Aphanipathes verticillata mauiensis, Opresko & Wagner & Montgomery & Brugler, 2012
|
publication ID |
https://doi.org/10.11646/zootaxa.3348.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:20C1097E-A60C-4C87-A203-B50E068DA204 |
|
persistent identifier |
https://treatment.plazi.org/id/053487FF-FFA1-FFE5-FF39-F1ACFE2BF616 |
|
treatment provided by |
Felipe |
|
scientific name |
Aphanipathes verticillata mauiensis |
| status |
subsp. nov. |
Aphanipathes verticillata mauiensis View in CoL subsp. nova
( Figures 1B View FIGURE 1 , 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 8A–D View FIGURE 8 )
Material examined. Holotype (USNM 1150095*; schizoholotype BPBM D-1877), Au‘au Channel, between the islands of Maui and Lāna‘i, Hawaiian Islands, 20.9409ºN, 156.7609ºW, 88 m, Pisces V, Dive 739, 7 April 2009, Tony Montgomery (large dry specimen labeled “bench”and small subsample with polyps preserved in alcohol for DNA analysis). Additional specimens all collected from the Au‘au Channel: four samples from Pisces V, Dive 739, 91– 127 m [ USNM 1150087* ( BPBM D-1875), 1150092* ( BPBM D-1876), 1150093* ( BPBM D- 1878), 1150094* ( BPBM D-1879)]; six samples from Pisces V, Dive 716, 88– 130 m ( USNM 1157687; 1157500*, 1157501, 1157502*, 1157503, 1157504*), two of which with subsamples deposited at the BPBM ( BPBM D-1873, D-1874); three specimens from Pisces IV, Dive 204, 90– 113 m ( USNM 1127629*, 1127632, 1157505); four specimens from Pisces IV, Dive 205, 97– 111 m ( USNM 1127630*, 1127631*, 1127633*, 1127635); and four samples from Pisces IV, Dive 206, 93– 114 m ( USNM 1128318*, 1128389*, 1128320*, 1157506*). [ NOTE: Specimens whose USNM number is followed by an asterisk were included in the DNA analysis].
Diagnosis. Colony size and shape similar to that of A. verticillata verticillata ; large, sparsely to densely branched to 12 th order or more. Branches long, straight or slightly curved, often disposed in single series on upper or lower side of next lower order branches. In places, successive orders of branches arising on the same, usually outer side of lower order branches. Spines on branchlets conical to slightly compressed laterally, arranged in longitudinal rows and in verticils, and covered with minute conical tubercles. Maximum size of polypar spines on branchlets typically 0.19−0.27 mm (up to 0.30 mm); abpolypar spines mostly 0.09−0.15 mm tall. Six to nine longitudinal rows of spines visible in lateral view on branchlets. Spines mostly 0.28−0.36 mm apart within each row (maximum about 0.5 mm). Tubercles usually simple, conical, with an average density of 2.4 per 1000 µm 2 (range 1.62−3.50 per 1000 µm 2; N = 7). Largest tubercles ( 0.012 −0.015 mm) on distal edge of spines; proximal edge sometimes with very few tubercles. Polyps mostly 1.2−1.5 mm in transverse diameter (range 0.72−1.81 mm; mean 1.35 mm; N = 421) with interpolypar space of 0.3−0.4 mm. Polyps arranged in single series on branchlets, with 5−8 polyps per centimeter (mean 5.6/cm; N = 90).
Description of holotype. Colony (USNM 1150095) about 80 cm tall ( Fig. 1B View FIGURE 1 ). Branches spread out in all directions so that overall width of colony about 70 cm. Basal plate 6 cm in diameter. Multiple stems arise from basal plate; thickest of these is about 1 cm in diameter. Colony branched to 12 th order; largest branches near base of corallum spread out somewhat laterally, with next highest order branches in many cases arranged uniserially on upper side, and extending vertically. Major branches are up to 35 cm in length; with 10 to 15 branches of next higher order occurring along 10 cm. Spacing of branches very variable, from 0.5 cm to 2 cm or more apart; generally, not more than two or three branches per 2 centimeters. On upper parts of corallum five or more orders of branches can arise successively on same side of each lower order branch, often on the convexly curved outer side. Most branches of corallum vertically directed although some on outer edges extend out somewhat horizontally. Distal branch angle very variable; mostly 30º to 60º; wider where lower order branches are upright, although in such cases higher order branches curved upward to become parallel to lower order branch from which they arose. Smallest terminal (unbranched) branchlets reaching maximum size of about 8 cm. Branch 7 cm long about 0.8 mm in diameter at proximal end, including spines, and about 0.4 mm in diameter excluding spines; diameter at midpoint 0.6 mm (total) and about 0.35 mm excluding spines. Average midpoint diameter of terminal branchlets 0.60 mm with spines included and 0.36 mm excluding spines (N = 10). Distal 3 cm of branchlets often collapsed when dried due to thinness of sclerenchyme.
