Asphondylia punica Marchal, 1897
|
publication ID |
https://doi.org/10.11646/zootaxa.3869.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:3C5EC936-DD36-4CE7-9B4C-452FA7BBE519 |
|
DOI |
https://doi.org/10.5281/zenodo.5119941 |
|
persistent identifier |
https://treatment.plazi.org/id/03F71F32-FFF3-E83B-FF7E-F285FD3DFAC5 |
|
treatment provided by |
Felipe |
|
scientific name |
Asphondylia punica Marchal |
| status |
|
Asphondylia punica Marchal View in CoL
Asphondylia punica Marchal, 1897: 20 View in CoL
Asphondylia conglomerata De Stefani 1900: 16 View in CoL — new synonym
Gall and biology: Asphondylia punica View in CoL induces one of the most common and conspicuous galls in Mediterranean desert and salt-marsh habitats. The galls develop in apical and axillary buds of Atriplex halimus View in CoL and are composed of numerous stunted leaves that form dense, spherical rosettes of up to 3cm in diameter ( Fig 2 View FIGURES 1–4 ) that are clumped together along shoots. Each rosette contains up to 20 larval chambers embedded in spongy tissue and each chamber contains one larva. The internal walls of the larval chambers are lined by a thick layer of white mycelium ( Fig. 3 View FIGURES 1–4 ). The fresh galls are green but after emergence of the gall midges they become yellowish and may remain on the plant until the next season. In such dry galls the internal complex of larval chambers becomes very rigid and is sometimes left on the shoot after the rosette leaves are shed. The galls are so common that on some bushes they may cover most of the branches and stunt their growth. Their size depends on the number of chambers in them and apparently on the condition of the individual plant. The galls appear on the plant around November–December and reach their final size in February. Development of the second and third larval instars is rapid, and adults emerge during February–March in Israel, and apparently also elsewhere (based on the literature). The galls support a rich community of Hymenoptera parasitoids of several families ( De Stefani 1900) but, despite the high mortality rates, it is easy to rear the gall midges in comparison to other Asphondylia species on Atriplex View in CoL , because the galls are so numerous and widespread. Rübsaamen, who examined galls collected in Israel in 1897, found pupae of another cecidomyiid species in the galls, which he suspected of being an inquiline ( Rübsaamen 1902). However, in years of collecting and rearing these galls in Israel, we never reared any species other than the gall inducer.
Very similar galls to those on A. halimus are found on A. leucoclada in Israel but are much smaller—up to 0.6 cm —and contain only 2–4 larval chambers per gall. We were able to rear only three adult gall-midges from galls on this host plant and only in October. At other times of the year galls were dry or contained tiny first instars. The differences in gall morphology and emergence times are not reflected in the gall-midge morphology, and therefore we refrain from describing the population from A. leucoclada as a separate species until further data become available. Atriplex leucoclada and A. halimus have overlapping distributions in salty Saharo-Arabian and Mediterranean habitats and are phylogenetically related ( Kadereit et al. 2010), hence it is plausible that Asphondylia punica uses both plants as hosts.
A species belonging to the genus Stefaniella (possibly S. atriplicis Kieffer ) galls stems and leaf mid-veins of A. leucoclada , including the leaves that compose the rosette galls of A. punica on this host. Thus, it may appear that adult Stefaniella emerge from the rosette galls, but on close examination one can find their inconspicuous galls in leaves surrounding the central Asphondylia chambers.
Adult: ( Fig. 4 View FIGURES 1–4 ) General color greyish brown. Head ( Fig. 8 View FIGURES 8–12 ): Eye facets round where not closely adjacent to hexagonal where closely juxtaposed. Palpus 3-segmented, with several strong setae and otherwise covered by microtrichia; first segment only slightly longer than wide, third segment 1.17–2.10 times as long as second. Labella rounded apically, setose and setulose. Antenna: Scape cylindrical, pedicel spherical. Male flagellomeres cylindrical, all covered by anastomosing loops of circumfila, numerous strong setae and microtrichia; first flagellomere 1.1–1.3 times as long as second. Female flagellomeres 1–9 cylindrical, with only two whorls of circumfila and two transverse connections, numerous strong setae and otherwise covered by microtrichia; first flagellomere 1.3–1.6 as long as second; flagellomeres 7–12 successively shorter; flagellomere 10 only slightly longer than wide; flagellomere 11 as long as wide; flagellomere 12 spherical, wider than long.
