Artibeus Glaucus THOMAS, 1893
|
publication ID |
https://doi.org/ 10.1093/mspecisev011 |
|
publication LSID |
lsid:zoobank.org:pub:97CDE6EE-CC9B-47E6-BD23-79AE322DD406 |
|
persistent identifier |
https://treatment.plazi.org/id/03F2AA3D-FFC2-2036-7EB5-FB532AB0FB86 |
|
treatment provided by |
Carolina |
|
scientific name |
Artibeus Glaucus THOMAS, 1893 |
| status |
|
Artibeus Glaucus THOMAS, 1893 View in CoL
Silvery Fruit-eating Bat
Artibeus glaucus Thomas, 1893:336 View in CoL . Type locality “Chanchamayo,” Junín, Perú.
[ Artibeus (Artibeus) View in CoL ] glaucus: Trouessart, 1897:160 View in CoL . Name combination.
Artibeus cinereus bogotensis: Andersen, 1906:421 View in CoL . Type locality “Curiche, [Cundinamarca] near Bogota, Colombia.” ( See Nomenclatural Notes ).
Artibeus pumilio Thomas, 1924:531 . Type locality “Tushemo, near Masisea, R. Ucayali [ Perú] Alt. 1000´.”
Artibeus cinereus glaucus: Hershkovitz, 1949:449 View in CoL . Name combination.
Artibeus cinereus pumilio: Hershkovitz, 1949:449 . Name combination.
Dermanura glauca: Owen, 1987:47 View in CoL . Name combination. (see Nomenclatural Notes).
Dermanura cinerea bogotensis: ( Owen, 1987:64) View in CoL . Name combination.
A [rtibeus]. g [laucus]. glaucus: Handley, 1987:166 View in CoL . Name combination.
Artibeus glaucus bogotensis: Handley, 1987:166 View in CoL . Name combination.
Artibeus (Dermanura) glaucus: Koopman, 1993:188 View in CoL View Cited Treatment . Name combination.
CONTEXT AND CONTENT. Order Chiroptera View in CoL , family Phyllostomidae View in CoL , subfamily Stenodermatinae View in CoL , tribe Stenodermatini View in CoL , genus Artibeus View in CoL . Synonymy modified from Marques-Aguiar (2008). A. glaucus View in CoL is monotypic ( Lim et al. 2008).
NOMENCLATURAL NOTES. Gervais (1856) suggested that members of the genus Artibeus should be split into 2 genera, Artibeus (the larger species in the taxa) and Dermanura (the smaller members of the group such as glaucus ). In 1908, Andersen concluded that the aspects of tooth morphology used by Gervais did not produce a natural phylogenetic relationship and thus synonymized Dermanura with Artibeus . Owen’s (1987) analysis of external, cranial, mandibular, and dental characters led him to resurrect the genus Dermanura ( Gervais 1856) for the smaller taxa. This conclusion was also supported by Van Den Bussche (1992) using ribosomal DNA restriction-site data. More recently, Wetterer et al. (2000) analyzed 150 morphological, karyological, and molecular characters and hypothesized that Dermanura was a subgenus of Artibeus based on its close phylogenetic affinities with Artibeus sensu stricto. Artibeus bogotensis (originally described by Andersen 1906) has recently been recognized as a distinct and allopatric species from A. glaucus ( Lim et al. 2008) , therefore we included only information pertaining to A. glaucus glaucus .
The generic name, Artibeus , comes from 2 Greek words: arti, meaning facial lines, and beus, in reference to the presence of evident facial lines (Alvarez-Castañeda and Alvarez 1996). The species name, glaucus , was derived from the Greek work glaus, meaning apparently without a color connotation.
