Anthomyza Fallén, 1810
|
publication ID |
https://doi.org/10.5281/zenodo.4272829 |
|
publication LSID |
urn:lsid:zoobank.org:pub:E95E58A5-E0F1-4237-9D7C-4A81BB3120DD |
|
DOI |
https://doi.org/10.5281/zenodo.4339863 |
|
persistent identifier |
https://treatment.plazi.org/id/03FB87A9-FF8B-FFF7-FE11-6AE7FBA4FDF7 |
|
treatment provided by |
Felipe |
|
scientific name |
Anthomyza Fallén, 1810 |
| status |
|
Genus Anthomyza Fallén, 1810 View in CoL View at ENA
Anthomyza Fallén, 1810:20 View in CoL [feminine]. Anthomyza: WILLISTON (1896) View in CoL : 105 (key); CZERNY (1902): 250 (diagnosis); ALDRICH (1905): 645 (catalogue); BECKER (1905): 230 (catalogue); WILLISTON (1908): 298 (key); COQUILLETT (1910): 507 (catalogue); MELANDER (1913): 286 (key); CZERNY (1928): 2 (redescription); CURRAN (1934): 329 (key); SÉGUY (1934): 301 (key); COLLIN (1944): 265 (key); STURTEVANT (1954): 557 (key); FREY (1958): 32 (key); TROJAN (1962): 37 (diagnosis); CURRAN (1965): 329 (key); SABROSKY (1965): 819 (catalogue); COLE (1969): 435 (key); DOSKOĆIL (1977): 257 (key); VOCKEROTH (1977): 241 (catalogue); SOÓS (1981):109 (diagnosis); ANDERSSON (1984b):50 (catalogue);VOCKEROTH (1987): 890 (key); ROHÁĆEK & FREIDBERG (1993): 64 (key); ROHÁĆEK (1998a): 172 (world checklist); ROHÁĆEK (1998b): 276 (key); ROHÁĆEK (2006a):83 –89 (diagnosis, key); ROHÁĆEK (2009a):24 –31, 106–114 (diagnosis, key, phylogeny).
Leptomyza Macquart, 1835: 580 View in CoL [feminine] (unnecessary new name for Anthomyza Fallén, 1810 View in CoL assumed preoccupied by Anthomyia Meigen, 1803 ). Type species: Anthomyza gracilis Fallén, 1823: 8 View in CoL (designated by COQUILLETT 1910: 560).
Leptomyza: SCHINER (1864) View in CoL : 281 (diagnosis).
Anthophilina Zetterstedt, 1837: 55 View in CoL [feminine] (unnecessary new name for Anthomyza Fallén, 1810 View in CoL assumed preoccupied by Anthomyia Meigen, 1803 ). Type species: Anthomyza gracilis Fallén, 1823: 8 View in CoL (by monotypy).
Anthophilina: RONDANI (1875) View in CoL : 186 (checklist, key); OSTEN SACKEN (1878): 198 (catalogue).
Type species: Anthomyza gracilis Fallén, 1823: 8 View in CoL (designated by WESTWOOD 1840: 152).
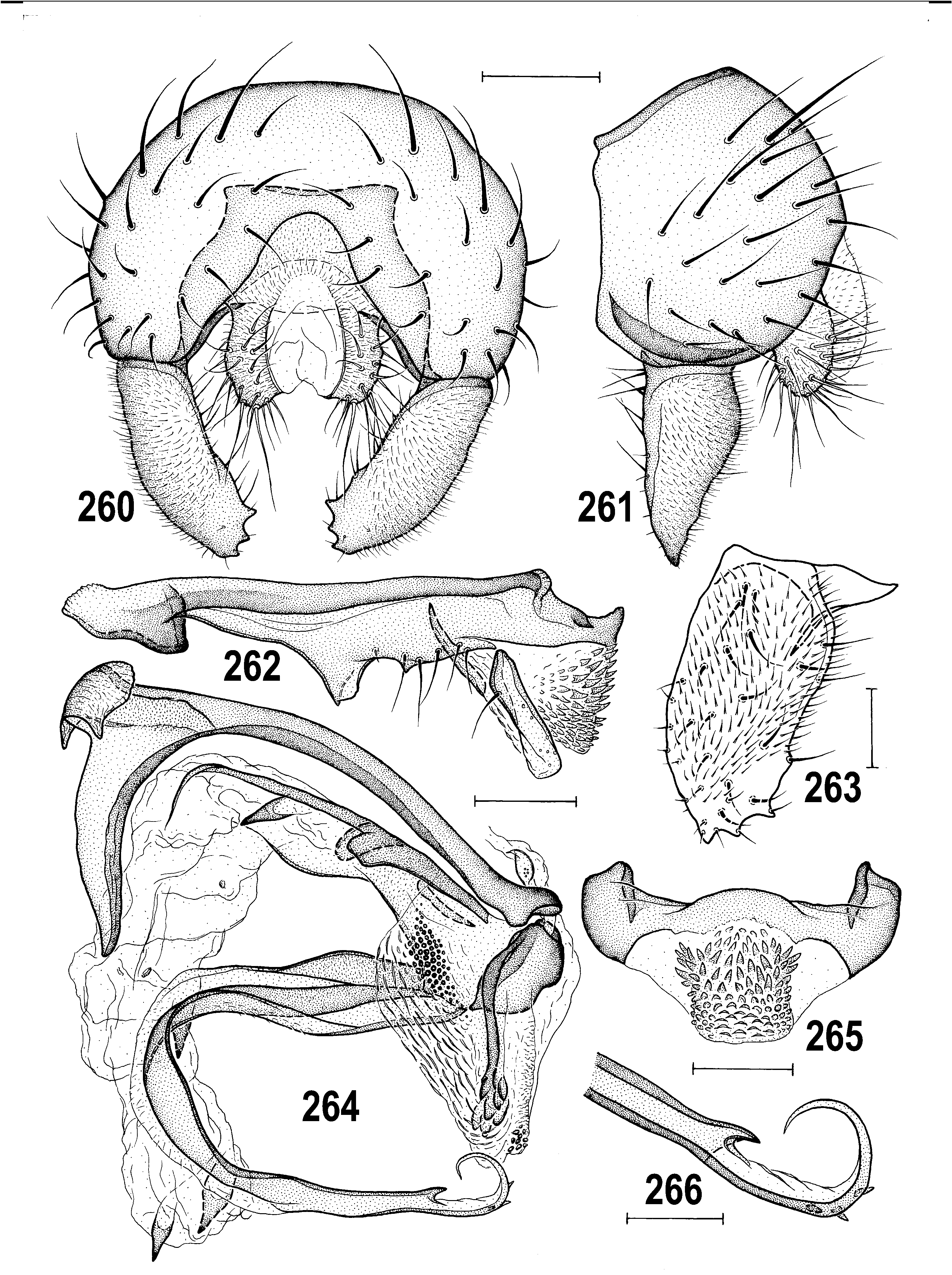
Diagnosis. (1) Head longer than high to slightly higher than long. (2) Eye large, suboval to ovoid, with longest diameter oblique. (3) Frons mostly dull, frontal triangle medium-sized or narrow, reaching to anterior half to third of frons. (4) Frontal lunule small but distinct. (5) Occiput slightly to distinctly concave. (6) Vertex (= top of head) usually without silvery microtomentose spots between frontal triangle and posterior part of orbits. (7) Antenna geniculate, pedicel simple; (8) arista short-ciliate to distinctly plumose. (9) Palpus usually yellow, with 1 subapical seta. Cephalic chaetotaxy: (10) pvt small, convergent to crossed; (11) vti usually longest, vte and oc also long; (12) 3 (aberrantly 4 due to duplication) ors, but the anterior short and/or reduced to a setula, 0–1 microsetulae in front of the latter; (13) a single row of small postocular setulae; (14) 1 long vi; subvibrissa usually small, somewhat longer than peristomals; (15) peristomal setulae small and sparse. (16) Posterior corner of head rounded. (17) Antenna often with darker 1st nagellomere in female; face usually with same colouring in both sexes.
(18) Thorax as wide as or narrower than head. Thoracic chaetotaxy: (19) 1 hu; 2 npl (anterior longer); (20) 1 distinct prs; (21) 1 short sa, 1 pa; (22) 2–3 long dc (if 3 then anterior markedly shorter), posterior dc longer than apical sc; (23) ac microsetae in 4 (rarely 2) rows on suture, in 2 more posteriorly; (24) 2 sc, basal very short and weak; (25) 1 minute ppl; (26) 2 stpl, posterior usually longer. (27) Legs mostly yellow, often with dark apical tarsal segments, rarely with darkened femora. (28) f 1 with posteroventral ctenidial spine. (29) t 2 with usual ventroapical seta (rarely duplicated). (30) Male f 3 simply setulose or with a posteroventral row of dense, short and thickened setae. (31) Wing long and relatively narrow; (32) wing membrane unicolourous, at most darkened at anterior margin. (33) C usually with distinct spinulae between apices of R 1 and R 2+3; (34) R 2+3, long, slightly sinuate, subparallel with C; (35) R 4+5 straight, very slightly bent or sinuous, subparallel with M apically; (36) cell dm long, widened distally, with r-m situated near (usually in front of) its middle; (37) distal part of CuA 1 usually longer than dm-cu and almost reaching wing margin; A 1 short, not reaching wing margin. (38) Alula small and narrow.
