Arctonoella Buzhinskaja, 1967
|
publication ID |
https://doi.org/10.12782/specdiv.28.147 |
|
persistent identifier |
https://treatment.plazi.org/id/03FF8796-FFDA-0815-B64E-F8AA9B033B90 |
|
treatment provided by |
Felipe |
|
scientific name |
Arctonoella Buzhinskaja, 1967 |
| status |
|
Genus Arctonoella Buzhinskaja, 1967 View in CoL
Arctonoёlla Buzhinskaja, 1967: 82–83.
Arctonoella View in CoL : Fauchald 1977: 60; Pettibone 1996: 637–638; Uschakov 1982: 123–124; Wu et al. 1997: 175.
Gender. Feminine.
Type species. Harmothoe sinagawaensis Izuka, 1912 View in CoL , fixed by original designation.
Diagnosis (emended after Pettibone 1996). Body with up to 41 segments, having 16 pairs of elytra on segments 2, 4, 5, 7, alternating to 23, 26, 29, 32, and 33 (or 35). Prostomium bilobed; anterior margin of each lobe with or without cephalic peaks. Two palps. Three antennae with distinct ceratophores; median antenna inserted in anterior notch of prostomium; ceratophores of lateral antennae inserted ventrally or terminoventrally, converging midventrally. Two pairs of eyes. First (tentacular) segment not distinct dorsally, with tentaculophores lateral to prostomium, achaetous, with dorsal and ventral tentacular cirri; without conical facial tubercle. Second (buccal) segment without nuchal fold, with first pair of elytrophores, biramous parapodia, and long ventral buccal cirri. Parapodia biramous; small notopodia with projecting acicular lobe at inferior edge; large neuropodia composed of longer prechaetal acicular lobe bifid distally, and shorter rounded postchaetal lobe. Dorsal cirri on non-elytrophorous segments, with cylindrical cirrophores and long distal styles. Notochaetae of two kinds: upper notochaetae stout, rod-like, with round tip; lower notochaetae slender, capillary. Neurochaetae all unidentate, tapering to fine tip, of two kinds: upper neurochaetae slender with long spinous region; lower neurochaetae shorter, with expanded subdistal spinous region. Nephridial papillae prominent, beginning on segment 6. Anal cirri as long as longest dorsal cirri.
Remarks. We have modified the generic definition of Pettibone (1996) to accommodate the variable presence of cephalic peaks in the type species (see below).
Arctonoella resembles Hesperonoe in having two types of chaetae both on notopodia and neuropodia (except H. andriashevi Averincev, 1990 , which has only one type of neurochaetae), but differs in having 16 instead 15 pairs of elytra ( 13 in H. andriashevi ) ( Hong et al. 2017). Arctonoella also differs from most species of Hesperonoe [except H. adventor (Skogsberg in Fisher and MacGinitie, 1928), see below] in the variable presence of cephalic peaks, which are consistently present in Hesperonoe , and in the bifurcated tips of the neuropodial prechaetal acicular lobe, which have a single tip in Hesperonoe . The particularly close relationships between Arctonoella and H. adventor are also discussed below.
Arctonoella sinagawaensis ( Izuka, 1912) [Japanese name: Shinagawa-urokomushi] ( Figs 1–6 View Fig View Fig View Fig View Fig View Fig View Fig ; Table 1)
Harmothoë sinagawaensis Izuka, 1912: 57–59 View in CoL , pl. 6, figs 8–12.
Harmothoe sinagawaensis View in CoL : Fauvel 1933: 10–11.
Gattyana sinagawaensis : Hartman 1959: 71, 78; Imajima and Hartman 1964: 32.
Hesperonoë View in CoL (?) sinagawaensis : Uschakov and Wu 1965: 172.
Arctonoella sinagawaensis View in CoL : Buzhinskaja 1967: 83–85, fig. lA–E; Uschakov 1982: 124, pl. 39, 1–8; Pettibone 1996: 632, 638, fig. 2; Wu et al. 1997: 175–177, fig. 108 (in part); Imajima 2001: 13, fig. 1; Buzhinskaja 2013: 37.
? Arctonoella sinagawaensis View in CoL : Wu et al. 1997: 175–177, fig. 108 (in part).
Hesperonoe sp. : Buzhinskaja 2013: 125, fig. E.
Hesperonoe urechis Marin and Antokhina, 2020: 7–11 , figs 1e, 8–10. syn. nov.
Not A. sinagawaensis View in CoL . “?? Harmothoë sinagawaensis View in CoL ”: Fauvel 1932: 23–24, text-fig. 3, pl. 1, figs 1, 2; same as “ Harmothoë sinagawaensis View in CoL (non Izuka), Fauvel, 1932 ”: Fauvel 1953: 48–49, fig. 21 [= Pararctonoella indica (Day, 1973) View in CoL , judged by Pettibone (1996), or Gattyana fauveli Misra, 1999: 135–136 View in CoL , fig. 1A–J].
Material examined. Mouth of Saba-gawa River ( 34°02′19.9″N 131°29′54.2″E), Hofu, Yamaguchi Prefecture, 31 May 2018, coll GoogleMaps . G GoogleMaps . Itani, 3 specimens (NSMT-Pol 113479–113481). The southern coast of Mukaishima Island ( 34°21′38.3″N 133°12′35.6″E), Onomichi, Hiroshima Prefecture, 21 October 2013, coll GoogleMaps . Y GoogleMaps . Henmi, 2 specimens (NSMT-Pol 113482–113483). Takasu-higata ( 34°26′58.7″N 133°52′32.1″E), Kojima-karakoto, Kurashiki, Okayama Prefecture, 7 April 2012, coll GoogleMaps . S GoogleMaps . Kobayashi, 1 specimen (NSMTPol 113484).