DIAGNOSIS
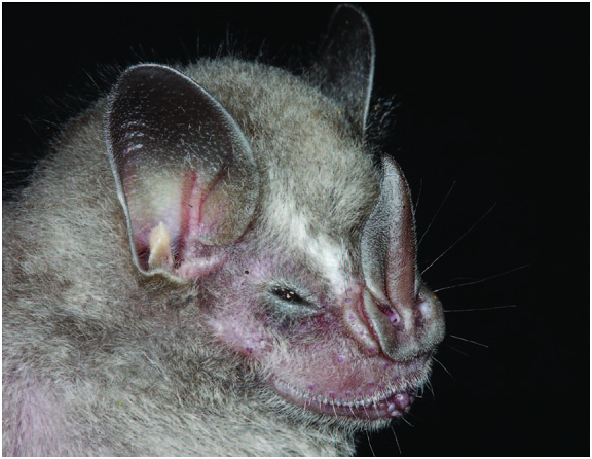
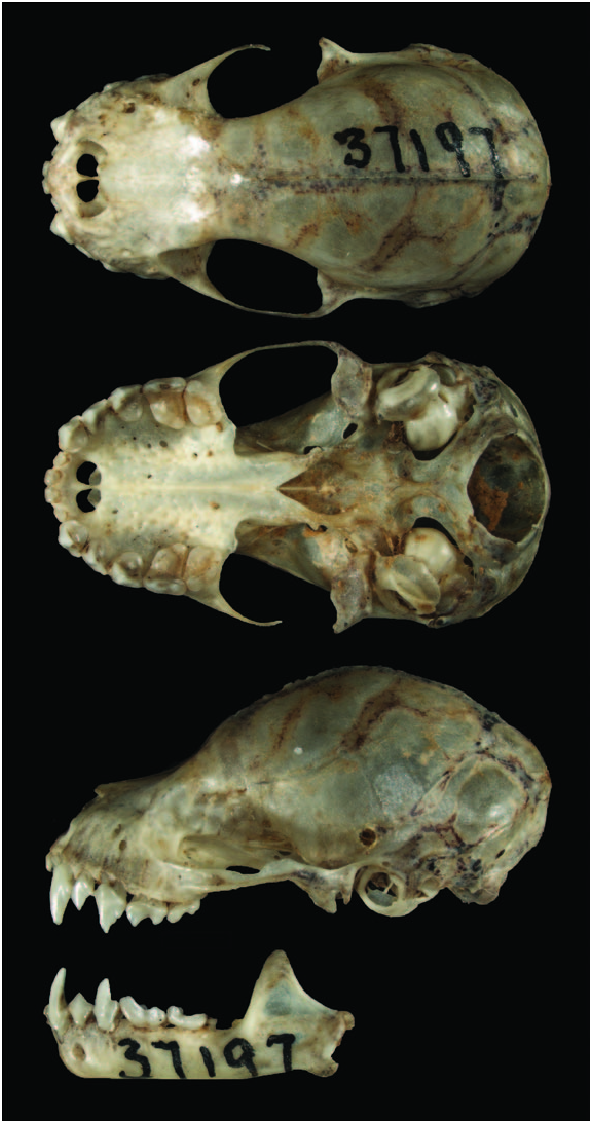
Artibeus glaucus is distinguished from other species of the genus by a combination of characters including size (e.g., members of the subgenus Dermanura present a forearm length of 46 mm and members of the subgenus Artibeus have a forearm length of 46 mm), pelage coloration, and morphology of the skull and teeth ( Figs. 1 View Fig and 2 View Fig ). A. glaucus is externally distinguished from A. bogotensis (Bogota fruit-eating bat) by the presence of faint or indistinct, white facial stripes and edging on the ears ( A. bogotensis has distinct facial markings), dark brown pelage ( A. bogotensis has pale brown), and dorsal fur that extends beyond the posterior margin of the uropatagium (in A. bogotensis hair does not extend beyond margin). Cranially, A. glaucus has a small 3rd lower molar and a robust orbitorostral region, whereas A. bogotensis does not have a 3rd lower molar and the orbitorostral region is poorly developed. In addition, specimens of A. bogotensis have a less furred uropatagia than A. glaucus . A. glaucus is distinguished from A. anderseni (Andersen’s fruit-eating bat) and A. gnomus (dwarf fruit-eating bat) by the absence of the 3rd lower molar in those species, and they are smaller in overall size: forearm length ranges from 34 to 36mm for A. anderseni and A. gnomus , and forearm length ranges from 36 to 39 in A. glaucus . Also edging on ears and noseleaf in A. glaucus is bright yellow compared with the subdued white in the smaller species. A. glaucus has supraorbital ridges that are parallel-sided, whereas in A. cinereus (Gervais’s fruit-eating bat), these ridges converge posteriorly, and A. cinereus does not have a small 3rd lower molar ( Lim et al. 2008; see Fig. 2 View Fig ).