Thorax: Legs: brownish-orange, covered by dark scales and hair; ventral part with silvery hair-like scales. Tarsal claws thick, evenly curved, untoothed; empodia as long as or longer than bend in claw, pulvilli minute ( Figs. 9–10 View FIGURES 8–12 ). Wing: hyaline, veins brownish-orange, with sparse microtrichia; length 2.56–3.14 mm in females (n=16), 2.34–2.99 mm in males (n=16); R 1 joins C at about mid-length of wing, R 5 joins C behind wing apex, M weak, CuA forked into CuA1 and CuA2.
Female abdomen ( Fig. 11 View FIGURES 8–12 ): Brownish-orange, pleuron and venter with silvery hair-like scales. Tergites 1–7 with posterior 1–2 rows of strong setae; tergite 8 much shorter than preceding, without setae. Sternites 2–6, with posterior row of setae and several setae on mid part; sternite 7 much longer than preceding, strongly setose on posterior half. Ovipositor relatively short: sclerotized part 1.20–1.66 as long as sternite 7 (n=14).
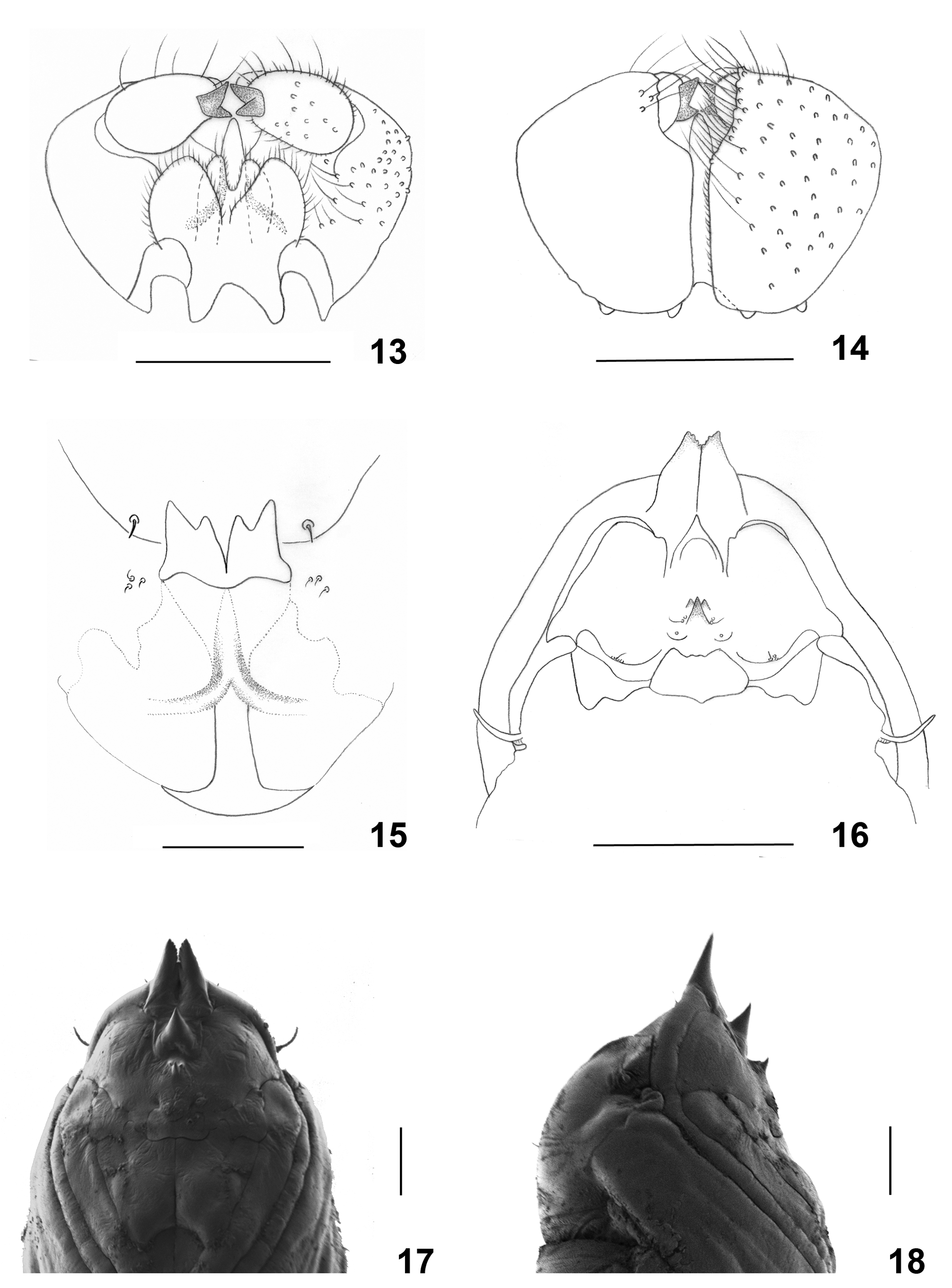
Male abdomen ( Fig. 12 View FIGURES 8–12 ): Color pattern as in female. Tergite 1 narrower than succeeding tergites without setae; tergites 2–7 rectangular, with posterior 1–2 rows of strong setae and evenly scattered scales; tergite 7 more setose on posterior half than preceding; tergite 8 much smaller than preceding, band-like, without setae. Sternites 2–6 rectangular, with 1–2 posterior rows of strong setae and several strong setae medially, otherwise evenly covered by scales. Sternite 7 more setose than preceding. Sternite 8 with small but strongly setose sclerotized area. Terminalia ( Figs. 13–14 View FIGURES 13–18 ): Gonocoxite compact, wide and short, bearing numerous strong setae particularly along medial margin and evenly setulose. Gonocoxal apodeme extending on both sides of aedeagus base forming elongate sclerotized