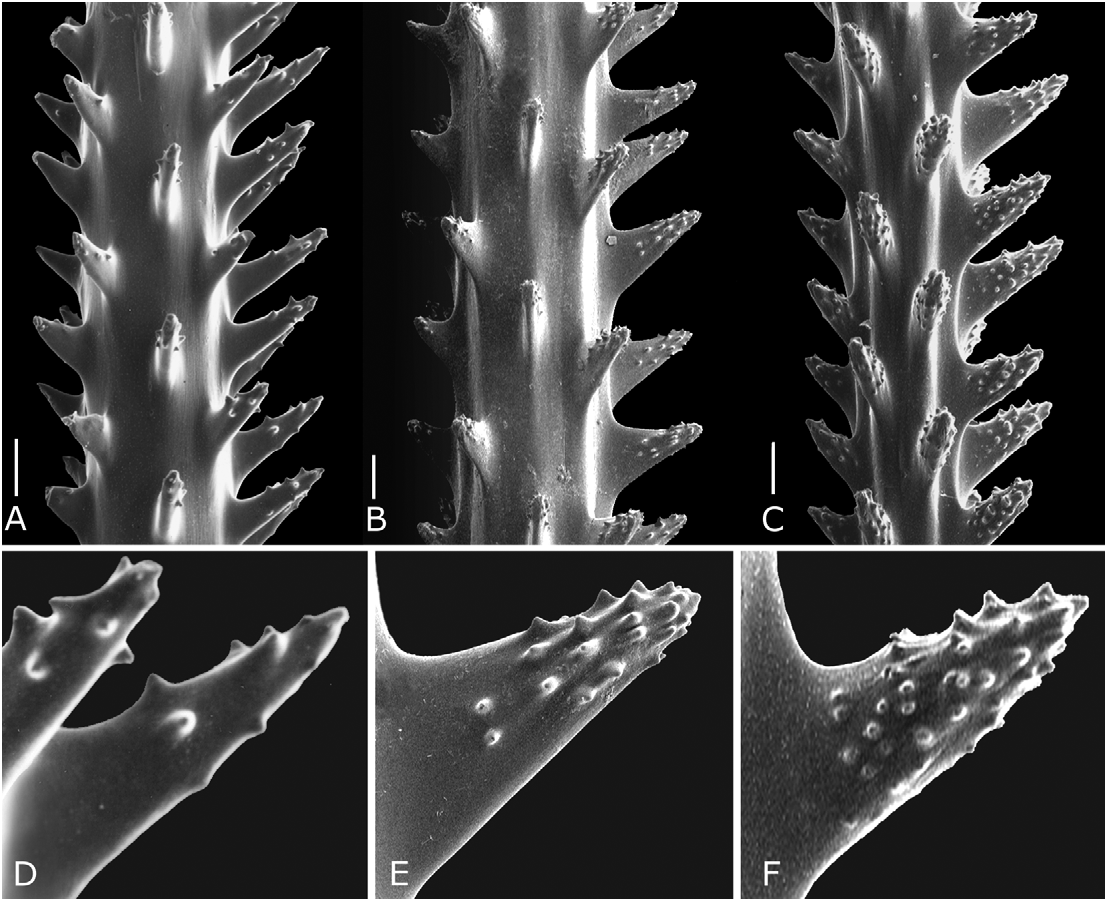
Spines on branchlets generally conical, but slightly compressed laterally, with relatively acute apex and covered over much of their surface with small conical tubercles ( Figs 4–5 View FIGURE 4 View FIGURE 5 ). Spines unequal in size around circumference of axis; polypar spines on terminal branchlets mostly 0.2 to 0.24 mm in height (from middle of base to apex), but up to 0.30 mm; abpolypar spines on branchlets mostly 0.14 to 0.18 mm in height (range 0.13−0.22 mm). On branchlets, six to nine longitudinal rows of spines visible in lateral view. Distance of adjacent spines in one row mostly 0.3−0.34 mm (range 0.22−0.38 mm). Polyps mostly 1.1−1.4 mm in transverse diameter, arranged on branchlets in a single series with 6−7 per centimeter.