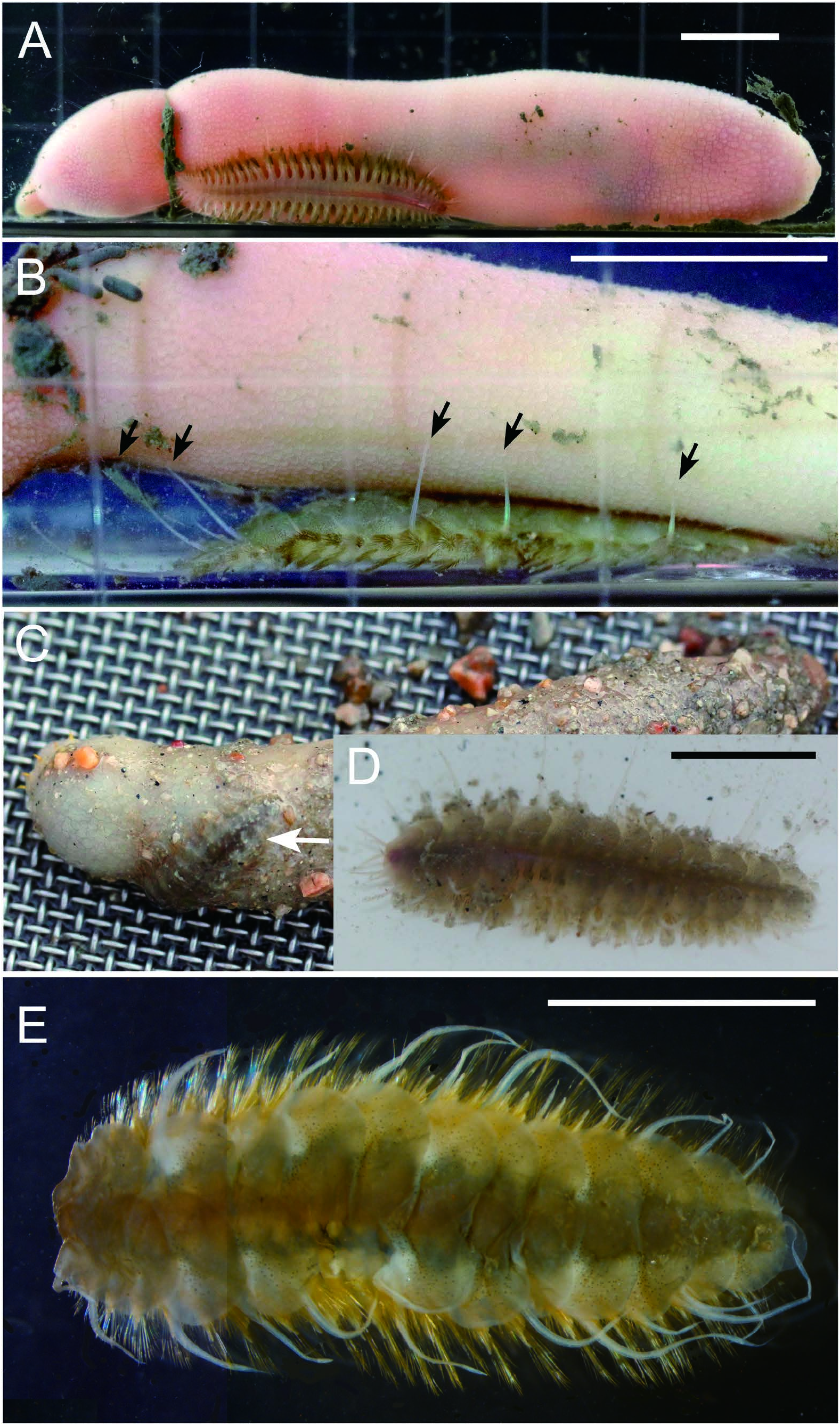
Description. All specimens complete. Largest specimen 60 mm BL, 6.0 mm BWa, 18 mm BWb, with 39 segments ( Table 1). Body dorso-ventrally flattened. Dorsum slightly convex; gray in vivo ( Fig. 2B–D View Fig ), grayish brown when preserved ( Figs 2E View Fig , 3A, C, D View Fig ). Venter flat, grayish, with longitudinal midventral pinkish band of ventral blood vessel in vivo ( Fig. 2A View Fig ), pale with longitudinal midventral brown band when preserved ( Fig. 3B View Fig ).
