins rectilinear constricted posteriorly (WPm/WPp 1.34±0.047). Anterior angles indistinct, posterior angles obtuse (123–134°), widely rounded. Width between posterior angles equals width between anterior angles (WPa/WPp 1.00±0.040). Basal margin slightly convex.
Elytra. Ovoid, moderately depressed along suture, comparatively long (LE/SBL 0.60±0.009) and moderately narrow (WE/LE 0.65±0.020). Humeri completely rounded. Lateral margins slightly divergent at basal half, subparallel at middle and evenly rounded to apex in apical third.
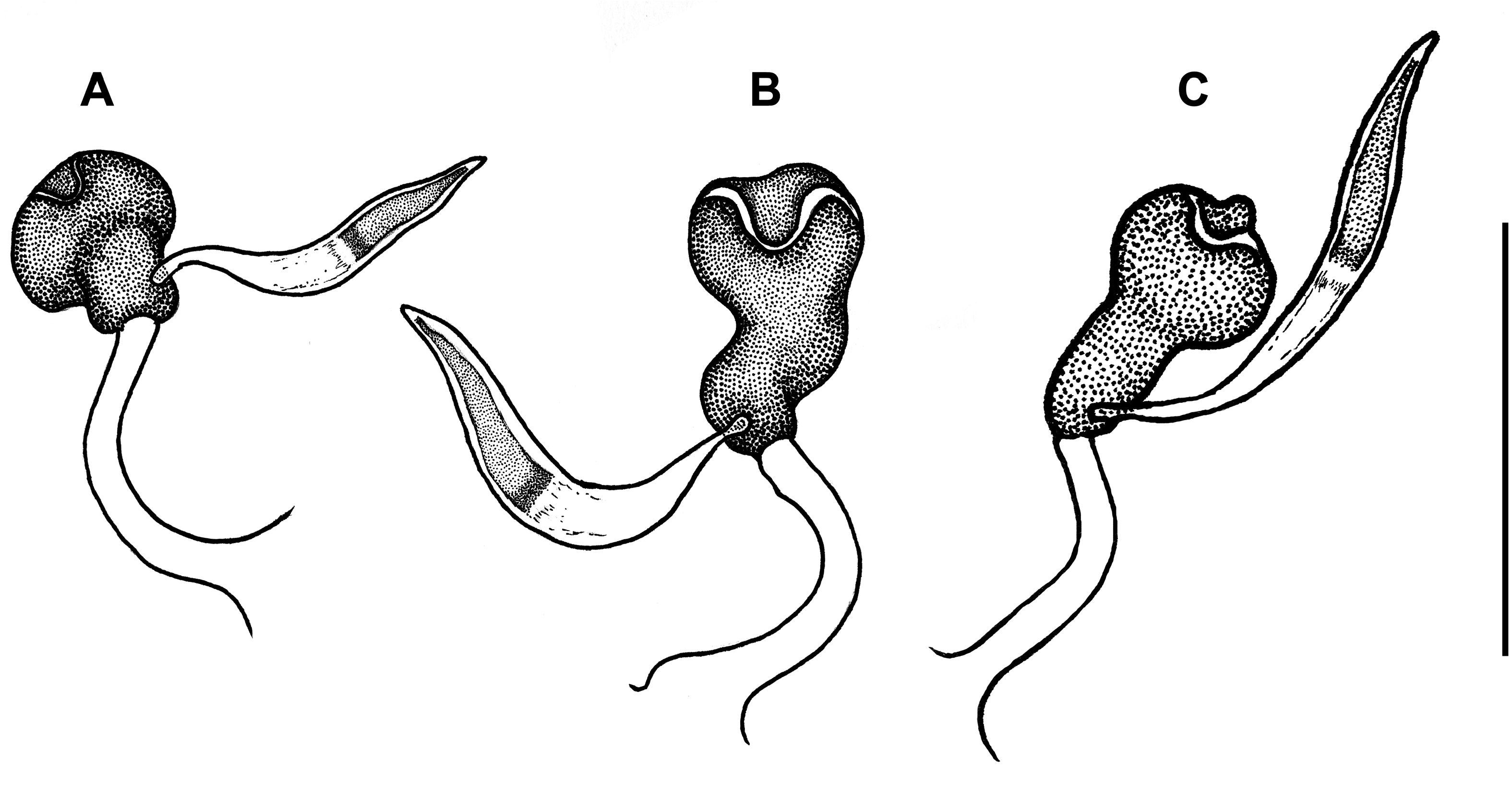
Male genitalia. Median lobe of aedeagus ( Fig. 15R View FIGURE 15 ) moderately arcuate and slightly twisted. Shaft almost subparallel in basal half, moderately tapering in apical half. Apex moderately curved ventrally with tapering narrowly rounded tip. Apical orifice of moderate length occupies one third of shaft length. Ventral margin of median lobe straight in basal two thirds, slightly curved downward in apical third. Walls of shaft with several poriferous canals scattered in apical and basal parts of shaft. Dorsal copulatory sclerites atypical for genus. Only one weakly sclerotized field present at the middle of shaft in a form of short, wide, and curved stripe ( Fig. 15R View FIGURE 15 ). V - and rC-sclerites lacking. Left paramere ( Fig. 15P View FIGURE 15 ) wide, apex not attenuate, bearing two long setae. Right paramere ( Fig. 15Q View FIGURE 15 ) narrow, of moderate length, bearing three long setae, which are slightly shorter than the length of paramere. Ring sclerite as in Fig. 17J View FIGURE 17 .