structures. Gonostylus ovoid, with numerous strong setae and otherwise evenly setulose, bearing bidentate apical tooth. Aedeagus cylindrical, tapered towards rounded apex. Hypoproct deeply divided apically into two lobes, setose and setulose, with two longer apical setae on each lobe. Cerci completely or almost completely separated, bulbous, strongly setose and setulose throughout.
Larva (third instar) ( Figs. 3 View FIGURES 1–4 , 15 View FIGURES 13–18 ): 1.9–2.5 mm long (n=3). Light to bright orange. Integument with pointed, shallow bumps. Antennae three times as long as wide; cephalic apodeme longer than head capsule. Spatula quadridentate, strongly scleortized; lateral teeth longer than median teeth, gap between median teeth much deeper than gaps between lateral and median teeth. Shaft long and wide at base, with two strongly sclerotized arms at midlength and weaker sclerotization throughout area posterior to teeth and around shaft. Three setose lateral papillae on each side of spatula. Pleural papillae with long setae.
Pupa ( Fig. 16 View FIGURES 13–18 ): 2.8–4.0 mm long (n = 13). Antennal horns slightly arched, apices tapered and serrated in frontal view. Cephalic seta minute. Upper facial horn large and tapered. Three lower facial horns arranged in a triangle, middle one much larger than two lateral, slightly curved anteriorly; lateral horns situated anteriorly from middle horn, pointed ventrally; on each side of middle horn two papillae, one bearing long seta. One asetose papilla on each side slightly posterior to three facial horns. Posterior part of frons on each side with three lateral papillae, one setose, two asetose. Prothoracic spiracle long and slender, with trachea ending close to base. Abdominal segments except for first, each with one posterior straight row and one anterior less ordered row of spikes.