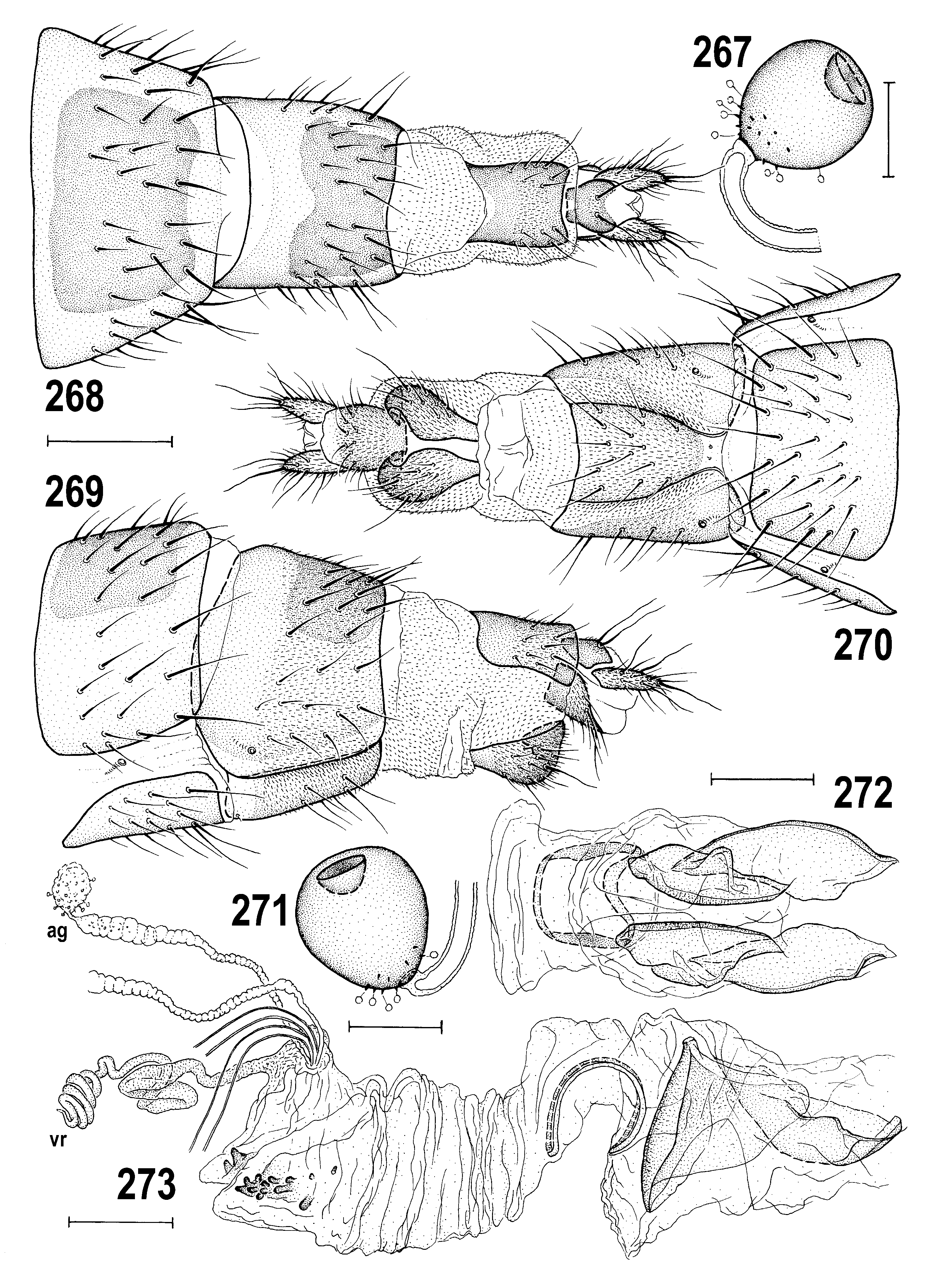
Male abdomen. (39) T1 and T2 partly fused. (40) T2–T5 large and broad, usually all uniformly pigmented, more rarely dark transversely banded or spotted. (41) S1–S5 narrow and usually paler than terga (S1 shortest and bare). Male postabdomen: (42) T6 reduced, short transverse, pigmented, medially unpigmented or entirely unpigmented, and bare. (43) S6 and S7 strongly asymmetrical, with 0–4 setulae each. (44) S8 less asymmetrical, long, setose in posterior half.
Male genitalia. (45) Epandrium as wide as high to strongly wider than high, with 1–2 pairs of longer setae in addition to short setae. (46) Medandrium of various form, usually broader ventrally and narrower dorsally. (47) Cercus small to medium long, weakly sclerotized, usually with nne pale setae. (48) Gonostylus of various size, usually relatively broad, with micropubescence on outer side, setose on inner side. (49) Hypandrium relatively robust, symmetrical and well sclerotized, with anterior inner lobes more or less developed; (50) transandrium of various form, without or with (sometimes extremely robust) caudal process. (51) Pregonite fused with hypandrial frame, low, often with 1 ventrally projecting lobe and with 2 (anterior and posterior) groups of setae; (52) postgonite slender, strap-like, with 1 anterior or lateral setula, usually in proximal half. (53) Phallapodeme slender to robust, basally (sometimes asymmetrically) bifurcate, apex usually bicuspidate. (54) Aedeagus with short and rather simple phallophore. (55) Distiphallus composed of largely membranous saccus and usually long, slender and sclerotized nlum. (56) Saccus armed with robust to small and short spines; (57) nlum sclerotized, formed by single sclerite, usually slender and distally attenuated but its apex may be secondarily widened and terminated in more projections. (58) Aedeagal part of folding apparatus with various structures externally and internally, usually spinose or tuberculate and striated; connecting sclerite usually distinct, rarely membranous. (59) Basal membrane usually densely spinose, unarmed when caudal process enlarged. (60) Ejacapodeme very small, usually with slender digitiform projection.
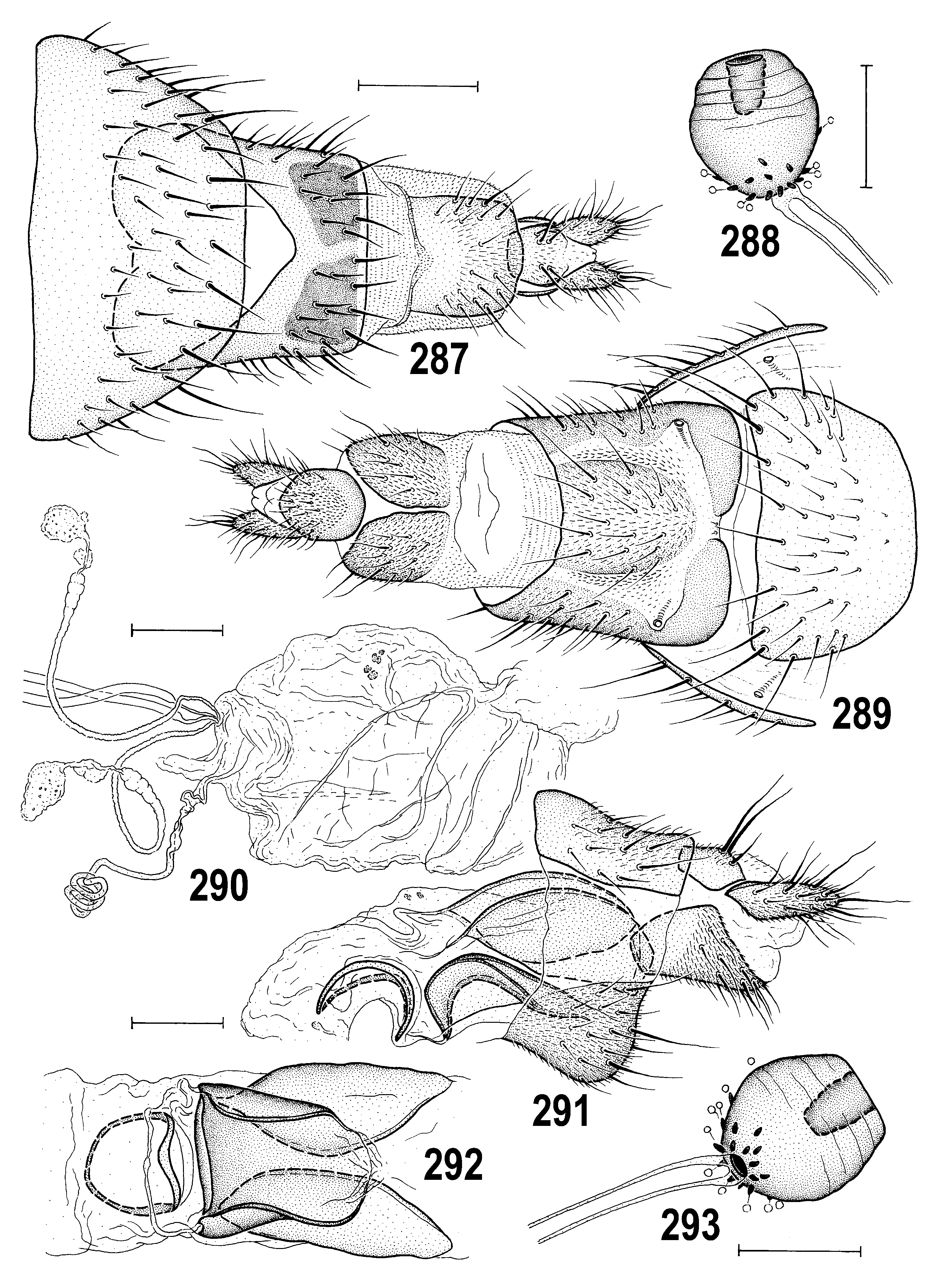
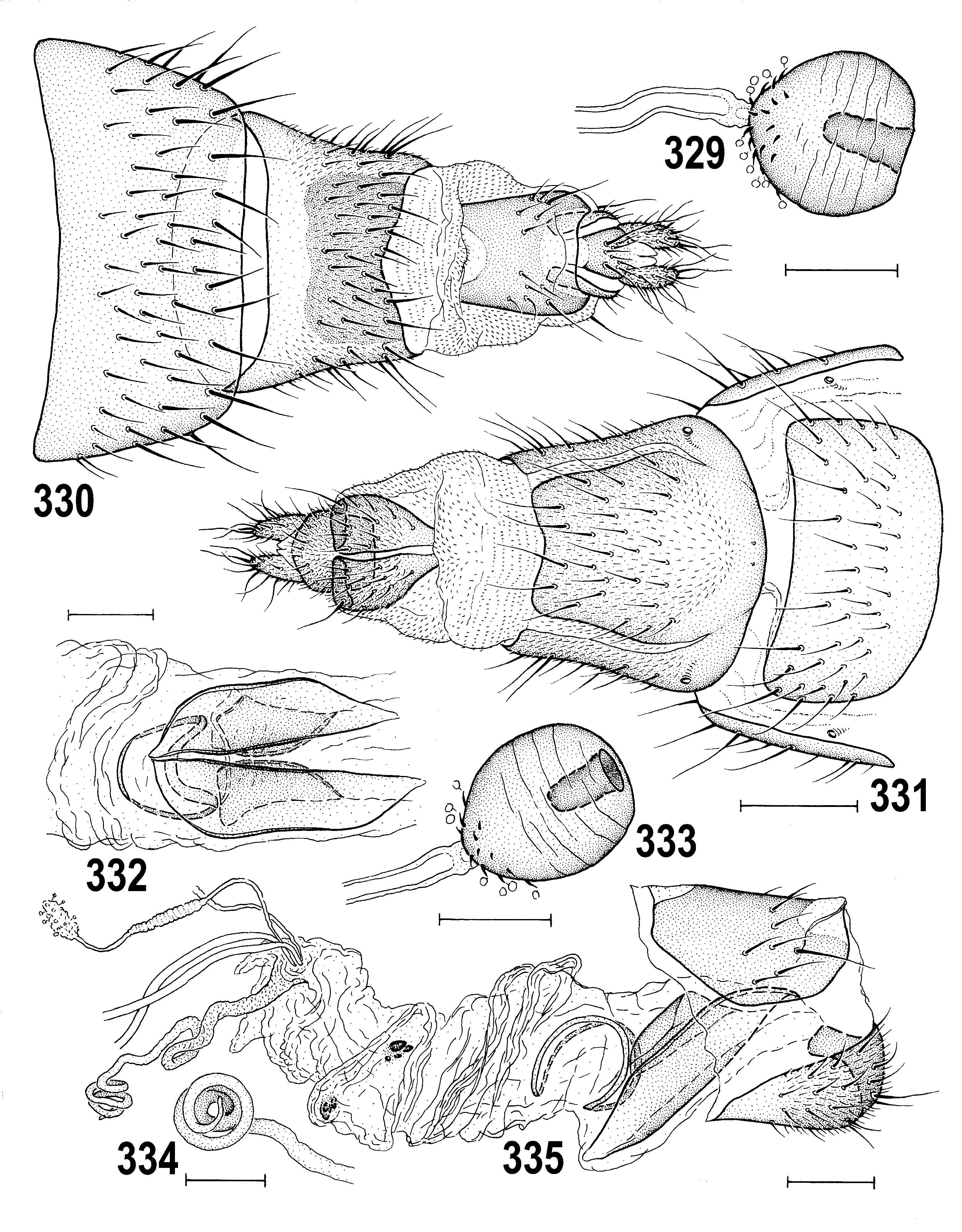
(61) Female abdomen with broader, more transverse preabdominal terga and narrower sterna than in male; T1–T5 usually uniformly pigmented dark brown to yellow. (62) Postabdomen long, tapered, telescopic. (63) T6 large, similar to T5, usually more trapezoidal in outline (seen dorsally); S6 largest sternum and usually paler than T6. (64) T7 and S7 forming or tending to form tergosternal ring-shaped cone, original S7 usually discernible by pale pigmentation; if S7 separate, it is reduced or (rarely) widened and overlapping lateral margins of T7; (65) T8 usually narrow, elongate, sometimes strongly tapered posteriorly. (66) S8 longitudinally divided, in 2 often elongate sclerites, having posterior part more or less bent dorsally and recurved internally. (67) Female genital chamber with 1 to 3 pairs of internal sclerites (often fused together, rarely asymmetrical) and with (68) one curved and usually elongate (never transverse) annular sclerite; (69) ventral receptacle very long, tubular and hyaline, with apex slender and curved, vermicular or helicoid; (70) accessory gland small, hyaline, at most with nnely granular structure and with minute stalked globulae on surface, on slender, subterminally slightly widened duct. (71) Spermathecae (1+1) on very long ducts, subspherical to elongately pyriform, usually with transversely ringed surface and minute spinulae, often also with terminal invagination. (72) T10 small, variable in shape, usually shorter and narrower than S10, with 1 medial pair of long setae and (sometimes) with 1–4 pairs of additional setulae. (73) S10 usually longer and wider than T10. (74) Cercus long and slender, rarely more robust, with numerous nne setae (apical and dorsopreapical longest).
Discussion. As stated by ROHÁĆEK (2006a, 2009a), this is likely the largest genus of Anthomyzidae and displays considerable morphological diversity in external as well as in male and female genitalic characters. This continues to be true after the revision of the Nearctic species with a number of species described as new below. The addition of these new species to Anthomyza required an update of the generic diagnosis (originally provided by ROHÁĆEK 2009a) and preparation of a key to the identincation of all the species now known in this region. Although the genus Anthomyza can be identined by external characters (see the generic key above), it is always necessary to verify the placement of unknown species by checking the male genitalic and female postabdominal characters stressed below.