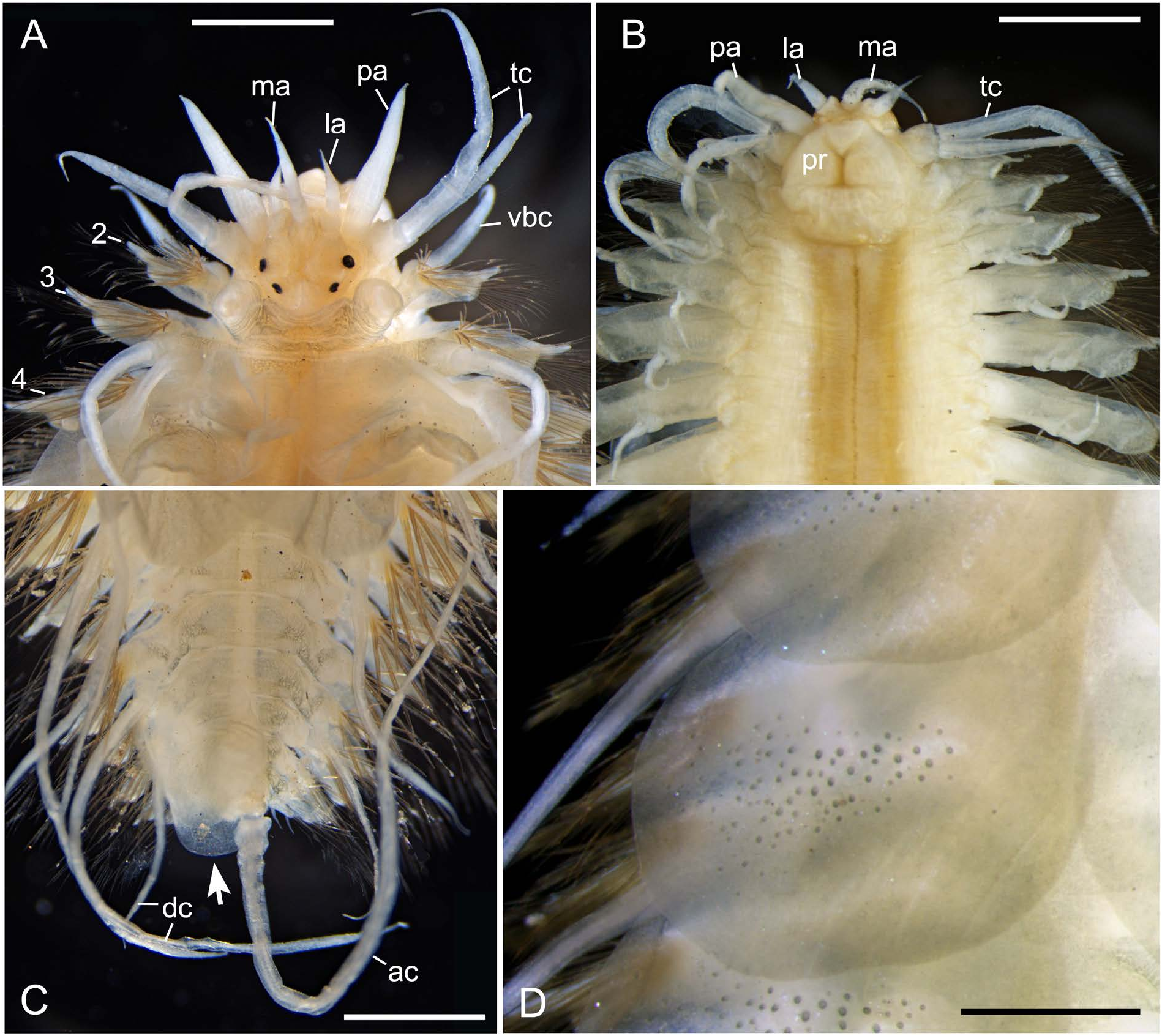
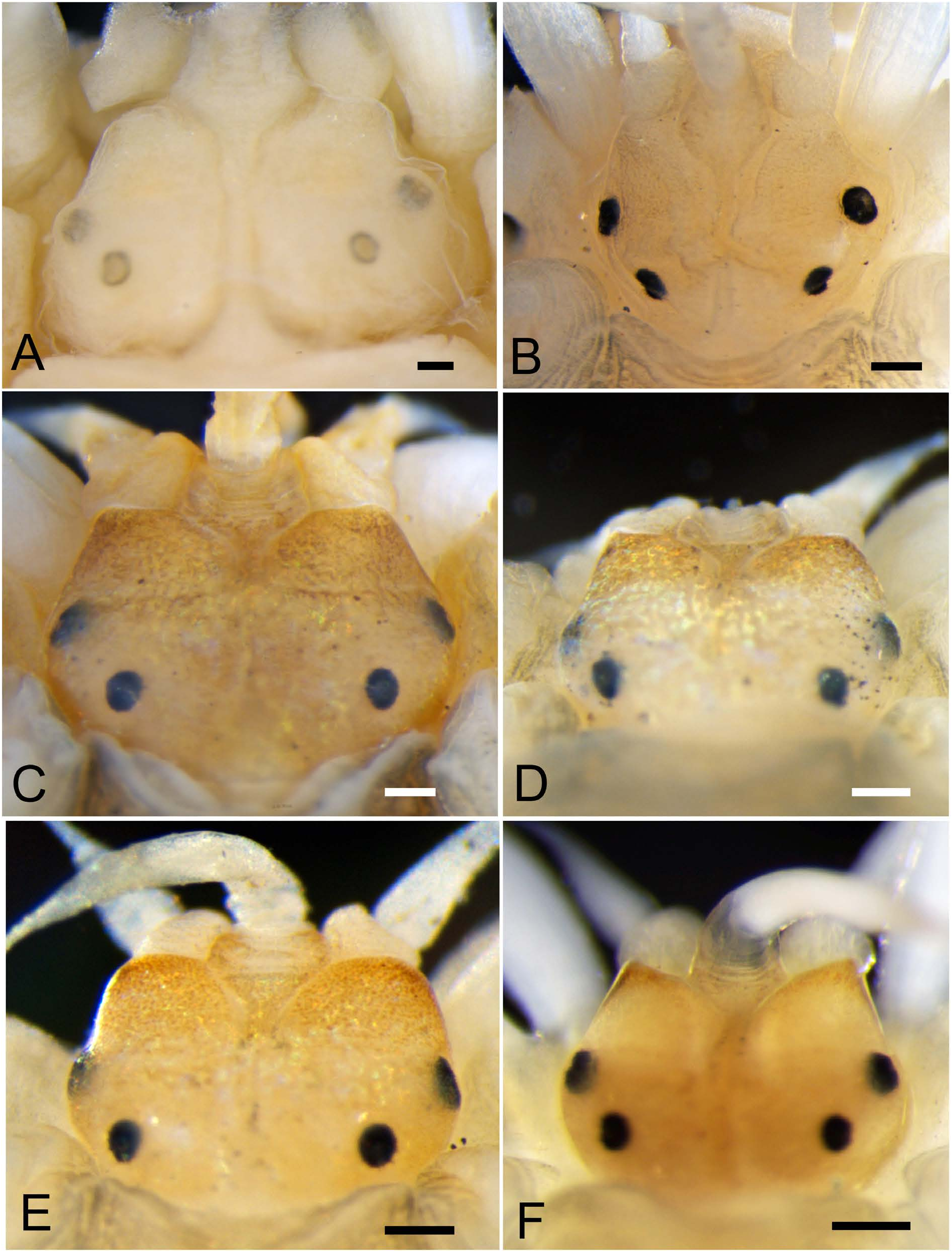
Prostomium 1.5 times wider than long, bilobed, with frontal margin showing tapering cephalic peaks or round protrusions ( four specimens 14–32mm BL: NSMT-Pol 113480– 113483; Fig. 4C–F View Fig ) or lacking marked peaks or protrusions ( two specimens 19, 60 mm BL: NSMT-Pol 113479, 113484; Fig. 4A, B View Fig ) ( Table 1); median antenna with ceratophore inserted into anterior middle notch and style 2–3 times longer than that in lateral antennae ( Fig. 3A, B View Fig ); lateral antennae with ceratophores inserted either beneath cephalic peaks (NSMT-Pol 113480–113483; Fig. 4C–F View Fig ) or terminoventrally (NSMT-Pol 113479, 113484; Fig. 4A, B View Fig ), converging midventrally ( Fig. 3B View Fig ); antennae with tapering tips, smooth; two pairs of round eyes, anterior near widest point of prostomium, 1.1–1.2 times larger than posterior (arranged closer together) ( Fig. 4A–F View Fig ); palps stout, longer than median antenna, with tapering tips, without papillae ( Fig. 3A, B View Fig ).