Material examined. The types of A. punica Marchal from Tunisia could not be found in the Muséum National d'Histoire Naturelle, Paris, where they were supposedly kept, and those of A. conglomerata De Stefani from Sicily are considered lost ( Gagné & Jaschhof 2014), hence we could not compare those types to our specimens. Nevertheless, the original descriptions and illustrations of the galls leave little doubt that the species we reared in Israel is indeed A. punica .
From Atriplex halimus : 3 exuviae, Israel, Park Yeroham, 16.iii.1995, N. Dorchin ; 4♀, 3♂, 2 exuviae, Israel, Nahal Nizzana , 17.iii.1995, N. Dorchin ; 2♀, 1♂, Israel, Qalya , 19.ii.1996, N. Dorchin ; 6♀, 5♂, Israel, Sede Boqer , 13.iii.2002, N. Dorchin ; 3♀, 4♂, Israel, Sede Boqer , 30.iii.2004, N. Dorchin ; 2♂, Israel, Enot Zuqim , 10.iii.2013, N. Dorchin; from Atriplex leucoclada : 1♂, 2 exuviae, Israel, Be’er Sheva , Zomet Eshel, 1.x.2001, A. Freidberg ; 2♀, 1 exuviae, 1pupa, Israel, Beer Sheva , Zomet Eshel, 17.x.2013, N. Dorchin .
Distribution. Circum-Mediterranean: Algeria, Tunisia, Libya, Egypt, Israel, Syria, Greece, Italy, Spain.
Comments. Asphondylia punica was described by Marchal (1897) from a single female that was reared in late March from galls collected in Tunisia. The description was accompanied by a good drawing of the galls. Three years later, De Stefani (1900) described Asphondylia conglomerata from Sicily from the same host, and attributed to it three types of galls: large rosettes on non-flowering twigs, small rosettes on flowering twigs, and round, bare galls in leaf axils. The detailed drawings of the rosette galls in that publication closely resemble those provided by Marchal, and the only morphological distinction De Stefani makes between the two species is that adults of A. conglomerata are generally red whereas those of A. punica are brown. Houard (1908, 1922), in his illustrated keys to galls of Africa, Europe, and the Mediterranean Region, separated A. conglomerata from A. punica based on the number of chambers in the galls and whether they are induced on flowering or non-flowering stems. His keys are accompanied by an excellent drawing of A. punica galls, showing multiple larval chambers in cross section. Yet another good description of A. conglomerata galls was given by Rübsaamen (1902), who examined galls that were collected in Israel, near Jericho. Rübsaamen compared these galls with those from De Stefani’s collection, but he does not say why he decided that the Israeli galls and the exuviae found in them belong to A. conglomerata rather than to A. punica . Color differences between adults and the diversity in gall size are hardly reliable characters to justify separation between gall-midge species. We therefore make A. conglomerata a junior synonym of A. punica .
In Israel, there is great diversity in the size and number of chambers of rosette galls on Atriplex halimus , which probably reflects the condition of the plants on which they develop. This can account for the size differences mentioned by De Stefani between rosette galls on flowering and non-flowering shoots. The third type of gall attributed by De Stefani (1900) to A. conglomerata is a small, spherical and glabrous gall in leaf axils. This is almost certainly a misidentification of the galls induced by Stefaniella atriplicis , which develops in stem and leaf mid-vein galls of various sizes on the same host plant. Rübsaamen (1902) examined such galls from Israel but they were already empty when he received them.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Asphondylia punica Marchal
| Dorchin, Netta, Mifsud, David & Askew, Richard 2014 |
Asphondylia conglomerata
| De Stefani, T. 1900: 16 |
Asphondylia punica
| Marchal, P. 1897: 20 |