GENERAL CHARACTERS
Artibeus glaucus is a small sized Neotropical bat ( Fig. 1 View Fig ). The species has indistinct facial stripes and obscure pale edging on the ears, dark brown pelage with less contrast on the shoulders and venter compared with other Artibeus . There is some external variation with the specimens from Amazonas, Peru, which have more prominent facial stripes and edging on the ears than those from Cuzco, Peru ( Lim et al. 2008).
Ranges of external measurements (mm) for 8 specimens from Peru (4 females and 4 males ––Swanepoel and Genoways 1979), 3 specimens from Ecuador and Peru ( Davis 1970), 2 specimens (including holotype) from Peru (females–– Andersen 1908; Carter and Dolan 1978), 4 specimens from Bolivia (3 females and 1 male ––Webster and Jones 1980), and specimens from Ecuador ( Tirira 2007) were: head and body length, 43–62; hind foot length, 8–12; ear length, 12–17; forearm length, 37.5– 43.8; and weight, 10–14 g.
Ranges of cranial measurements (mm) of 7 specimens from Amazonas and Cuzco, Peru ( Lim et al. 2008), 8 specimens from Peru (4 females and 4 males ––Swanepoel and Genoways 1979), 9 specimens from Ecuador and Peru ( Davis 1970), 2 specimens (including holotype) from Peru (females––Carter and Dolan 1978), and 4 specimens from Bolivia (3 females and 1 male – –Webster and Jones 1980) were: greatest length of skull, 19.0– 20.9; zygomatic breadth, 10.8–12.1; mastoid breadth, 9.7–11.0; rostral length, 8.3–9.1; postorbital constriction, 4.7–6.0; palatal length, 7.2–7.5; maxillary toothrow length, 5.9–6.7; breadth across upper molar, 7.9–8.9; width across upper canines, 5.1– 5.8; and coronoid height, 4.9–5.3.
DISTRIBUTION
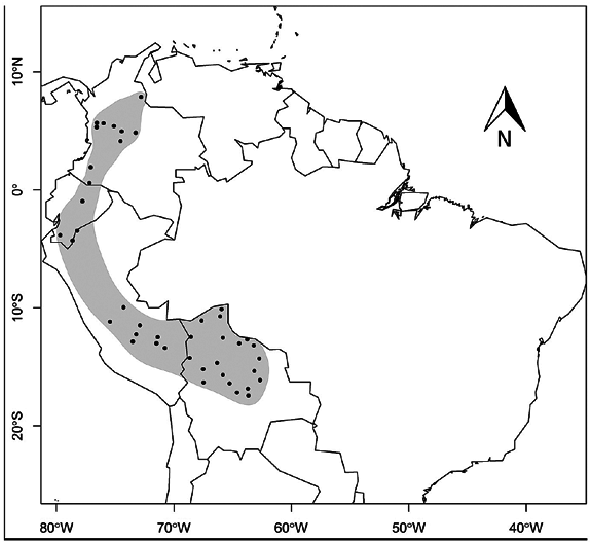
Artibeus glaucus is found in Ecuador, Perú, and Bolivia ( Fig. 3 View Fig ; Moya and Arteaga 2007; Marques-Aguiar 2008). The species can be found in lowland habitats but is mainly found at intermediate elevations in the Andean mountains (between 200 and 2,000 m, but most often found below 1,200 m in
Ecuador — Tirira 2012). There are no known fossil records for the species.
FORM AND FUNCTION
Dental formula is i 2/2, c 1/1, p 2/2, m 2/3, total 30. Proximate, caloric, nitrogen, and mineral composition values were obtained for Artibeus glaucus by Studier et al. (1994). Males (n = 7): live mass (g) = 8.60, comprised of: water, 69.08; fat, 2.39; ash, 4.74; nonfat organic, 23.78; caloric density (kcal/g dry mass), 3.95; water index, 2.43; and fat index (g/g lean dry mass), 0.084. Concentrations of indicated minerals were (mg/g dry mass): nitrogen, 163.23; iron, 0.376; calcium, 12.31; magnesium, 0.951; sodium, 4.29; and potassium, 10.56. Females (n = 5): live mass = 9.32 g, comprised of: water, 69.47; fat, 2.609; ash, 4.24; nonfat organic, 23.67; caloric density (kcal/g dry mass), 4.065; water index, 2.515; and fat index (g/g lean dry mass), 0.096. Concentrations of indicated minerals were (mg/g dry mass): nitrogen, 164.58; iron, 0.347; calcium, 13.24; magnesium, 1.056; sodium, 4.543; and potassium, 10.464.