The monophyly of Anthomyza is only supported by several apomorphic characters in the male and female genitalia, the most important of which are the following: (56) saccus armed by spines; (57) nlum of distiphallus compact, formed by a single sclerite; (60) ejacapodeme very small; (64) female T7 and S7 forming (or tending to form) a tergosternal ring; (66) female S8 longitudinally divided into 2 sclerites which are posteriorly partly invaginated; (68) annular sclerite more or less curved and elongate; (69) ventral receptacle very long, tubular, with apex attenuated and curved or twisted. None of these apomorphies is unique within the family Anthomyzidae and some (64, 66, 68) are shared with relatives of Anthomyza , viz., Arganthomyza , Ischnomyia and Fungomyza . In addition, the genus Epischnomyia Roháček, 2006 has recently been hypothesized as being the closest relative of Anthomyza in a multigene molecular analysis ( ROHÁĆEK & TÓTHOVÁ 2014). This relationship also seems to be supported by the internal structures of the male and female genitalia, as some peculiar (and highly modined) characters of Epischnomyia could probably be derived from those of a common ancestor of these two genera or even from a basal clade of Anthomyza (as results of ROHÁĆEK & TÓTHOVÁ 2014 indicate), e.g. the enormously enlarged spines in the saccus of the distiphallus or the sclerotized curved apex of the otherwise tubular ventral receptacle of the female. Nevertheless, the genus Anthomyza in the present concept (without Epischnomyia ) seems to be a monophyletic group as was also demonstrated by molecular data analysis of (only) the 12S + 16S mitochondrial gene markers (ROHÁĆEK et al. 2009). However, the hypothesis based on analysis of seven combined mitochondrial and nuclear gene markers ( ROHÁĆEK & TÓTHOVÁ 2014: Fig. 1 View Fig ) placed Epischnomyia within Anthomyza as a sister group of the Anthomyza macra group (albeit with low support), thus rendering Anthomyza paraphyletic. Because the apomorphic aedeagal characters 56, 57 (although modined in Epischnomyia ) are shared by these two genera, their close (sister-group) relationship is highly plausible; moreover, even the possibility that Epischnomyia species belong, in fact, to Anthomyza cannot be rejected and this hypothesis is to be tested in future phylogenetic studies. Besides the apomorphic features in the male aedeagal complex (56, 57) shared with Epischnomyia , all being clearly derived states with respect to those found in other genera of the Anthomyza clade, the monophyly of Anthomyza is further demonstrated by the very reduced ejacapodeme (60) and by the membranous elongate tubular ventral receptacle (69), with a slender and twisted to coiled apex. The latter character is considered to be the most derived state of a transformation series where the more ancestral, but also still apomorphic, states (relative to taxa outside the Anthomyza clade) of this character occur in Fungomyza , Ischnomyia and Arganthomyza species; the plesiomorphic state, viz. the short membranous ventral receptacle, is known e.g. in the genus Amygdalops Lamb, 1914 . In Epischnomyia , as expected, the ventral receptacle is peculiarly modined: although not terminally tapered (plesiomorphic) it has a short and robust U-curved sclerotized apex (apomorphic). The dissimilarity of Epischnomyia and Anthomyza is further demonstrated by the female S8 which is not longitudinally divided medially in Epischnomyia (in contrast to all other taxa of the Anthomyza clade) but broad and only posteromedially incised.
According to ROHÁĆEK (2009a), the sister pair Fungomyza + Arganthomyza could form a sister clade to Anthomyza . Although this hypothesis disagrees with results of the most recent molecular study by ROHÁĆEK & TÓTHOVÁ (2014), particularly regarding the placement of Fungomyza , the morphological data suggest that these genera plus Ischnomyia and Epischnomyia (see above) are related. The poorly known monotypic genus Receptrixa Roháček, 2006 from the Asian Near East also resembles this group of genera in having the female S8 divided and the tergosternum T7+S7 well developed, and, like Fungomyza + Arganthomyza , it also has shortened spermathecal ducts. The genus differs, however, in having a strongly modined female genital chamber with an extremely enlarged and sclerotized ventral receptacle (see ROHÁĆEK 2006a: Figs 516, 517), reduced spermathecae and fused female cerci.
Species included: At present the genus Anthomyza includes 20 species in the Palaearctic Region plus one species in the Oriental Region. ROHÁĆEK (2009a) divided these species among groups delimited by synapomorphic features as revealed by cladistic analysis of morphological characters. These species [and species groups] are as follows: A. macra Czerny, 1928 , A. pleuralis Czerny, 1928 , A. decolorata Roháček, 2009 [ A. macra group]; A. umbrosa Roháček, 2006 , A. baezi Roháček, 1999 , A. clara Roháček, 2006 [ A. umbrosa group]; A. pallida ( Zetterstedt, 1838) , A. dissors Collin, 1944 [ A. pallida group]; A. neglecta Collin, 1944 , A. paraneglecta Elberg, 1968 , A. orineglecta Roháček, 2006 [ A. neglecta group]; A. collini Andersson, 1976 , A. anderssoni Roháček, 1984 [ A. collini group]; A. drachma Sueyoshi & Roháček, 2003 [ungrouped]; A. ssavosterna Sueyoshi & Roháček, 2003 [ungrouped, possibly related to A. bellatrix group]; A. bellatrix Roháček, 1984 , A. trifurca Sueyoshi & Roháček, 2003 , A. cuneata Roháček, 1987 [ A. bellatrix group]; A. tschirnhausi Roháček, 2009 [originally tentatively placed in A. collini group, now in A. tschirnhausi group, established here]; A. gracilis ( Fallén, 1823) and A. elbergi Andersson, 1976 [ A. gracilis group].
