Syncerus caffer (Sparrman, 1779)
|
publication ID |
https://doi.org/10.5281/zenodo.6512484 |
|
DOI |
https://doi.org/10.5281/zenodo.6636729 |
|
persistent identifier |
https://treatment.plazi.org/id/03F50713-9945-FFFF-0375-F62EF68AF855 |
|
treatment provided by |
Conny |
|
scientific name |
Syncerus caffer |
| status |
|
Cape Buffalo
French: Buffle d'Afrique / German: Kaffernbuffel / Spanish: Bufalo cafre
Other common names: East African Buffalo
Taxonomy. Bos caffer Sparrman, 1779 ,
Sunday River, Algoa Bay, South Africa.
In the late 1800s and early 1900s, the various forms of the African buffalo were classified initially as distinct species, often under Bos , based largely on skull and horn characteristics, but that was followed quickly by their unification under a single species with numerous named subspecies (e.g. 21 subspecies under Bos [Bubalus] caffer by Lydekker in 1913). The latter approach persisted through the 20™ century, but with a steady reduction in the number of subspecific names, to the point where 4-5 subspecies were recognized most commonly ( aequinoctialis , caffer , brachyceros , mathewsi , and nanus ). However, the general impression that African buffaloes form a single species from the large black buffalo in the savanna grading into the small red ones in the central rainforests is misleading. The taxa in this group are probably best described as a ringed-shaped morphocline from the central African rainforest (considered ancestral) via West Africa and the Sudan to the eastern and southern African savanna. Because of recent disparate craniometrical (skull and horn characteristics) and genetic analyses, caffer , brachyceros , mathewsi, and nanus have been elevated to specific standing here. Intermediate forms occur in areas of range convergence in, for example, East Africa, particularly in the Western Rift west of Lake Victoria (southern Uganda). Now considered monotypic.
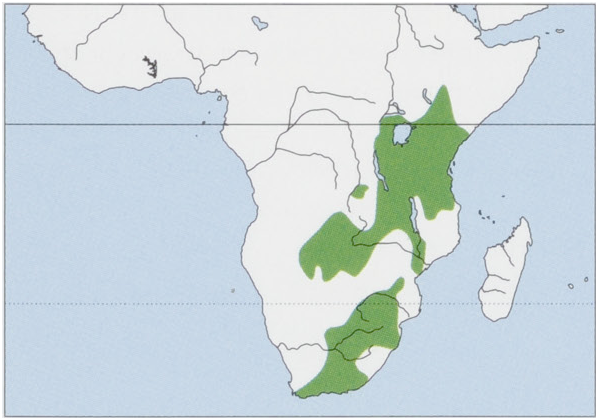
Distribution. S Ethiopia, extreme S Somalia, most Kenya (except extremes NW & NE), S Uganda (Lake Mburo), Rwanda, Burundi, extreme E & SE DR Congo (Rutshuru Plains and Katanga), Tanzania, Malawi, Zambia, N Botswana, NW and extreme S Zimbabwe, NE Namibia, N, C & extreme SW Mozambique, Swaziland, NW Lesotho, and South Africa. View Figure
Descriptive notes. Head-body 240-340 cm, tail 50-110 cm, shoulder height 148-175 cm; weight 500-900 kg (males) and 350-620 kg (females). These measurements are general for the non-forest African buffalo species and should be considered provisional until further information is available for individual species. The Cape Buffalo, particularly the male,is the largest of the African buffalo species and comparable to the largest of the Asian wild oxen. They are stocky in build, with relatively short legs. Both sexes have smooth,jet black coats; the pelage of old males may become scant, exposing areas of dark skin, and grizzled hairs may be present on their heads. Neonates may be black or dark brown and go through various changes in color (e.g. dirty yellowish-brown to chocolate brown) before becoming the typical adult black. Both sexes have dark horns that are widely spread laterally. Each horn, larger in males, curves strongly downward below the base of the skull and then up, so that the length of each horn by itself nearly equals the span. Horn span is 72:5-134 cm, and horn length along the curve is 66-116 cm. In males, the bases of the horns are greatly expanded and nearly meet at the midline of forehead, where each base forms a convex boss. The skull is massive but short and broad, and convex in profile. The greatest length of the skull is 44.8-57. 5 cm, and the mastoid breadth is 24-1-31: 6 cm. The mouth is wide, and the nose is moist and bare. The ears are large, droopy, and meagerly fringed, and the tail is tufted. No scent glands have been described. Dental formulais 10/3, C0/1, P3/3,M 3/3 (x2) = 32. The eruption pattern of the permanent teeth makesit possible to determine the age of young Cape Buffaloes up to age six, when all the permanent teeth are in. The permanent molars all erupt by about 3-5 years of age. The lower canine teeth are the last and most variable, erupting between 4-5 and 5-5 years of age. One cementum annulus develops each year, making their counts on molars useful for aging, and wear patterns of molar crowns correlate well with ages determined from annuli counts up to 15-16 years of age.