Female internal genitalia. Not examined.
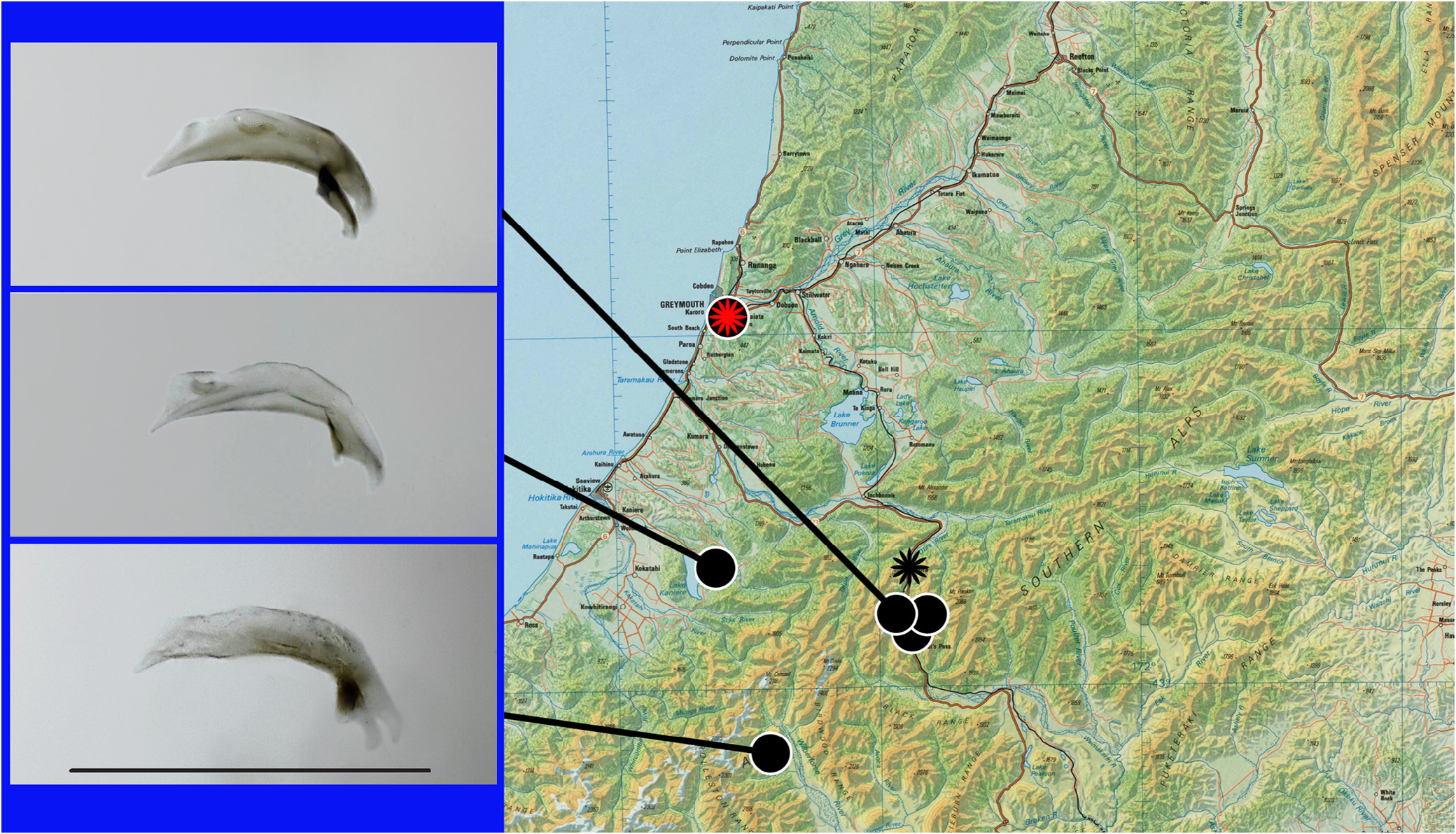
Geographical distribution. The range of the species occupies foothills and a part of the Southern Alps approximately from the catchment of Moa Stream and the Arthur’s Pass area in the central Canterbury to the mouth of Grey River in the central West Coast region ( Fig. 21 View FIGURE 21 ).
Habitat. Specimens were collected from moss, litter, leaf mold, litter and [plant] mats samples.
Relationships. The structure of the male genitalia of N. subcaecus suggests its relatedness to the species with reduced and simple armature of the internal sac. In having only one sclerotized copulatory sclerite N. subcaecus demonstrates affinity to and presumably forms one group with N. viator , described below.
| BR |
Embrapa Agrobiology Diazothrophic Microbial Culture Collection |
| T |
Tavera, Department of Geology and Geophysics |
| DSIR |
Department of Scientific and Industrial Research |
| MC |
Museo de Cipolleti |
| LP |
Laboratory of Palaeontology |
| LE |
Servico de Microbiologia e Imunologia |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Trechinae |
|
Tribe |
Anillini |
|
SubTribe |
Nesamblyopina |
|
Genus |
Nesamblyops subcaecus (Sharp)
| Sokolov, Igor M. 2023 |
Cillenum subcaecum
| Sharp, D. 1886: 375 |