In the Nearctic Region, 18 species of Anthomyza (including 15 new) are recognized here and assigned to species groups as follows: A. tenuis ( Loew, 1863) , A. oblonga sp. nov., A. silvatica sp. nov. [ A. macra group]; A. pengellyi sp. nov., A. mcalpinei sp. nov., A. pullinotum sp. nov., A. concolor (Thomson, 1869) , A. occidentalis sp. nov., A. vockerothi sp. nov. [ A. pallida group]; A. variegata ( Loew, 1863) , A. dichroa sp. nov., A. gibbiger sp. nov., A. orthogibbus sp. nov. [ A. neglecta group]; A. shewelli sp. nov., A. gilviventris sp. nov. [ A. tschirnhausi group, established here]; A. vulgaris sp. nov., A. furvifrons sp. nov., A. equiseti sp. nov. [ A. gracilis group].
The Nearctic species of Anthomyza are treated below following their presumed phylogenetic relationships. Diagnoses of the particular species groups of Anthomyza are updated as a consequence of the inclusion of the Nearctic species; this is done in separate paragraphs in front of the treatment of the nrst species of each group. The species groups represented in the Nearctic Region are keyed below. Keys to the identincation of the species within groups are given under the diagnosis of each group.
Key to identincation of species groups of Nearctic Anthomyza
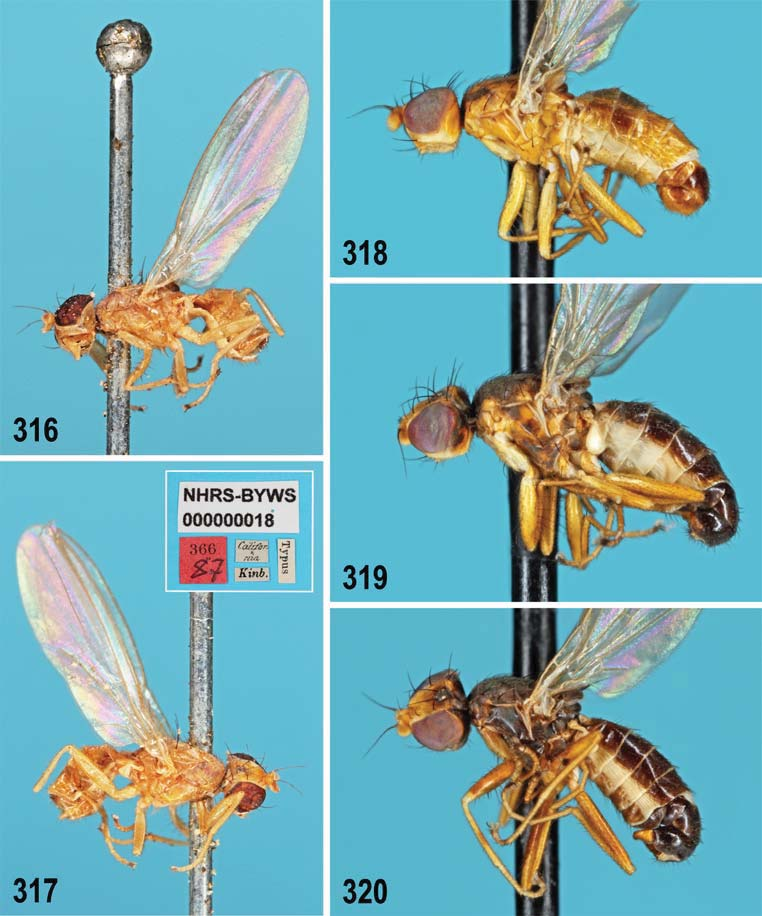
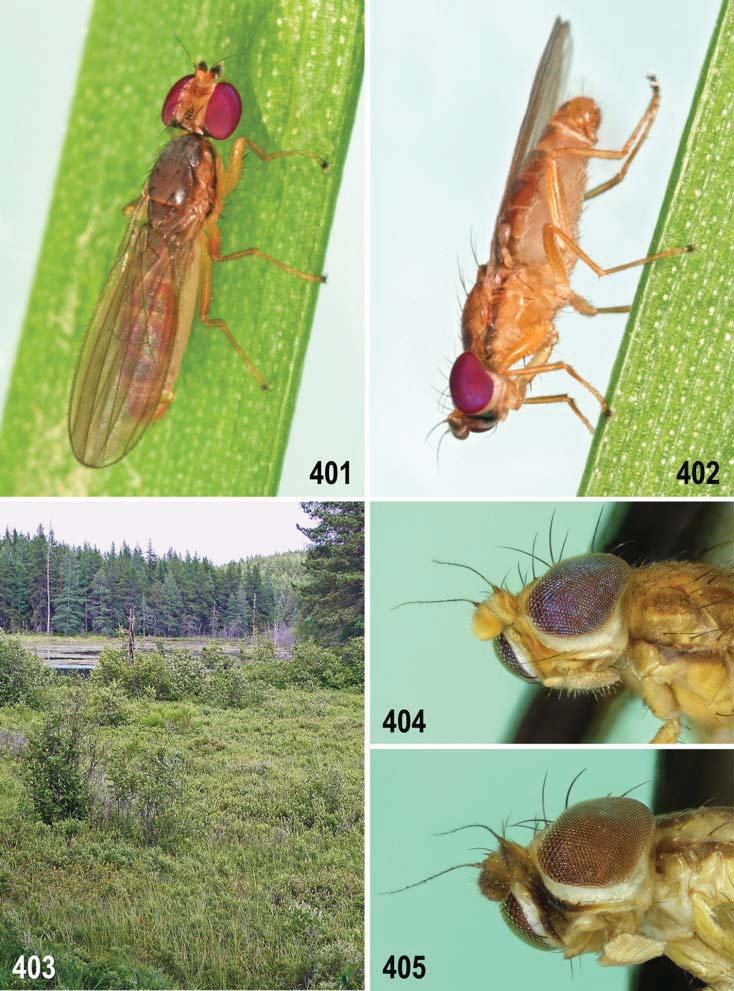
1 Silvery white microtomentum above occipital foramen in a large medially divided patch (best observed obliquely from above in pale species, Figs 294, 297 View Figs 294–298 , 319, 320 View Figs 316–320 , 357 View Figs 357–360 , 401 View Figs 401–405 ) almost reaching to bases of pvt; occiput dark, yellow, or variegated; male f 3 with or without shortened and thickened setae posteroventrally on distal part. ......... 2