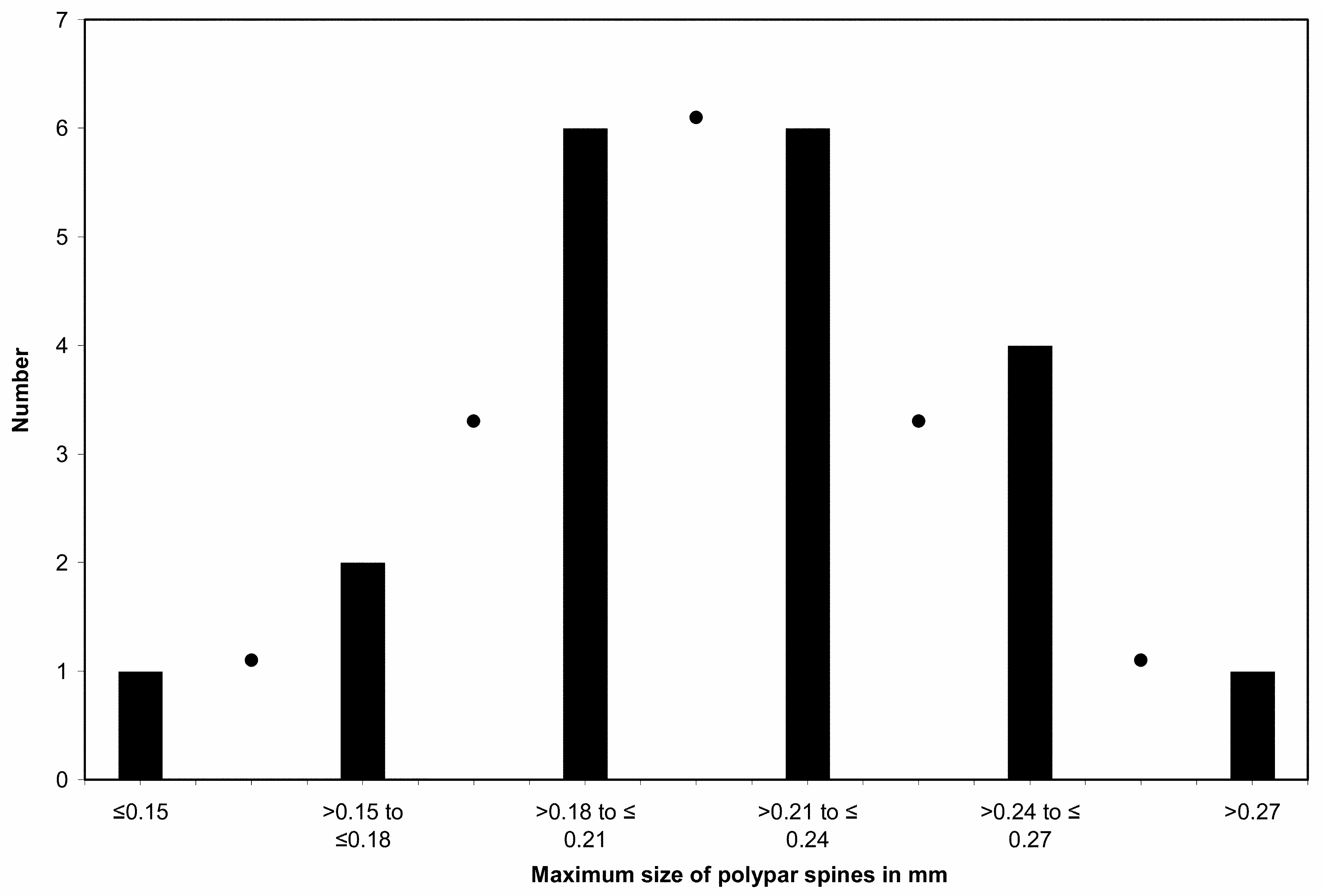
Morphological variation. Twenty samples of A. verticillata mauiensis have been collected to date, including 10 complete colonies, ranging in height from 25 to 150 cm. A summary of the major morphometric features of these specimens is presented in Table 1. As has been described for other species of antipatharians, characters associated with the skeleton and polyps can be quite variable. Several of the specimens are sparsely branched with little evidence of a uniserial arrangement of higher order branches ( Fig. 8C View FIGURE 8 ), whereas in others this feature is evident throughout the colony ( Fig. 8A View FIGURE 8 ). In the samples examined, the height of the polypar spines (from middle of base to apex) ranged from 0.09 to 0.30 mm (mean 0.17 mm), that of the abpolypar spines from 0.05 to 0.20 mm (mean 0.12 mm); the spacing of the spines ranged from 0.20 to 0.51 mm (mean 0.32 mm); the transverse diameter of the polyps from 0.72 to 1.81 mm (mean 1.35 mm); and the density of the polyps from 4.49 to 7.79 per centimeter (mean 5.85 per centimeter). Wide variation in one character can occur within the same colony. For example, the size of the polypar spines of the holotype (USNM 1150095) ranges from 0.19 to 0.30 mm, and the polyp density in specimen B2 from Pisces V, Dive 739 ranges from 5 per centimeter on some branchlets up to 8 per centimeter on others. However, there are also specimens where a character may be consistently above or below the average as in a specimen from Pisces V, Dive 716 (specimen No. 6) where the polypar spines consistently measure, at least in the subsamples examined, not more than 0.15 mm in height. To estimate the typical range in maximum size of the polypar spines, the maximum size for each of the 20 specimens was recorded and an evaluation was made of the size frequency distribution ( Fig. 6 View FIGURE 6 ). For ninety percent of the colonies the maximum size of the polypar spines falls in the range of 0.19−0.27 mm, with a mean and median of about 0.22 mm.
Genetic Variation. Given the morphological variation in colony appearance and in characters such as the polyps and size of the spines, the possibility existed that a cryptic species was present among the suite of specimens examined. To address this issue, DNA studies of the new subspecies were carried out by coauthor M. Brugler.