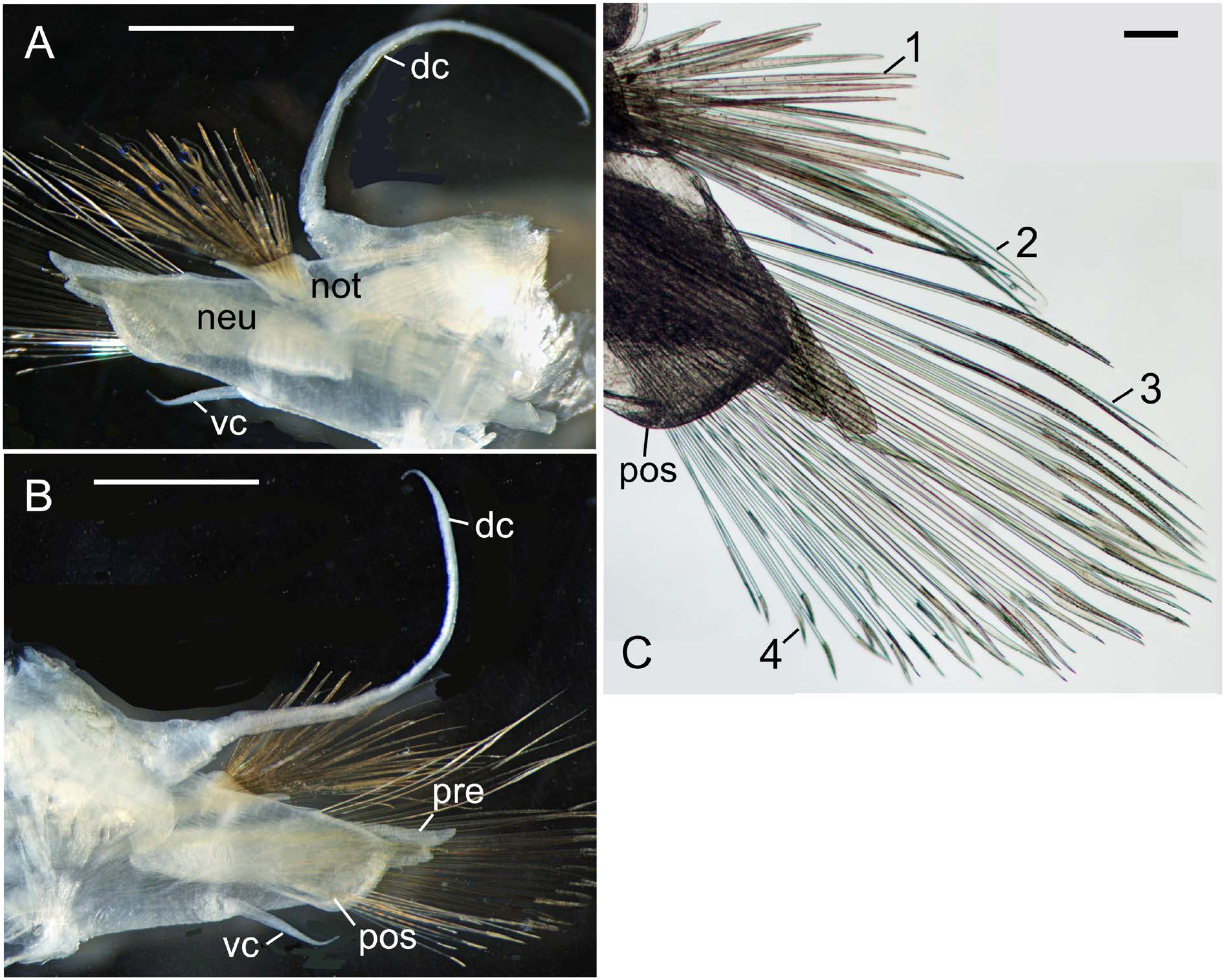
First segment with one pair of tentaculophores, each with digitiform acicular lobe containing single stout aciculum, without chaetae, with two tentacular cirri; dorsal tentacular cirri 1.5 times longer than ventral one ( Fig. 3A, B View Fig ), all with tapering tips, smooth.