– No large silvery white microtomentose patch between occipital foramen and ocellar triangle, or small microtomentose areas restricted to dorsolateral margins of foramen ( A. gracilis View in CoL group, Figs 521, 523 View Figs 518–523 ); occiput otherwise entirely dark; male f 3 simply setose posteroventrally. .................................................................................................. 3
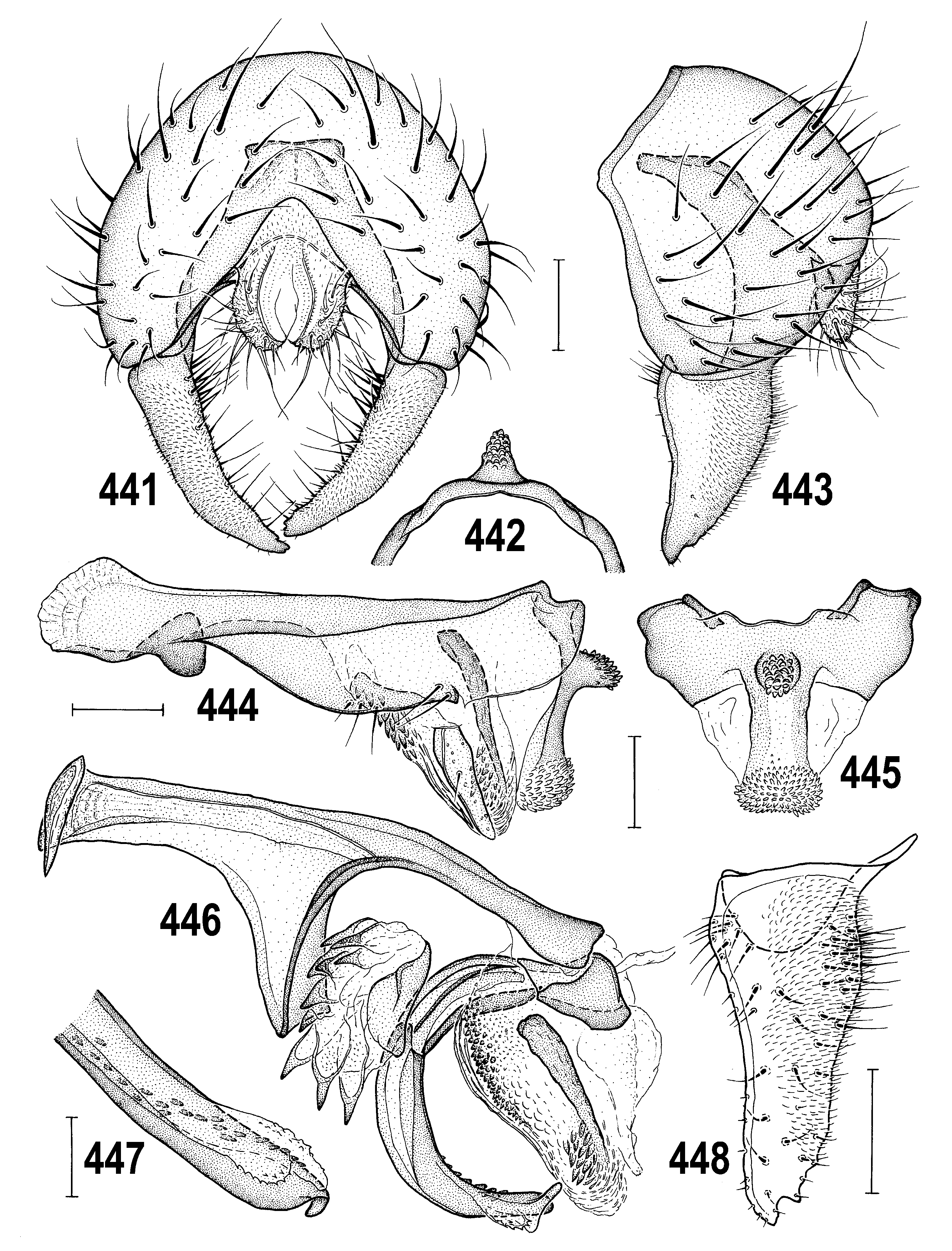
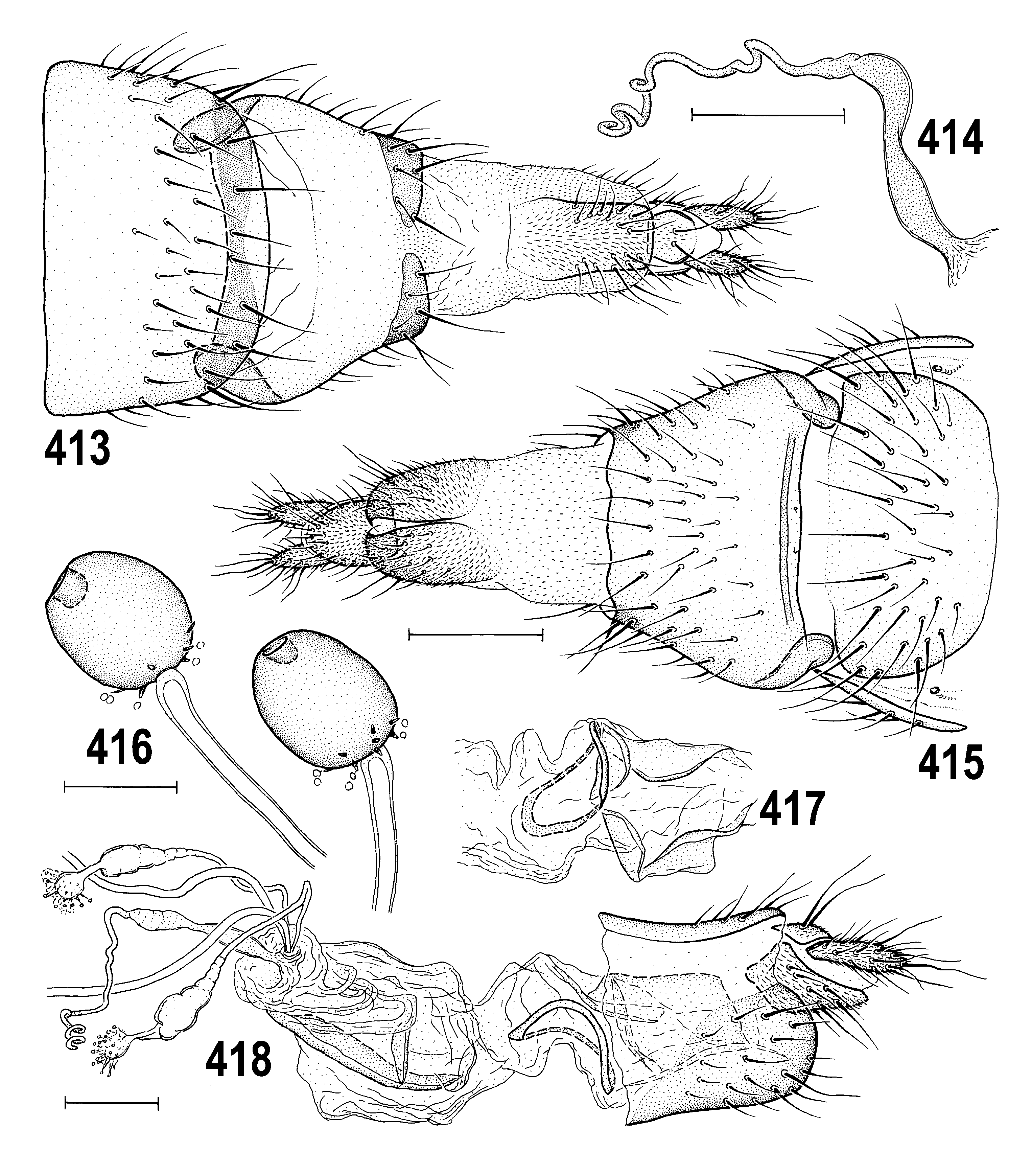
2(1) Primarily yellow species; pleura yellow with narrow brown band on dorsal margin of mesopleuron (least developed in Anthomyza gibbiger View in CoL ) and including greater ampulla of pteropleuron ( Fig. 382 View Figs 381–384 ); male epandrium invariably completely yellow ( Figs 384 View Figs 381–384 , 402 View Figs 401–405 ); male often with face darkened medially ( Fig. 405 View Figs 401–405 ), always yellow in female (cf. Fig. 404 View Figs 401–405 ); 1st antennal nagellomere long-ciliate on anteroventral margin ( Fig. 395 View Figs 392–395 ); male f 3 simply setose; nlum of distiphallus with short, digitiform curved apex ( Figs 410 View Figs 406–412 , 447 View Figs 441–448 ); female T7+S7 with ventrolateral, usually distinctly pouch-like lobes ( Figs 393 View Figs 392–395 , 415 View Figs 413–418 ). .................................. A. neglecta View in CoL group (p. 229; key to species on p. 230)
– Overall colouring wide-ranging; pleura dark brown, yellow, or both but if yellow, never with brown dorsal margin on mesopleuron and greater ampulla ( Figs 295 View Figs 294–298 , 342 View Figs 339–342 ); male epandrium usually at least partly darkened ( Figs 294, 295 View Figs 294–298 ), sometimes yellow in two variably coloured species ( Anthomyza concolor View in CoL , A. vockerothi View in CoL ); face yellow in both sexes; 1st antennal nagellomere