Due to the low molecular weight of DNA obtained from several individuals, only a 16-member subset of the colonies analyzed morphologically were screened at the molecular level. Although sequence coverage was slightly reduced, the same four gene regions sequenced in Wagner et al. (2010) were analyzed (mitochondrial [mt] cox3 - IGR- cox1 [GenBank accession number HM 060617 View Materials ], mt trnW -IGR- nad2 [ HM 060612 View Materials ], nuclear ITS1 [ HM 060619 View Materials ], nuclear ITS2 [ HM 060627 View Materials ]). Four individuals screened at mt cox3 -IGR- cox1 shared identical haplotypes (alignment length: 1,168 bp; includes 124 bp cox3, 259 bp cox3-cox1 IGR, and 785 bp cox1). The same held true for fifteen individuals screened at mt trnW -IGR- nad2: all individuals shared identical haplotypes (alignment length: 614 bp; includes 448 bp trnW -nad2 IGR and 166 bp nad2). Three segments of the nuclear ribosomal cistron were analyzed for two individuals, both of which shared identical sequences (noncontiguous alignment length: 791 bp; segment 1 includes 145 bp 18S and 156 bp ITS1 ; segment 2 includes 9 bp ITS1 , 158 bp 5.8S, and 67 bp ITS2 ; segment 3 includes 256 bp 28S). Given that mt and nuclear DNA revealed no differences and that the internal transcribed spacers are currently the most variable markers within anthozoans, the hypothesis that can be advanced is that the specimens included in the description of A. verticillata mauiensis do not include any cryptic species .
Comparison of Genetic and Morphological Variation. There were fourteen specimens for which we had both morphological and genetic data. Across these fourteen specimens the diameter of the terminal branches ranged from 0.84 to 1.23 mm; the maximum transverse diameter of the polyps varied from 1.4 to 2.3 mm; the polyp density from 5.6 to 7.8 per centimeter; and the maximum size of the polypar spines from 0.15 to 0.30 mm. Thus, the genetic uniformity seen in these specimens is not reflected in morphological uniformity, and this is a clear indication of the morphological plasticity of this subspecies. The presence of such morphological plasticity necessitates the use of extreme care in describing new antipatharian taxa from a limited number of specimens as these may represent the extreme ranges of a single species. As in this case, future taxonomic studies of antipatharians would benefit greatly from using both morphological and genetic characters.