Habitat. Cape Buffaloes occur in a variety of habitats and elevations throughout eastern Africa from southern Ethiopia to South Africa. They are most abundant in wellwatered savannas, swamplands, and floodplains, but they are capable of inhabiting drier savannas and riparian areas of arid landscapes if permanent water is available. Cape Buffaloes occur in montane forests above 3000 m on Mount Kenya. Optimal habitat for the Cape Buffalo, as well as the Lake Chad Buffalo (S. brachyceros ), includes open grazing areas in a mosaic of dense cover afforded by thickets or reed beds, but more open savanna woodlands are frequently occupied.
Food and Feeding. All non-forest African buffaloes are predominantly grass eaters, regularly consuming genera such as Cynodon, Sporobolus, Heteropogon, Digitaria, and Pancium. Given the size of Cape Buffaloes, they are often foodand protein-limited, leading to malnutrition at high population densities and during periods of drought. Adults require about 14 kg of forage/day. At Lake Manyara National Park, Tanzania, Cape Buffaloes adopt a bulk feeding strategy when forage is abundant, whether it is of high or low quality; they are more selective when forage is scarce and of poor quality, even though they cannot crop plants as closely or as selectively as smaller sympatric ungulates, given their large mouths and wide incisor row. In the Serengeti National Park, Tanzania, grass leaves were selected over other plant parts, but as the dry season progressed and grasses senesced, such selective foraging was more difficult; the buffaloes then moved toward riverine habitats where forage was less desiccated. In some unique habitats such as the “succulent thicket” of the Eastern Cape, South Africa, Cape Buffaloes consume a greater variety of woody species because their normally preferred grasses and herbaceous species are underrepresented in the sparse understory; during the dry season, the diet may contain 33% browse species such as Acacia karroo, Plumbago auriculata, Grewia robusta , and Ptaeroxylon obliqguum. Cape Buffaloes in the bamboo forest zone on Mount Kenya at elevations of 2800-3050 m use their horns to expose soil, which they consume, a behavior thought to be related to their enhanced need for iron living at the high elevations. Elsewhere, Cape Buffaloes are fond oflicking salty surfaces, including sweating conspecifics. Availability of permanent water is a critical habitat component because Cape Buffaloes need to drink daily. In dry areas, they are usually never farther than 8-20 km from permanent water.
Breeding. Breeding and birthing of the Cape Buffalo may occur at any time of the year, but in areas with pronounced dry and rainy seasons, and the resulting variability in forage quality and quantity, they tend to peak seasonally. In the Serengeti National Park, Tanzania, most breeding behavior was observed from November through early July, when it peaked. In the Limpopo Province of South Africa, monthly births were associated with rainfall and forage conditions 12-13 months prior to births, suggesting that the condition of the female determines her optimum time to breed. Many male Cape Buffaloes of all ages stay in the highly gregarious herds throughout the year, but some may associate in small bachelor groups and senescent males are often alone. Males establish a dominance hierarchy relative to age and size that confers breeding rights, and they do not reach their full breeding potential until 8-9 years of age. To establish dominance, mature males interact regularly with a variety of displays and threats, seemingly designed to minimize serious combat. The head-on threat display is most common when a dominant male encounters immature males; he stands still with his head and shoulders up but his nose pointing to the ground, presenting his horns directly at an opponent. He also may thrash his head up and down with hooking motions of the horns. The same head position is used in lateral displays that emphasize the size of the entire body; this behavior is most common when mature males of equal rank encounter one another. Such displays play a significant role in establishing dominance because head-on combat between mature male Cape Buffaloes is relatively rare. When it does occur, males charge each other, often from distances of 30 m or more, with their head stretched out, making a deep growling call. Right before impact, they tuck and turn their heads slightly and take the full impact on the bosses between their horns. This constitutes the complete fight. Speed, weight, and the strength of impact immediately determine the winner, and the loser turns and runs, frequently chased for up to 100 m by the winner. Unlike mature males, immature males frequently spar with each other. Both