Segment 2 with first pair of elytrophores, biramous parapodia, and ventral buccal cirri with tapering tips, smooth ( Fig. 3A View Fig ).
Elytra 16 pairs, covering dorsum almost completely, on segments 2, 4, 5, 7, alternating to 23, 26, 29, 32, and 33 ( Table 1). Elytra oval, thin, semitransparent, without marginal fringing papillae, with up to around one hundred small rounded microtubercles ( 0.01–0.05 mm in diameter) in central area of outer surface ( Fig. 3D View Fig ).
Parapodia biramous. Notopodia small, with projecting acicular lobe at inferior edge ( Fig. 5A View Fig ). Neuropodia large, composed of slender prechaetal acicular lobe and rounded postchaetal lobe ( Fig. 5A–C View Fig ); prechaetal acicular lobe slightly longer than postchaetal lobe, bifid distally, with longer finger-like supraacicular and shorter infraacicular conical processes and protruding tip of neuroacicula. Dorsal cirri with short thick cirrophores and long slender cirrostyles extending beyond chaetae, longest in several posterior chaetigers ( Fig. 3C View Fig ), e.g., about 5 mm in longest dorsal cirri (2.4– 2.5 times as long as BWa, 1/4–1/3 as long as BL) in NSMTPol 113480 and 113482 (22 and 15 mm BL, respectively). Ventral cirri short. All dorsal and ventral cirri with tapering tips, smooth.
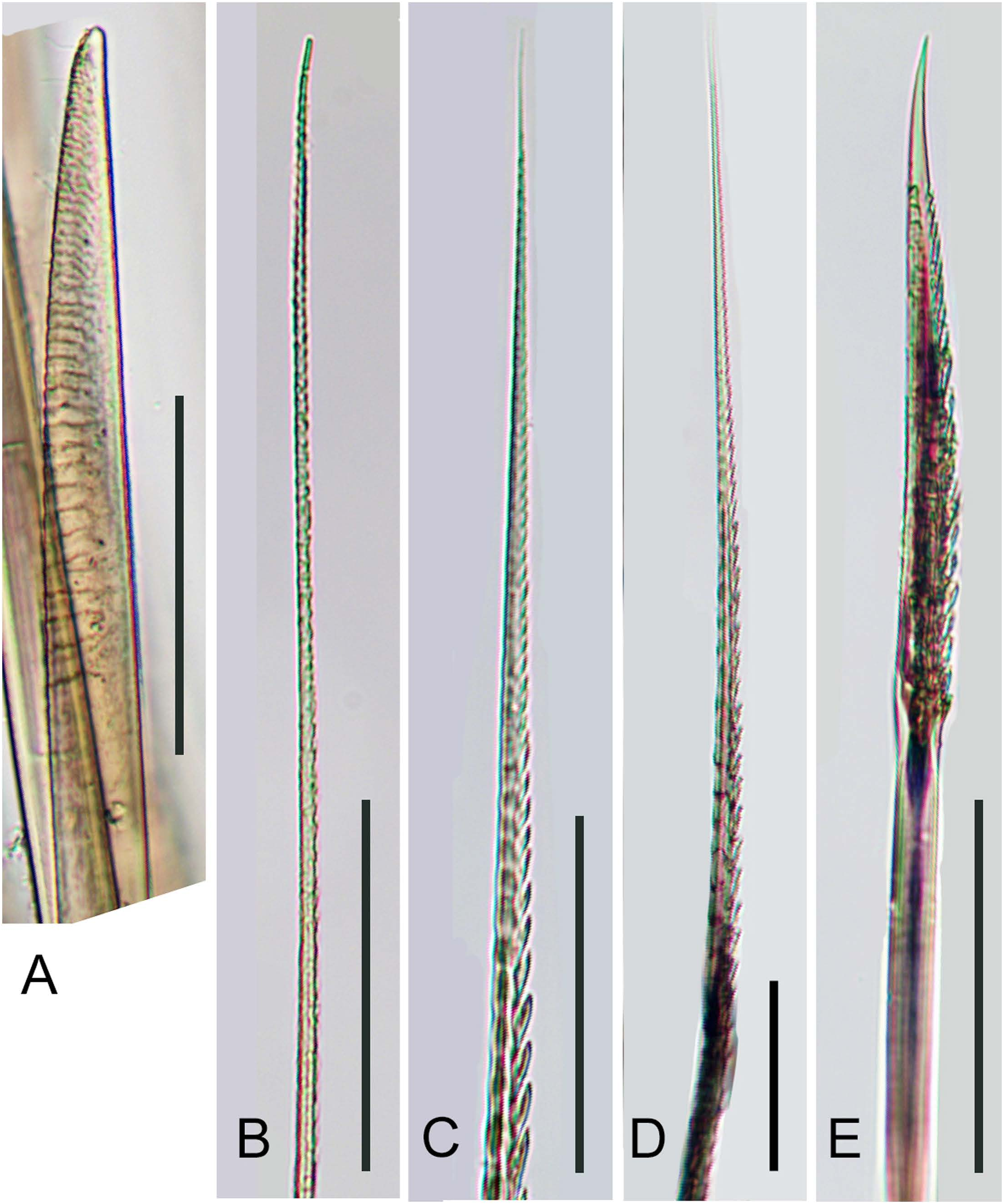
Notochaetae faintly serrated, of two types ( Fig. 5C View Fig ): superior notochaetae stout, rod-like, with round tip and fine transverse striations on distal portion ( Fig. 6A View Fig ); inferior notochaetae slender, capillary, faintly serrated ( Fig. 6B View Fig ). Neurochaetae all unidentate, markedly serrated, tapering to fine tips, of two types ( Fig. 5C View Fig ): supra-acicular neurochaetae slender, with long spinous region ( Fig. 6C, D View Fig ); infraacicular neurochaetae gradually shortening downwards, with expanded subdistal shorter spinous region and marked smooth non-serrated area around slightly curved tip ( Fig. 6E View Fig ); intermediate form in middle position ( Fig. 5C View Fig ).