short-ciliate on anteroventral margin; male f 3 with shortened and thickened setae posteroventrally in distal part; nlum of distiphallus with slender, curved, gradually attenuate and pointed apex ( Figs 266 View Figs 260–266 , 362 View Figs 361–368 ); female T7+S7 ventrolaterally without pouch-like lobes ( Figs 270 View Figs 267–273 , 289 View Figs 287–293 , 331 View Figs 329–335 , 374 View Figs 369–375 ). ................. ................................................... A. pallida View in CoL group (p. 141; key to species on p. 142)
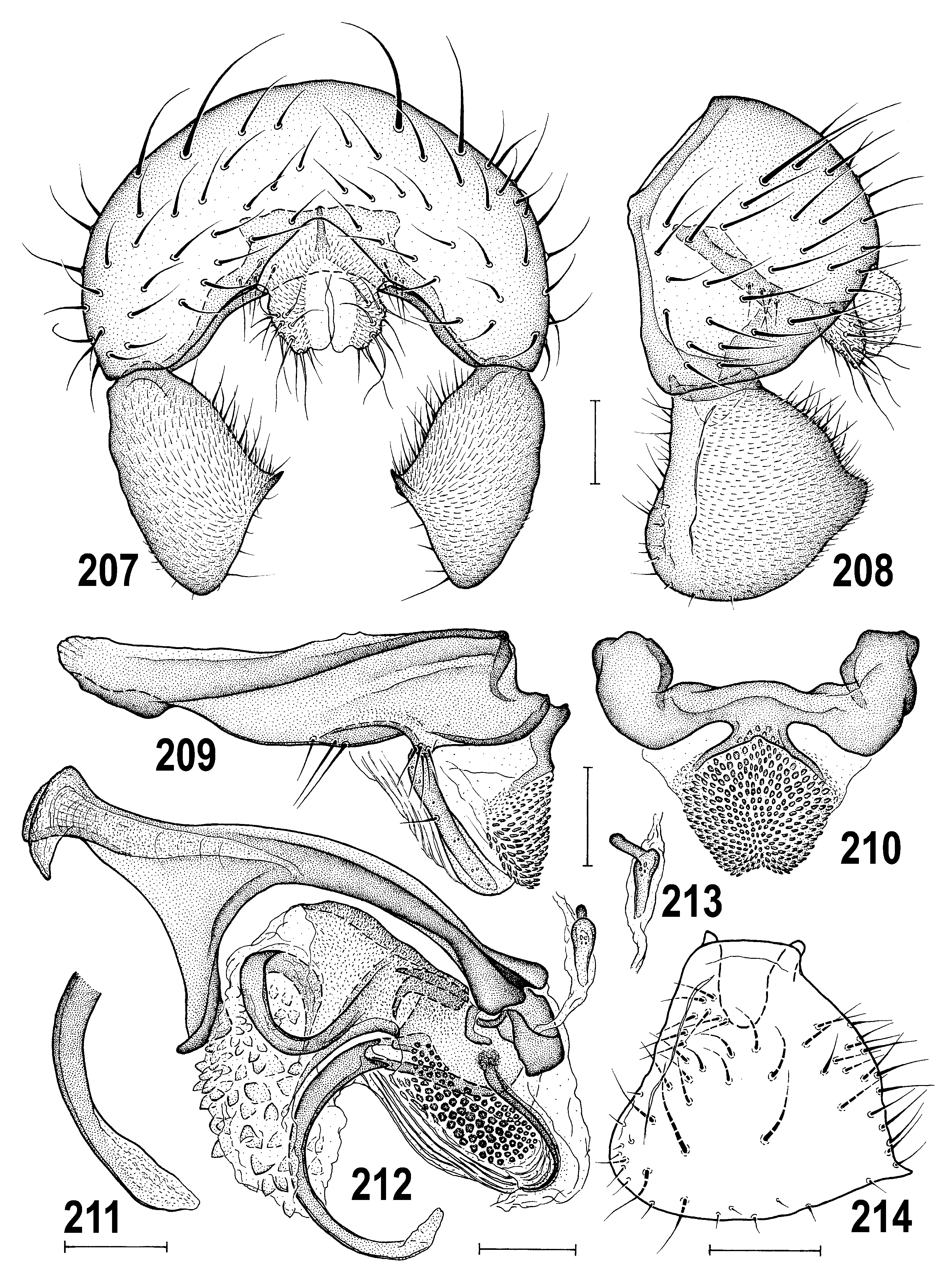
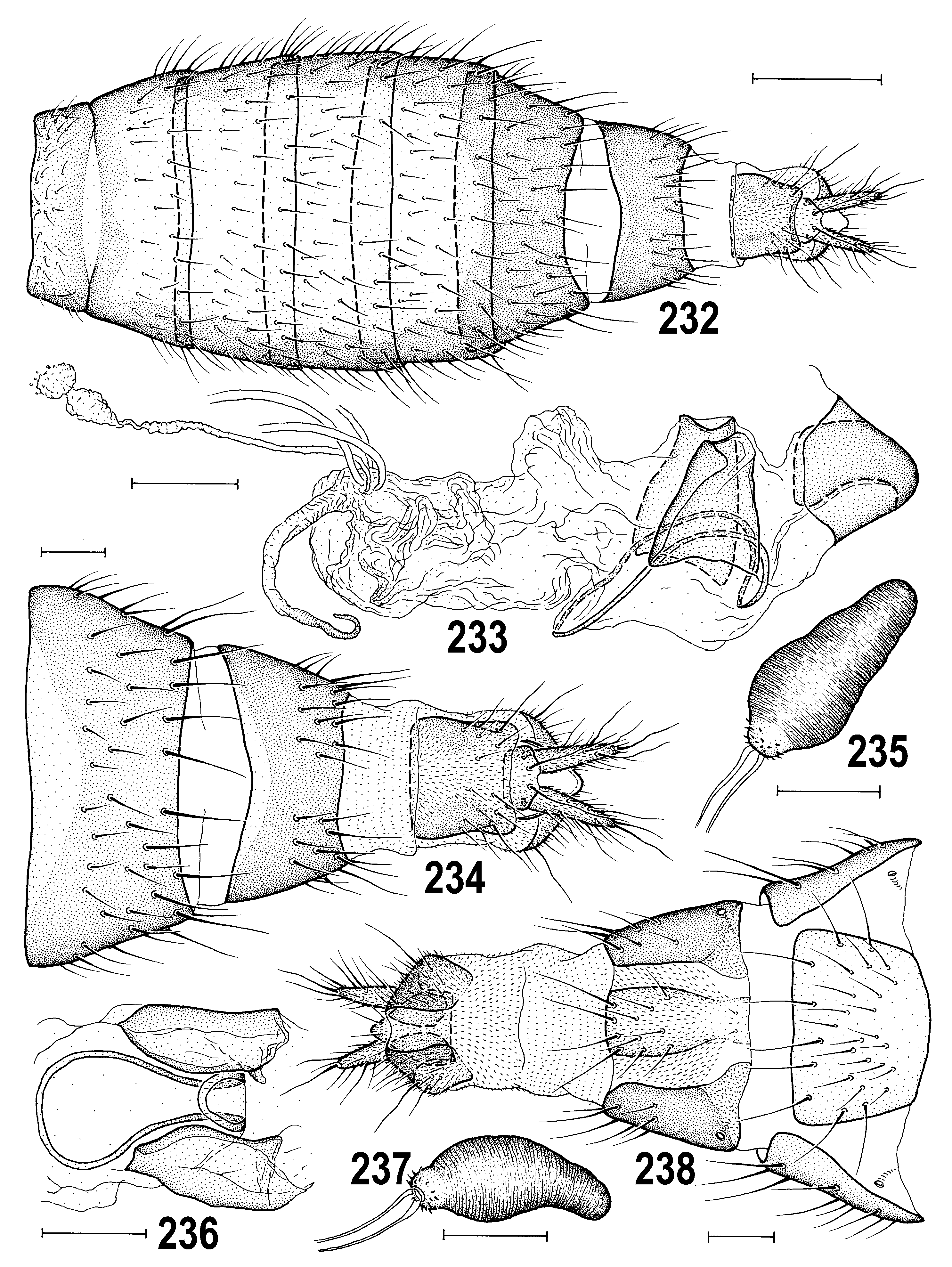
3(1) Pleura pale (yellow) usually with dorsal brown band; male surstylus distally broad, spatulate ( Figs 214 View Figs 207–214 , 231 View Figs 225–231 ); female T7 and S7 separate ( Figs 217 View Figs 215–221 , 238 View Figs 232–238 ); paired internal sclerites of female genital chamber short ( Figs 218–219 View Figs 215–221 ). ............................................ ..................................................... A. macra View in CoL group (p. 111; key to species on p. 111)
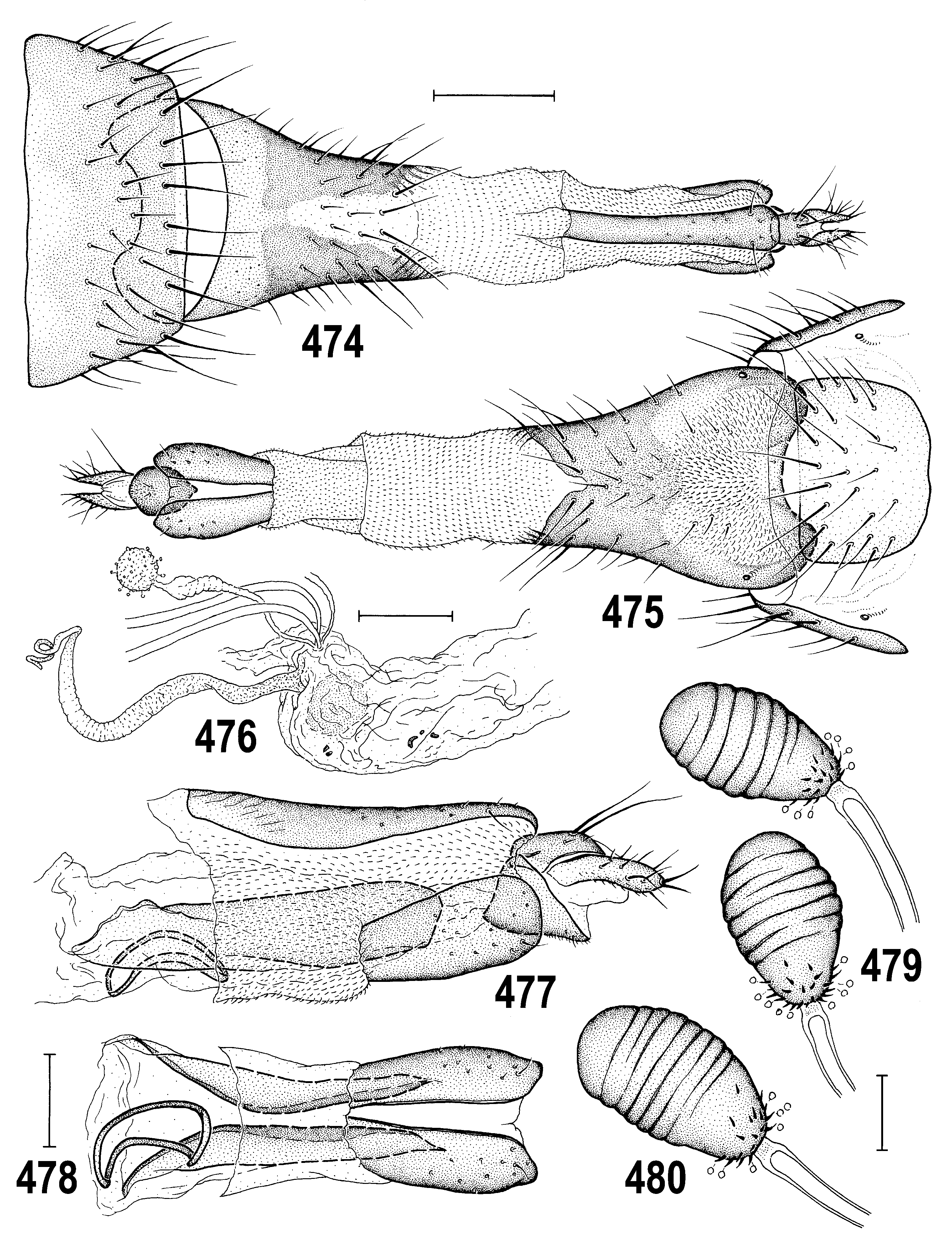
– Pleura entirely dark as in notum; male gonostylus narrowing to apex ( Figs 466 View Figs 460–466 , 535 View Figs 527–535 ); female T7 and S7 fused to form conical ring-shaped tergosternum ( Figs 474, 475 View Figs 474–480 , 536–538 View Figs 536–543 ); paired internal sclerites of female genital chamber very elongate ( Figs 477, 478 View Figs 474–480 , 571, 573 View Figs 566–573 ). ............................................................................................................ 4
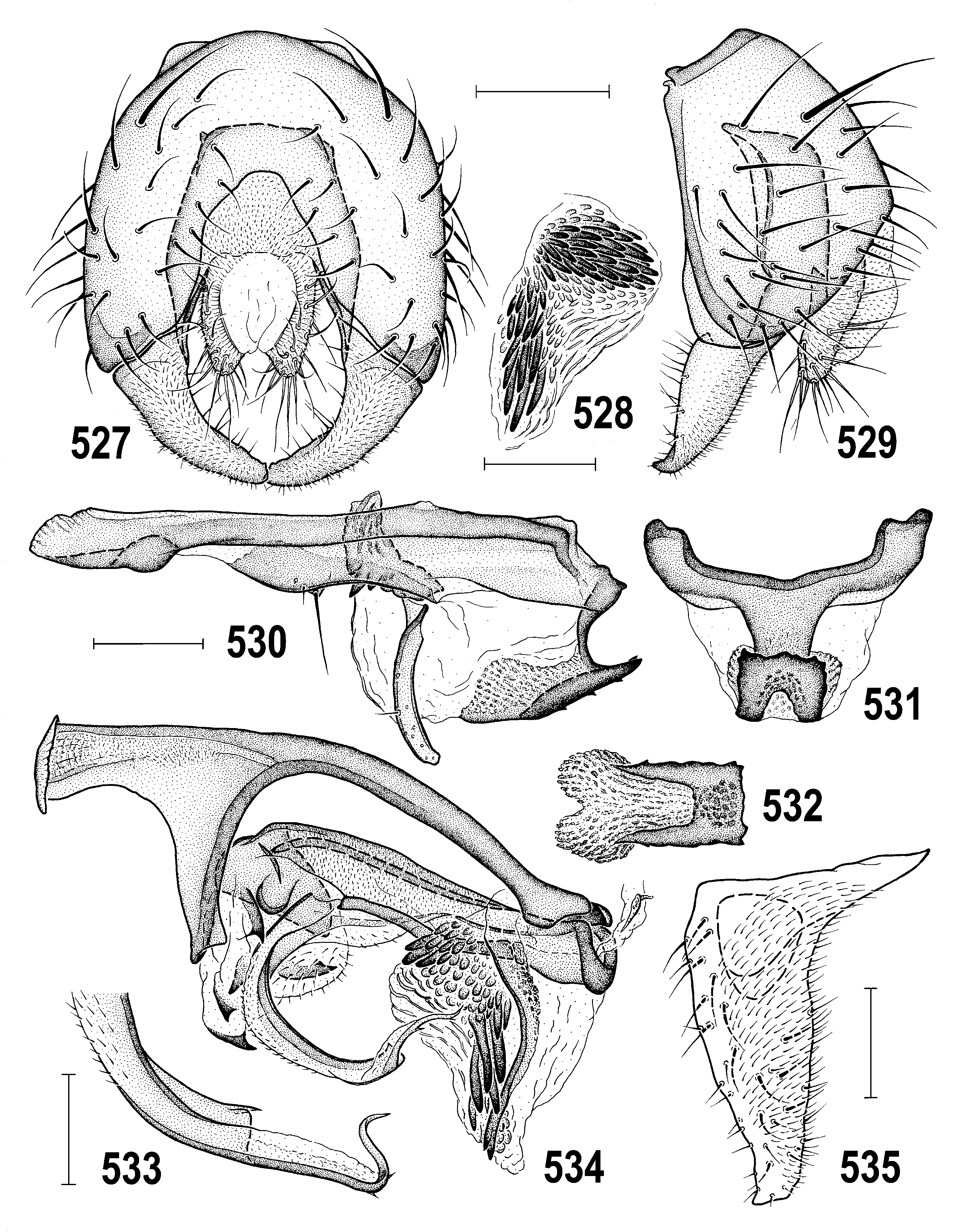
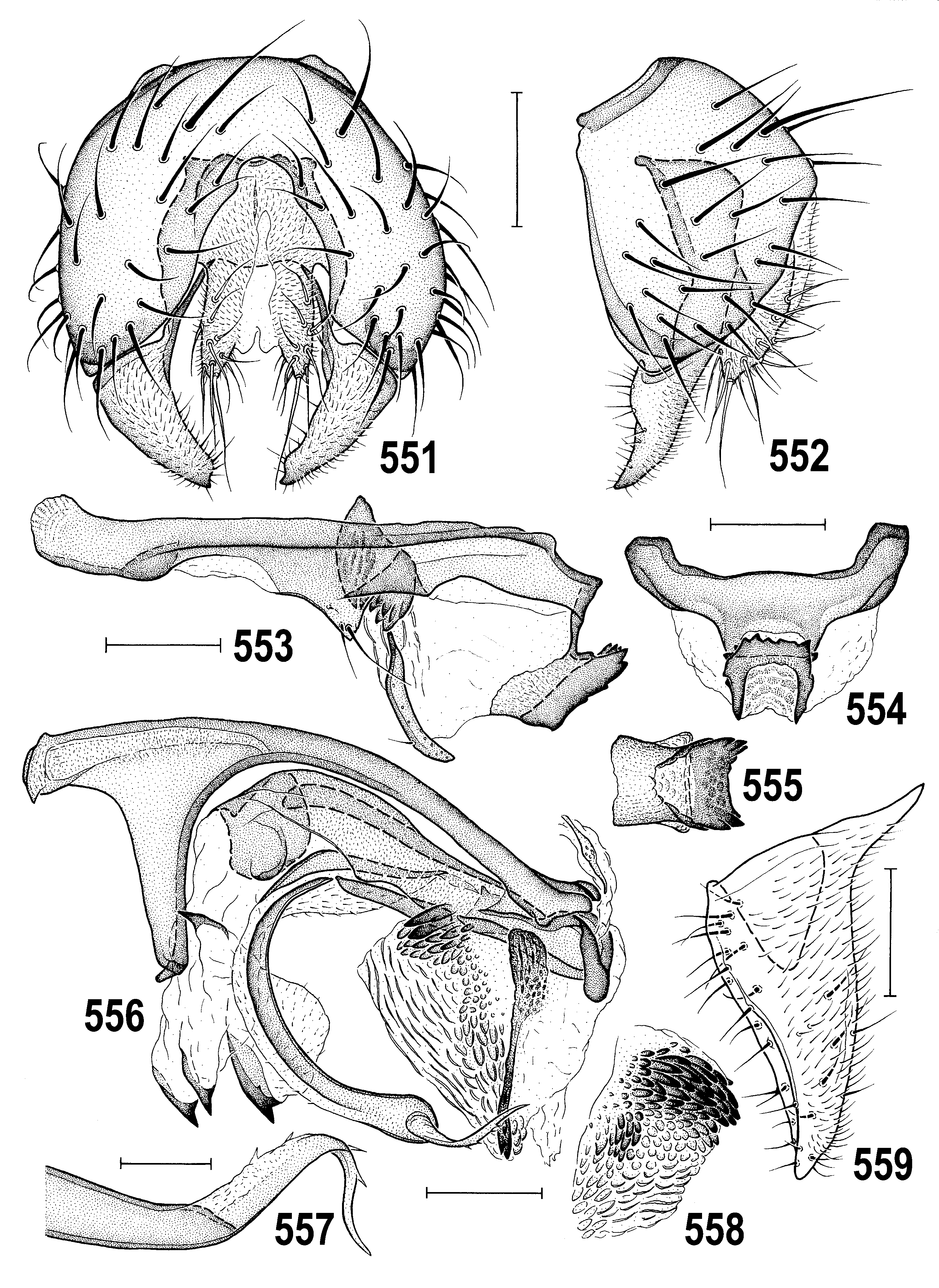
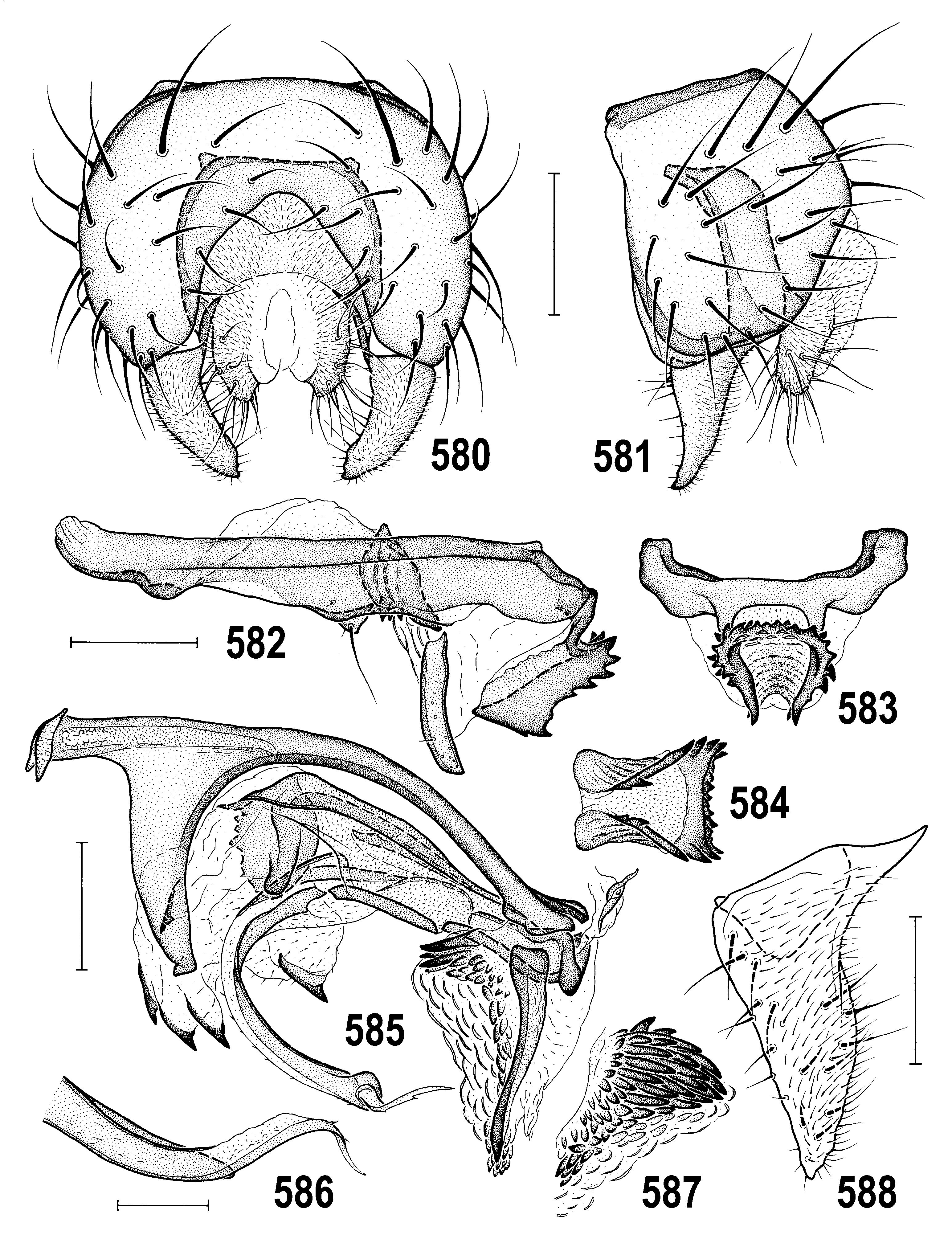