Genetic Comparisons. Unfortunately, soft tissue material suitable for DNA analysis was not available from the type specimen of A. verticillata verticillata or from any specimens that could be referred to the typical form; therefore, a genetic comparison of the two subspecies was not possible. However, a specimen of A. verticillata mauiensis was included in a large phylogenetic analysis of the Antipatharia conducted by Brugler (2011). In that study, representatives of the genus Aphanipathes did not form a monophyletic clade. A. verticillata mauiensis (referred to in that work as ‘ Aphanipathidae n.sp. 1, Pisces IV-205-7 [new ID – Aphanipathes cf. verticillata ]’), grouped sister to the largest clade of antipathids ( Antipathidae was polyphyletic). A specimen of Aphanipathes pedata from the Gulf of Mexico (DFH11-8A), a specimen identified as Antipathes sp. (USNM-1086470), and a specimen identified as Aphanipathes n.sp. from Hawaii (USNM-1010741) all grouped within the larger clade of aphanipathids ( Aphanipathidae was also polyphyletic), but the first specimen was more closely related to Phanopathes spp. while the latter two were more closely allied with Stichopathes spiessi and S. dissimilis , two species currently assigned to the family Antipathidae , but with morphological affinities to the Aphanipathidae . These results suggest that the current toolbox of morphological characters used to differentiate taxa may need to be reevaluated in light of recent ( Brugler 2011) and upcoming phylogenetic reconstructions of the order Antipatharia (i.e., Sánchez, J.A., Brugler, M.R., Miller, K., Umaña, C., Dueñas, L., Opresko, D., in prep. The evolutionary history of the order Antipatharia [black corals] as inferred from the predicted secondary structure of ITS2).
Distribution. Presently only known from the Keyhole Pinnacle in the Au‘au Channel between the Islands of Maui and Lāna‘i, in 88− 130 m.
Comparison of A. verticillata mauiensis with A. verticillata verticillata . The only differences between the two subspecies are the density and morphology of the tubercles on the spines. On the schizotypes of A. verticilllata verticillata , as well as on the specimen from Okinawa, the tubercle density is 2.9 per 1000 µm 2, whereas measurements made on the holotype of A. verticillata mauiensis (USNM 1150095) from Hawai‘i gave an average density of 2.4 per 1000 µm 2; a difference that is statistically significant at p = 0.005 (Mood’s Median). Furthermore, there is a distinct difference between the two subspecies in the occurrence of fused tubercles; with the Hawaiian subspecies usually having only simple tubercles (very rarely a double tubercle may be found) and the typical form having, on some branchlets, fused tubercles with two or three apices, which in some cases form wide, shelf-like edges.
The recognition of the Hawaiian form as a subspecies is based on our current state of knowledge that the morphological differences described above are confined to geographically separated populations. In all other respects, and particularly in the verticillate arrangement of the spines, these two forms are more closely related to each other than to other members of the genus (see discussion below). The designation of infraspecific forms is not unusual in antipatharians (see Schultze 1902; van Pesch 1914), and is a reflection of the typical wide range of variation seen in most morphological characters, especially when dealing with large suites of specimens. Furthermore, it has been reported that geographic dispersion of antipatharians is likely to be limited due to the planulae being negative bouyant, non-feeding, and short-lived ( Miller 1996); therefore, it would not be unexpected to find isolated populations forming unique morphotypes. Testing this hypothesis would require DNA markers for the analysis of intra-individual and inter-individual (population level) genetic variation; however, such markers are not yet available for antipatharians. In the present case, such investigations would also be limited by the lack of fresh tissue samples of A. verticillata verticillata . We consider this a very important area for future investigation, not only for the species discussed here, but for antipatharian taxonomy in general.