Nephridial papillae cylindrical at post-ventral base of each parapodium, from segment 6 to posterior end, except at few posteriormost small parapodia.
Anal cirri about 6 mm long (about 1/3 as long as BL) in NSMT-Pol 113479 ( 19 mm BL) ( Fig. 3C View Fig ).
Variations. The shape of anterior margin of prostomium varied from having typical cephalic peaks with tapering tips ( Fig. 4F View Fig ) to lacking any peak (i.e., anterior margin of prostomium connecting to dorsal side of ceratophores of lateral antennae) ( Fig. 4A, B View Fig ), with intermediate forms having round or slightly tapering protrusions ( Fig. 4C–E View Fig ). The last pair of elytra on the segment 33 was extremely small in five specimens ( 14–32 mm BL; Fig. 3C View Fig ).
Reproduction. No intracoelomic egg or sperm were found in our specimens, which were collected in April, May, and October, while Izuka (1912: 58) described “a large mass of eggs is borne under elytra at the end of March”.
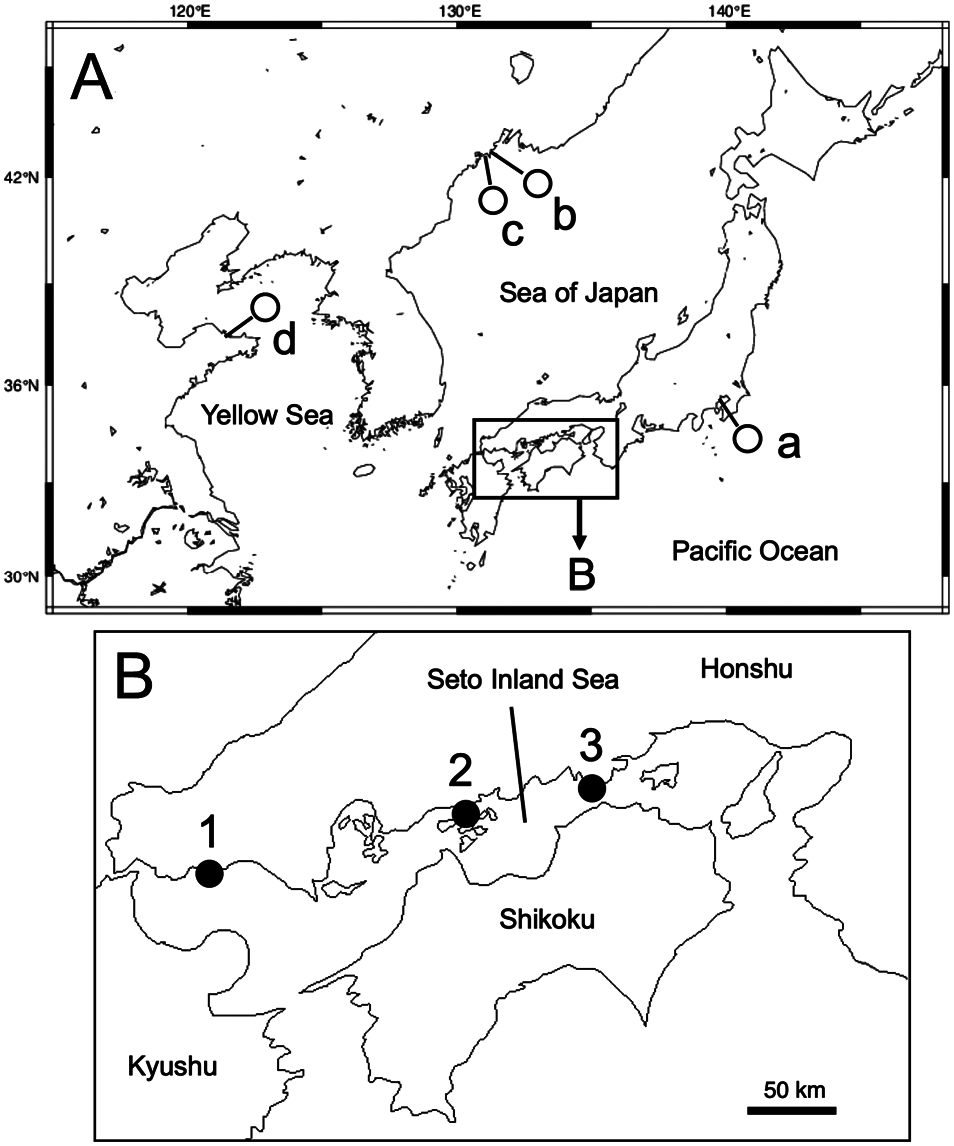
Geographic distribution. Tokyo Bay ( Izuka 1912) and the Seto Inland Sea (present study) in Japan, Possjet Bay ( Buzhinskaja 1967; Marin and Antokhina 2020) and Peter the Great Bay ( Marin and Antokhina 2020) in the Russian Far East, and the Yellow Sea in China ( Fauvel 1933) ( Fig. 1 View Fig ).
Habitat. All our specimens were collected on sandy intertidal-flat sediments inhabited by U. unicinctus . One specimen (NSMT-Pol 113479) was collected together with a spoon worm (NSMT-Ec 188), by sucking with a yabby pump ( Fig. 2A, B View Fig ) and another (NSMT-Pol 113482) was found adhered to the body surface of a spoon worm ( Fig. 2C, D View Fig ). The largest specimen (NSMT-Pol 113484) was collected together with a large spoon worm (more than 30 cm BL in vivo) by digging around an opening of its burrow. Accordingly, the scale worm seems to reside within the burrows of the spoon worm, being possible symbiotic (i.e., commensal), as suggested by Marin and Antokhina (2020), who also collected their specimens from spoon-worm burrows using a yabby pump.