4(3) Thorax densely brownish to bluish-grey microtomentose and dull ( Figs 518, 522 View Figs 518–523 , 547 View Figs 547–550 ); epandrium small, round-quadrate in lateral pronle ( Figs 518, 520 View Figs 518–523 ); gonostylus small (cf. Fig. 552 View Figs 551–559 ) and short with more or less acute apex ( Figs 535 View Figs 527–535 , 559 View Figs 551–559 ); caudal process with peculiar dentate ventral appendage ( Figs 530–532 View Figs 527–535 , 582–584 View Figs 580–588 ); female S7+T7 not elongate or obviously nattened ( Figs 566–568 View Figs 566–573 ); female 8th abdominal segment with sclerites shorter ( Figs 536–538 View Figs 536–543 ), setose and microtomentose; spermathecae with deep invagination ( Figs 569, 572 View Figs 566–573 ). ..... A. gracilis View in CoL group (p. 313; key to species on p. 314)
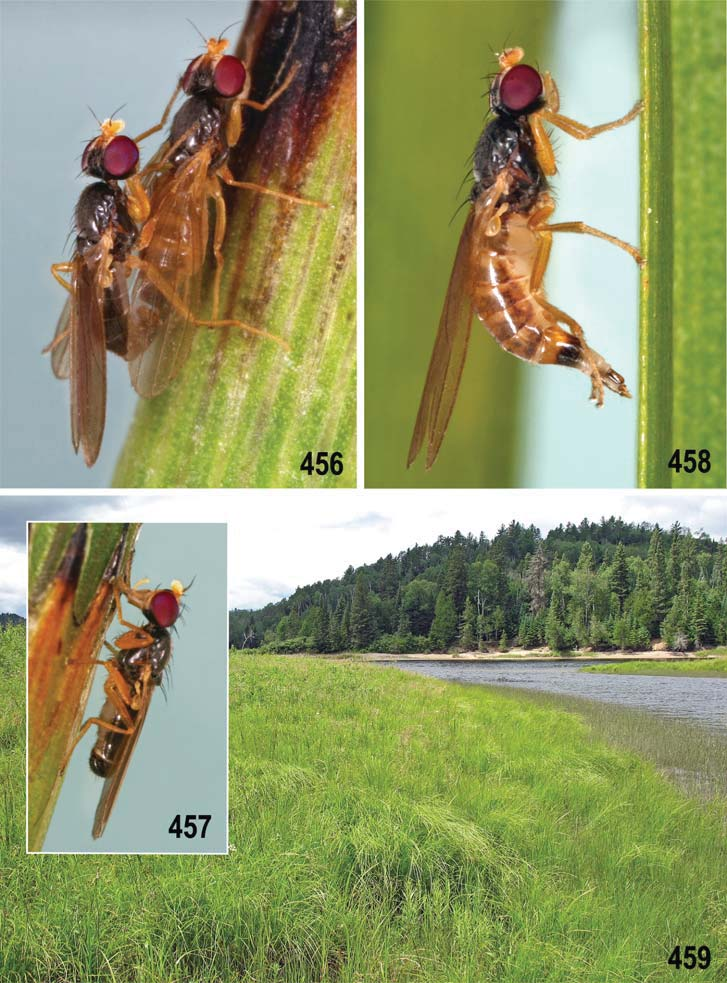
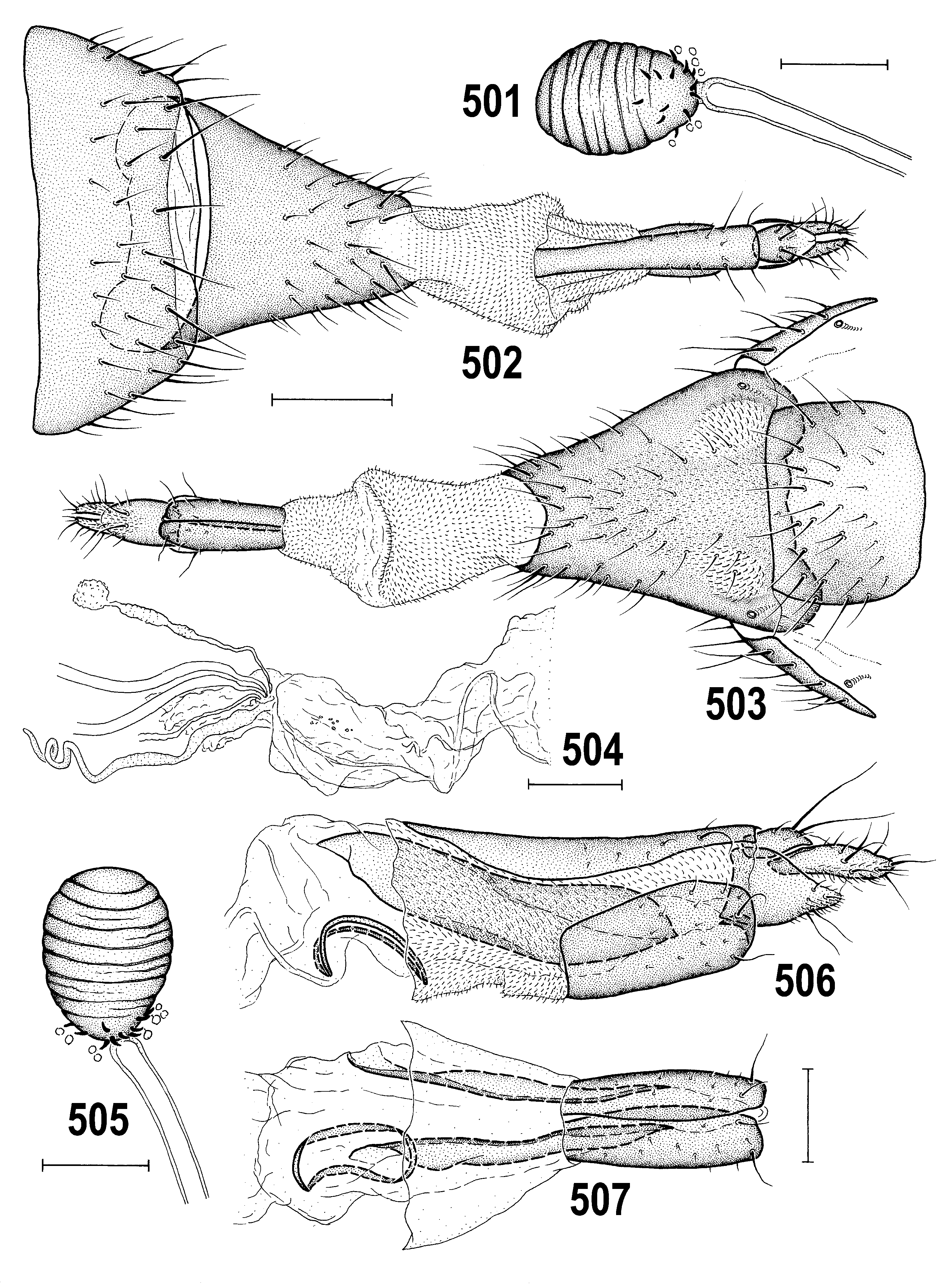
– Thorax less microtomentose, more shining ( Figs 458 View Figs 456–459 , 481 View Figs 481–484 ); epandrium large, ovoid in lateral pronle ( Figs 457 View Figs 456–459 , 483 View Figs 481–484 ); gonostylus long, slender, elongate ( Figs 508–511 View Figs 508–515 ); caudal process of transandrium simple ( Figs 463, 464 View Figs 460–466 ); female S7+T7 elongate, laterally nattened (with incomplete posteromedial split or desclerotization, Figs 474 View Figs 474–480 , 502 View Figs 501–507 ); female 8th abdominal segment with sclerites extremely elongate ( Figs 506, 507 View Figs 501–507 ), with reduced setosity and microtomentum; spermathecae without invagination ( Fig. 479 View Figs 474–480 ). ........................................... A. tschirnhausi View in CoL group (p. 277; key to species on p. 278)
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Anthomyza Fallén, 1810
| Roháćek, Jindřich & Barber, Kevin N. 2016 |