Comparisons to other species of Aphanipathes . The genus as revised by Opresko (2004) consists of four nominal species: Aphanipathes sarothamnoides Brook 1889 ; Antipathes salix Pourtales 1880 ; Aphanipathes verticillata Brook 1889 ; and Antipathes pedata Gray 1857 . All these species have an irregularly branched, bushy corallum, with relatively straight and usually elongated, upright branches. In all species except A. salix , the branches and branchlets tend to be arranged uniserially, at least on parts of the corallum. Morphometric comparisons of the four species are presented in Table 2. A lack of a sufficient number of specimens prevents a detailed statistical comparison of the taxa, and, in fact, the information presented for A. verticillata verticillata , A. sarothamnoides , and A. salix is based on just a few specimens. Therefore, differences seen in some of the values listed cannot be assumed to be definitive, since examination of additional specimens of each species is likely to increase the range of the values for each of the characters. There are, however, differences among the species in the morphology of the spines, particularly in the extent that the surface of the spines is covered with tubercles ( Fig. 7 View FIGURE 7 ). In general (exceptions are likely to occur), the tubercles on the spines of A. salix tend to be few in number and mostly near the apex, but with a few extending down to about half the distance to the base with an average tubercle density of 2.2 per 1000 µm 2 (range 1.8−2.4 per 1000 µm 2, N = 6); those of A. sarothamnoides cover about one-third to one-half the distance from the apex with an average tubercle density of 2.3 per 1000 µm 2 (range 1.6−3.0 per 1000 µm 2, N = 6); those of A. pedata cover one-half to three-quarters of spine surface with an average tubercle density of 3.2 per 1000 µm 2 (range 2.8−3.6 per 1000 µm 2, N = 7); whereas those of both forms of A. verticillata cover one-half to the entire surface with an average tubercle density of 2.9 per per 1000 µm 3 in the typical form and 2.4 per 1000 µm 2 in A. verticillata mauiensis . Aphanipathes verticillata is the only species in which the spines are arranged in verticils, although on some branches in some colonies the verticillate arrangement may not be very distinct.
Comparison of Aphanipathes verticillata mauiensis with Antipathes griggi . The two species are generally indistinguishable in the field; both reach a maximum size of 1 m or more, both are a reddish or reddish-orange when alive, and both tend to grow with upright branches which tend to be uniserially arranged on the lower order branches ( Fig. 8 View FIGURE 8 ). Specimens can occasionally be discerned in the field by a close evaluation of the polyp color. Polyps of A. verticillata mauiensis often have tentacles that are lighter in color at the tips and this can be a distinguishing character when present. The two species are similar in the size of the polyps, mostly 1.2−1.3 mm in A. griggi and 1.2−1.5 mm in A. verticillata mauiensis , and in the size of the polypar spines, generally 0.20−0.26 mm tall in A. griggi and 0.19−0.27 mm in A. verticillata mauiensis . The major differences in the two species occur in the arrangement and morphology of the spines. The spines of A. verticillata mauiensis are arranged in distinct verticils ( Fig. 8D View FIGURE 8 ) whereas those of A. griggi are not ( Fig. 8H View FIGURE 8 ). Furthermore, in A. griggi the spines are often bifurcated, multi-lobed, or jagged at the apex, with small, round to elongate papillae on the surface ( Fig. 8H View FIGURE 8 ), whereas those in A. verticillata mauiensis are pointed or rounded at the tip, and covered with very distinct conical tubercles ( Fig. 8D View FIGURE 8 ). In addition to the above described morphological differences, a recent comparison between A. griggi and A. verticillata mauiensis (identified only as an undescribed species of Aphanipathidae ) using nuclear and mitochondrial DNA sequences also revealed substantial molecular differences between these two species (see Wagner et al. 2010, Fig. 10).
There may be significant ecological differences as well. A. griggi has a wider reported depth range (< 30–120 m) than A. verticillata mauiensis . A. verticillata mauiensis may extend deeper ( 88–130 m) than A. griggi and may have higher densities at the deeper depths. However, more surveys are needed to determine the ecological differences between these Hawaiian species, because A. verticillata mauiensis and A. griggi may well have been misidentified in previous surveys due to their similarities in gross morphology ( Fig. 8 View FIGURE 8 ).
Antipathes griggi is an important component of the commercial black coral trade in Hawaii. While it is not known if or to what extent specimens of A. verticillata mauiensis have been utilized commercially, the typical depth range of commercial harvesting is shallower than the shallowest reported specimen. No published data are available on the relative hardness of the skeletal axis of the two species, but gross observations of the samples used in this study would suggest that specimens of A. verticillata mauiensis are not substantially different in skeletal density from those of A. griggi .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.