sexes will wallow in muddy areas, but given the limited availability of such wallows in most areas, this behavior appears to have a social function,likely related to rank among mature males who dominate the wallows’ use. Males frequently urinate in wallows. During the extended breeding season in KwaZulu-Natal, South Africa, mature males alternate their time between seeking receptive females among mixed herds and resting in small all-male groups, likely a reflection of the high cost of breeding activities. Like many bovids, males constantly check the readiness of females to breed by examining their genitals, testing their urine, and performing a lip curl. Breeding males tend estrous females, which draws the attention of other mature males, who may displace the tending male. A tending male frequently rests his chin on the female’s rump; she will signal her readiness to breed by standing still and moving her tail to the side. Copulation is brief, and it may occur multiple times between the same pair over 30 minutes. Mock-mounting is common among females and subadult males. Females typically do not have their first offspring until 4-5 years of age, rather late among the Bovini . Gestation is as long as 11-5 months, and birth intervals are 15-24 months, depending on maternal and forage conditions. Females give birth to a single offspring, commonly among the herd. Neonates typically weigh 45 kg but range up to 55-60 kg. Offspring always nurse from between their mothers’ rear legs irregularly for 3-10 minutes/bout. They may nurse, or try to nurse, until the birth of the next offspring, but females tend to stop lactating during their seventh month of pregnancy when their previous offspring are ten months old. Fetal sex ratios in the Cape Buffalo were not related to maternal age, condition, rainfall, or density in Kruger National Park, South Africa. Recent evidence suggests that fetal sex ratios can be influenced by sex-ratio distorter genes in males that function during spermatogenesis and even post-copulation in the females’ genital tract and differ in their effect depending on rainfall patterns; i.e. fetal sex ratios will be male-biased during wet periods when food resources are going to be most abundant. A newborn Cape Buffalo needs several hours to become strong enough to stand and follow its mother, and it remains slow and clumsy for several weeks, during which the pair may become somewhat separated from their herd. During this period, the mothers are particularly protective of their offspring. Cape Buffaloes are notorious for vigorously protecting herd members, particularly offspring, from common threats, so predation of young is relatively low. Cases of herds of buffaloes holding Lions (Panthera leo) at bay in trees, and overrunning and stomping them to death have been reported. Predation of males, particularly old non-breeding males that live alone, is common. In a study in the Serengeti, Lions killed 44 males compared to six females, six young, and six of unknown sex. Hyenas typically do not pose much of a threat to African buffaloes. Maximum longevity in the wild is 18-20 years, sometimes more, and at least four known individuals lived almost 30 years in captivity.
Activity patterns. Cape Buffaloes follow the same general activity patterns as other ruminants, with feeding periods followed by resting/ruminating periods throughout the day and night, but their lengths vary depending on location, forage availability, competition with other ungulates, and secondarily, predation pressure. In Lake Manyara National Park, Tanzania, Cape Buffaloes generally grazed from about 10:00 h to 14:00 h, but that period varied from 1-5 hours of intense feeding in July to 4-5 hours in April. Resting/ruminating periods occur before and after feeding. In South Africa, 74% of the 24 hour cycle was spent feeding, resting/ruminating, and moving; 48% of the time was spent actively grazing. Nocturnal activity was highest during the dry season. Throughout the day during the breeding season at Hluhluwe-Umfolozi Game Reserve, South Africa, adult female Cape Buffaloes spent 21-28% oftheir time feeding and 66% of their time resting/ruminating. In contrast, mature males in mixed groups spent 14-18% of their time grazing and 74% of their time resting; when in all-male groups, they grazed more (26-29%). Breeding activities in mixed groups accounted for a small part of their activity budget overall (2:5-3%). Throughout the year in the Lower Sabie region of Kruger National Park, South Africa, Cape Buffaloes fed more at night (44-5%) than during the day (32%) and, concomitantly, rested less at night (16%) than during the day (28-4%). Where heavily poached, such as Mount Kilimanjaro, Cape Buffaloes become largely nocturnal and very wary.