Anthomyza Fallén, 1810:20
| ROHACEK J. 2009: 24 |
| ROHACEK J. 2006: 83 |
| ROHACEK J. 1998: 172 |
| ROHACEK J. 1998: 276 |
| ROHACEK J. & FREIDBERG A. 1993: 64 |
| ANDERSSON H. 1984: 50 |
| VOCKEROTH J. R. 1977: 241 |
| COLE F. R. 1969: 435 |
| CURRAN C. H. 1965: 329 |
| SABROSKY C. W. 1965: 819 |
| TROJAN P. 1962: 37 |
| FREY R. 1958: 32 |
| STURTEVANT A. H. 1954: 557 |
| COLLIN J. E. 1944: 265 |
| CURRAN C. H. 1934: 329 |
| SEGUY E. 1934: 301 |
| CZERNY L. 1928: 2 |
| MELANDER A. L. 1913: 286 |
| COQUILLETT D. W. 1910: 507 |
| COQUILLETT D. W. 1910: 560 |
| WILLISTON S. W. 1908: 298 |
| ALDRICH J. M. 1905: 645 |
| BECKER T. 1905: 230 |
| CZERNY L. 1902: 250 |
| WILLISTON S. W. 1896: 105 |
| OSTEN SACKEN C. R. 1878: 198 |
| RONDANI C. 1875: 186 |
| SCHINER J. R. 1864: 281 |
| WESTWOOD J. O. 1840: 152 |
| ZETTERSTEDT J. W. 1837: 55 |
| MACQUART J. 1835: 580 |
| FALLEN C. F. 1823: 8 |
| FALLEN C. F. 1823: 8 |
| FALLEN C. F. 1823: 8 |
Anthomyza :
| ROHACEK J. 2009: 24 |
| ROHACEK J. 2006: 83 |
| ROHACEK J. 1998: 172 |
| ROHACEK J. 1998: 276 |
| ROHACEK J. & FREIDBERG A. 1993: 64 |
| ANDERSSON H. 1984: 50 |
| VOCKEROTH J. R. 1977: 241 |
| COLE F. R. 1969: 435 |
| CURRAN C. H. 1965: 329 |
| SABROSKY C. W. 1965: 819 |
| TROJAN P. 1962: 37 |
| FREY R. 1958: 32 |
| STURTEVANT A. H. 1954: 557 |
| COLLIN J. E. 1944: 265 |
| CURRAN C. H. 1934: 329 |
| SEGUY E. 1934: 301 |
| CZERNY L. 1928: 2 |
| MELANDER A. L. 1913: 286 |
| COQUILLETT D. W. 1910: 507 |
| WILLISTON S. W. 1908: 298 |
| ALDRICH J. M. 1905: 645 |
| BECKER T. 1905: 230 |
| CZERNY L. 1902: 250 |
| WILLISTON S. W. 1896: 105 |
Anthophilina :
| OSTEN SACKEN C. R. 1878: 198 |
| RONDANI C. 1875: 186 |
Leptomyza :
| SCHINER J. R. 1864: 281 |
Anthophilina
| ZETTERSTEDT J. W. 1837: 55 |
Leptomyza
| MACQUART J. 1835: 580 |