ECOLOGY
Artibeus glaucus is a common species in secondary abandoned crop fields and in logging forest, but it also can be found in montane mature forestand primary lowland rain forest (Vargas- Espinoza et al. 2008). It is a common species in lowland forest of Ecuador and Peru ( Bravo Cabezas et al. 2003; Lim et al. 2008); in Ecuador, it is found in tropical and subtropical humid forests and lowland temperate forest ( Tirira 2012). A. glaucus has a mean relative abundance of 0.78 individuals per 4,500 m of riverine tropical forest of Peru ( Pacheco et al. 2011).
Artibeus glaucus is considered a functional foliage gleaner because it feeds on fruits of the canopy section ( Loayza et al. 2006). It has been found roosting under the cut leaves of Xanthosoma (Araceae) in Ecuador ( Timm 1987).
Artibeus glaucus has a bimodal polyestry reproductive pattern ( Wilson 1979). Pregnant or lactating females have been found from January to August in Ecuador and from October to December ( Albuja 1983).
Ectoparasites are not known for A. glaucus , but several reports exist for A. bogotensis , the sister species (Tipton and Machado-Allison 1972; Wenzel 1976; Guerrero 1997). Prevalence of Bartonella infection was not found in specimens of A. glaucus , after an extensive survey in 19 different bat species from the Amazon region of Peru ( Bai et al. 2012).
GENETICS
The diploid number (2n) of chromosomes for Artibeus glaucus is 30–31, the fundamental number (FN) is 56, with a Y
1
and Y
2
chromosome system (X-autosome fusion). The X chromosome is subtelocentric and the Y 1 and Y 2 chromosomes are both acrocentrics ( Gardner 1977).
Lim et al. (2008) studied the molecular and morphological variation of A. glaucus and found strong evidence that A. bogotensis and A. glaucus should be recognized as distinct and allopatrically occurring species. Molecular phylogenies using the mitochondrial cytochrome b gene found strong support for the relationship of A. glaucus sister to the clade conformed by A. bogotensis + A. gnomus ( Lim et al. 2008; Solari et al. 2009). The average Kimura-2 parameter genetic distance for cytochrome b is 7.3% between A. bogotensis and A. gnomus , 9.5% between A. bogotensis and A. glaucus , and 10.4% between A. gnomus and A. glaucus ( Lim et al. 2008) .
Using cytochrome b sequences, the haplotype diversity for the species is 0.9000, the nucleotide diversity is 0.0281, the maximum interspecific diversity is 0.0428, and the minimum intraspecific diversity is 0.089, last 2 parameters by using the K2P model ( Redondo et al. 2008).
CONSERVATION
Artibeus glaucus is classified as “Least Concern” in the 2008 International Union for Conservation of Nature and Natural Resources Red List of Threatened Species ( Sampaio et al. 2008); however, when this classification was assigned, A. bogotensis was considered a subspecies of A. glaucus . A. glaucus is considered common and frequent with stable populations in Ecuador ( Tirira 2007).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Artibeus Glaucus THOMAS, 1893
| Ortega, Jorge, Arroyo-Cabrales, Joaquín, Moreno-Santillán, Norberto Diana, Velazco Martínez-Mendez, Paúl M. & Real-Monroy, Melina Del 2015 |
Dermanura glauca:
| OWEN, R 1987: 47 |
Dermanura cinerea bogotensis: ( Owen, 1987:64 )
| OWEN, R 1987: 64 |
Artibeus glaucus bogotensis:
| HANDLEY, C 1987: 166 |
Artibeus cinereus glaucus:
| HERSHKOVITZ, P 1949: 449 |
Artibeus cinereus pumilio:
| HERSHKOVITZ, P 1949: 449 |
Artibeus pumilio
| THOMAS, O 1924: 531 |
Artibeus cinereus bogotensis:
| ANDERSEN, K 1906: 421 |
Artibeus (Artibeus)
| TROUESSART, E 1897: 160 |
Artibeus glaucus
| THOMAS, O 1893: 336 |