Movements, Home range and Social organization. Cape Buffaloes are non-migratory, and groups move in regular and repeated patterns throughout their relatively exclusive, but often extensive, traditional home ranges, which vary greatly in size from 126 km * to more than 1000 km ®. These regular movements over the same ground throughout a traditional range trample and turn up the soil, which encourages regrowth of vegetation and repeated foraging in the same areas. Movements typically involve travel to water, and daily movements of groups are greatest where permanent wateris widely dispersed. In the Lower Sabie region of Kruger National Park, water is readily available, and Cape Buffaloes only moved an average of 3-35 km/day. Groups are made up of clans of related females and their offspring, accompanied by males of various ages. Males often occur in small bachelor groups of 5-10, or alone; home ranges of male groups can be remarkably small, e.g. 3-4 km?®. Sizes of traditional ranges and densities of Cape Buffaloes within them are directly related to rainfall and its effect on forage and water availability; herd home ranges are larger and densities lower in drier areas. Densities vary widely depending on habitat conditions and protection from excessive poaching; they are typically 0-6-3 ind/km?, but as high as 15 ind/km? in Lake Manyara National Park and 10-8 ind/km? in Ngorongoro Crater, Tanzania. All African buffaloes are highly gregarious. Cape Buffalo herds number in the dozens to thousands, depending on habitat type, time of year, and forage and water availability; very large groups tend to be temporary aggregations. A group occupying a traditional range in northern Botswana was thought to be stable, but seven of 45 radio-collared females switched groups during the wet season, when herds moved extensively and often splintered into smaller groups; in one case, a female moved 133 km during the switch, and no females rejoined their original group during the period of study. Recent assessments of fission-fusion events among known groups of Cape Buffaloes in the Satara region of Kruger National Park show, for example, that regular associations of pairs of adult females and juveniles for a period of time are not good predictors that they will remain together when groups split or aggregate. When Cape Buffaloes relocate, rather than feed, groups assume characteristic column formations with pathfinders and dominant females in the lead, subgroups of females and young clustered in the center, infirm individuals behind them, and bachelor males surrounding the entire group. The animals’ vision is poor, but hearing is keen, and Cape Buffaloes emit low-pitched moos as they move and feed. Group structure and regular communication protect infirm (e.g. blind) individuals, often permitting their survival for extended periods.
Status and Conservation. Classified as Least Concern on The IUCN Red List (included under S. caffer , which includes all four African buffalo species identified here). Range-wide, numbers of African buffaloes have been greatly reduced from historic levels because of land-use changes, competition with livestock and associated disease transmission (notably livestock-transmitted anthrax, rinderpest, and bovine tuberculosis), poaching, and extended drought, and these are still common threats in many areas. In the late 1990s, numbers of non-forest African buffaloes were estimated at 500,000-1,000,000, with 70% of them occurring in and around protected areas, but free-ranging populations of the Cape Buffalo in southern Africa reportedly decreased by about 50% from 1991 to 1996. Currently, stable to increasing populations of the Cape Buffalo occur in many protected areas, particularly in Tanzania, Zambia, northern Botswana, Zimbabwe, and South Africa. Populations in Mozambique are less stable. The buffaloes have been successfully reintroduced in Swaziland. Efforts to domesticate Cape Buffaloes have been largely unsuccessful, but the species is managed as a free-ranging ranch animal on some private lands in southern Africa. Well-managed hunting and safari zones have the potential to bring needed revenue to enhance conservation of the Cape Buffalo and other wildlife species.
Bibliography. Beekman & Prins (1989), Blancou (1935), Brown et al. (1991), Caron et al. (2003), Cribiu & Popescu (1980), Cross et al. (2005), East (1999), Estes (1991a), Grimsdell (1973), Grobler & Van Der Bank (1996), Groves & Grubb (2011), Grubb (1972, 2000), Halley et al. (2002), van Hooft et al. (2010), Huffman (2010d), IUCN/SSC Antelope Specialist Group (2008bb), Kingdon (1982), Lydekker (1913), Mahaney (1987), Mloszewski (1983), Nowak (1999), Prins (1996), Roberts (1996), Ryan (2006), Ryan & Jordaan (2005), Ryan et al. (2007), Schaller (1972), Sinclair (1977), Sinclair & Gwynne (1972), Skinner & Chimimba (2005), Taylor (1988), Tshabalala et al. (2009), Turner et al. (2005), Van Hooft et al. (2000, 2002), Vilder et al. (1963), Visscher et al. (2004), Weigl (2005), Whyte (1996), Winterbach (1998), Winterbach & Bothma (1998).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.