In the aquarium, the scale worm was usually located underneath the spoon worm, with its dorsal side facing the host’s body, lifting up its dorsal cirri with their tips in contact with the host body surface ( Fig. 2A, B View Fig ).
The holotype of A. sinagawaensis was collected at the end of March “near Shinagawa” (inner part of Tokyo Bay, central Japan) ( Izuka 1912), a well-known clam-digging intertidal flats during the spring tides around March ( Izuka 1921) that were densely populated by U. unicinctus . Around Shinagawa, several-hundred individuals of the spoon worm were collected per day by local fishermen to be used as fishing baits ( Takamatsu 1894; Nishikawa 2007). Thereafter, the tidal flats around Shinagawa and all other areas in the inner part of Tokyo Bay were almost completely land reclaimed ( Furota 1997; Sato 2010).
The Chinese coast of the Yellow Sea inhabited by the specimens of A. sinagawaensis collected by Fauvel (1933) also falls within the distribution range of U. unicinctus ( Zhou et al. 2007) .
Remarks. Morphological characteristics of the six specimens ( 15–60 mm BL) from the Seto Inland Sea almost agree well with Izuka’s (1912) original description, which was based on a single specimen ( 23 mm BL) from Tokyo Bay, as well as with Fauvel’s (1933) description ( three specimens from the Yellow Sea, 37–57 mm BL), and Buzhinskaja’s (1967) and Pettibone’s (1996) descriptions (same specimen from Possjet Bay, 60 or 58 mm BL). However, Izuka (1912) described the last two elytra attached on segments 32 and 35, contrasting to on segments 32 and 33 ( Buzhinskaja 1967; Pettibone 1996; present study). The disagreement could represent an interpopulation variability or a misjudgment by Izuka (1912), which lead us to describe this character as “segments 32 and 33 (or 35)” in the generic diagnosis. Conversely, the presence on “segments 31 and 32” reported by Fauvel (1933) for the Chinese specimens seems to be a mistake, meaning “chaetigers” instead of “segments”; Uschakov (1982) examined both Buzhinskaja’s (1967) and Fauvel’s (1933) specimens and confirmed their morphology fully agrees.
Izuka (1912) described the in vivo body color of its mature female as pinkish-yellow, in contrast to the gray of our immature specimens that also have small rounded tubercles in the central part of the outer elytral surface, instead of “neither cilia nor papillae” as described by Izuka (1912). Most likely, he may have overlooked the tubercles due to the large egg masses attached under elytra in his specimen. It would have been desirable to re-examine the Izuka’s (1912) type specimen. However, A. sinagawaensis is not included in the species list of Akira Izuka’s type and non-type polychaete collection of The University Museum, The University of Tokyo ( Nishi and Tanaka 2011). The first author visited this museum in September 2022 and could not find other specimens than those in Nishi and Tanaka (2011), including the holotype of A. sinagawaensis . However, it may be outside the museum. In the description of “ Harmothoë sinagawaensis (non Izuka) Fauvel, 1932 ”, Fauvel (1953: 49) wrote “I have since had the opportunity to observe H. sinagawaensis specimens from Japan, which is a different species, with two kinds of dorsal setae and 16 pair of elytra”. This study was carried out after another one on the Japanese polychaete fauna (i.e., Fauvel 1936), based on the personal collection of professor Yo K. Okada ( Kyoto Imperial University). For this occasion, the type of A. sinagawaensis might have been transported to France together with Okada’s collection. All Fauvel’s collection (150 vials with 80–90 species) is deposited at the Muséum national d’Histoire naturelle of Paris and requires further revision ( Solís-Weiss et al. 2004), which might reveal the presence of the holotype of A. sinagawaensis .
Hesperonoe urechis Marin and Antokhina, 2020 , which was originally described based on two specimens from Posjeta (=Possjet) Bay ( holotype) and Peter the Great Bay (Sea of Japan), is here considered as synonymous with A. sinagawaensis based on our morphological and molecular data. Despite the holotype of H. urechis [ 71 mm BL; illustrated in Marin and Antokhina (2020: fig. 8b, c) without indication of holotype, according to I. Marin, personal communication] was described as having 15 pairs of elytra, the preserved fragmented holotype actually has 13 pairs of elytra ( Marin and Antokhina 2020). Also, the photo of the living holotype ( Marin and Antokhina 2020: fig. 8c; its original explanation “not collected” is a mistake, according to I. Marin, personal communication) has 16 pairs of elytra, with the last one as large as the largest one at midbody ( Fig. 7A View Fig ). The non-type living specimen illustrated in Marin and Antokhina (2020: figs 1e, 8a) also shows 16 pairs of elytra ( Fig. 7B View Fig ). This specimen was lost and thus not included in the “Material examined” section in Marin and Antokhina (2020: 7) (I. Marin, personal communication). It is the same individual illustrat- ed in Buzhinskaja (2013: fig. E) as Hesperonoe sp. (I. Marin, personal communication). This specimen is yellow-brown, instead of the gray of our specimens, which is here considered as intraspecific variation.
The report of A. sinagawaensis from Hainan Island (South China Sea) was not supported by a morphological description ( Wu et al. 1997) and thus cannot be here confirmed.
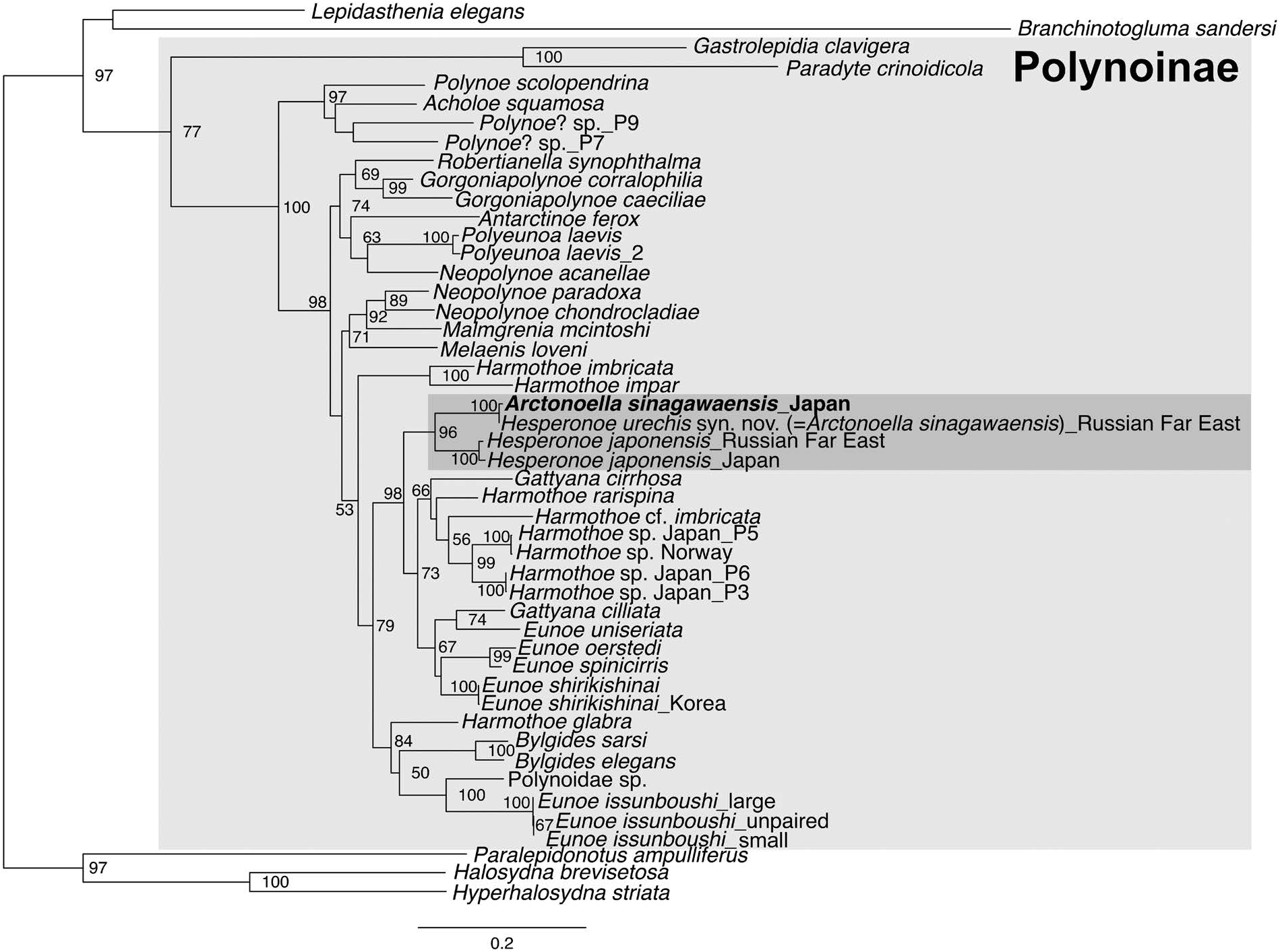
Molecular analyses. The maximum-likelihood phylogenetic analysis of Polynoinae based on COI, 16S, 18S, and 28S sequences ( Fig. 8 View Fig ) shows: (1) A. sinagawaensis from Yamaguchi grouping within Polynoinae with a 77% bootstrap support [BS] and being sister to H. urechis (100% BS), (2) H. japonensis from Hokkaido ( Japan) and Peter the Great Bay ( Russia) being sister (100% BS), and (3) the Arctonoella and H. japonensis clades being sister (96% BS). The interspecific genetic distances between the Japanese and Russian specimens of A. sinagawaensis and H. japonensis ranged from 15.1% to 16.0%, while the intraspecific distances ranged from 0.4% to 1.0%, respectively ( Table 3).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Arctonoella Buzhinskaja, 1967
| Sato, Masanori, Jimi, Naoto, Itani, Gyo, Henmi, Yumi & Kobayashi, Shuji 2023 |
Hesperonoe urechis
| Marin, I. & Antokhina, T. 2020: 11 |
Hesperonoe sp.
| Buzhinskaja, G. N. 2013: 125 |
Arctonoella sinagawaensis
| Wu, B. & Wu, Q. & Qiu, J. & Lu, H. 1997: 175 |
Arctonoella
| Wu, B. & Wu, Q. & Qiu, J. & Lu, H. 1997: 175 |
| Pettibone, M. H. 1996: 637 |
| Uschakov, P. V. 1982: 123 |
| Fauchald, K. 1977: 60 |
Arctonoella sinagawaensis
| Buzhinskaja, G. N. 2013: 37 |
| Imajima, M. 2001: 13 |
| Wu, B. & Wu, Q. & Qiu, J. & Lu, H. 1997: 175 |
| Pettibone, M. H. 1996: 632 |
| Uschakov, P. V. 1982: 124 |
| Buzhinskaja, G. N. 1967: 83 |
Hesperonoë
| Uschakov, P. V. & Wu, B. L. 1965: 172 |
Gattyana sinagawaensis
| Imajima, M. & Hartman, O. 1964: 32 |
| Hartman, O. 1959: 71 |
Harmothoe sinagawaensis
| Fauvel, P. 1933: 10 |
Harmothoë sinagawaensis
| Izuka, A. 1912: 59 |