Onychostoma dongnaiensis, Hoang, Huy Duc, Pham, Hung Manh & Tran, Ngan Trong, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3962.1.6 |
|
publication LSID |
lsid:zoobank.org:pub:5872B302-2C4E-4102-B6EC-BC679B3645D4 |
|
DOI |
https://doi.org/10.5281/zenodo.5628884 |
|
persistent identifier |
https://treatment.plazi.org/id/03EFB841-B56D-FFBA-06FE-F9D4FCC0D41B |
|
treatment provided by |
Plazi |
|
scientific name |
Onychostoma dongnaiensis |
| status |
sp. nov. |
Onychostoma dongnaiensis View in CoL sp. nov.
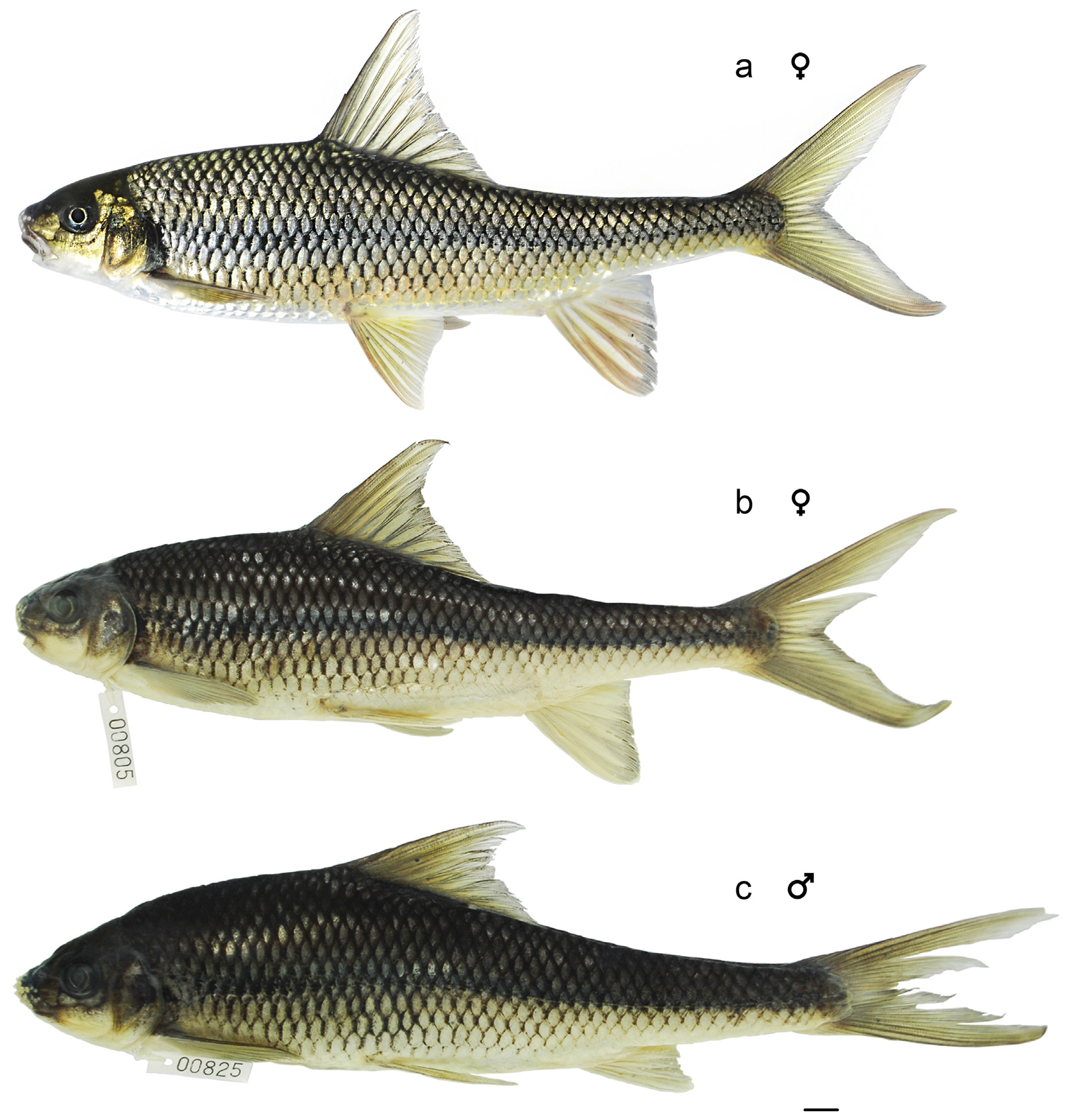
Holotype: UNS 00851, adult female, 172 mm SL; upper Da Teh main stream: middle Dong Nai drainage in montane evergreen forest in Lam Dong Province, Vietnam (11°36’13.80”N, 107°35’49.60”E, 172 m) 11 March 2014 by Pham Manh Hung and Hoang Duc Huy ( Fig. 1 View FIGURE 1 ).
Paratypes: NSMT-P 121249 (adult female, 155 mm SL), 11 March 2014, collected at same locality as holotype; UNS 00852 (adult female, 196 mm SL), UNS 00853 (adult female, 180 mm SL), UNS 00854 (adult female, 166 mm SL), ZRC 54625 (adult male, 157 mm SL), 7 March 2014, collected at same locality as holotype; ZRC 54626 (adult female, 173 mm SL), 11 April 2014, collected at same locality as holotype (11°38’13.79”N, 107°37’26.14”E, 195 m).
Etymology. Specific epithet is in reference to the type locality of middle Dong Nai drainage. Suggested common name: Dongnai srang (English), Cá srang Đồng Nai (Vietnamese). Srang is the vernacular name of this species of Onychostoma by the Mạ people in the Da Teh catchment of the middle Dong Nai River, Lam Dong Province.
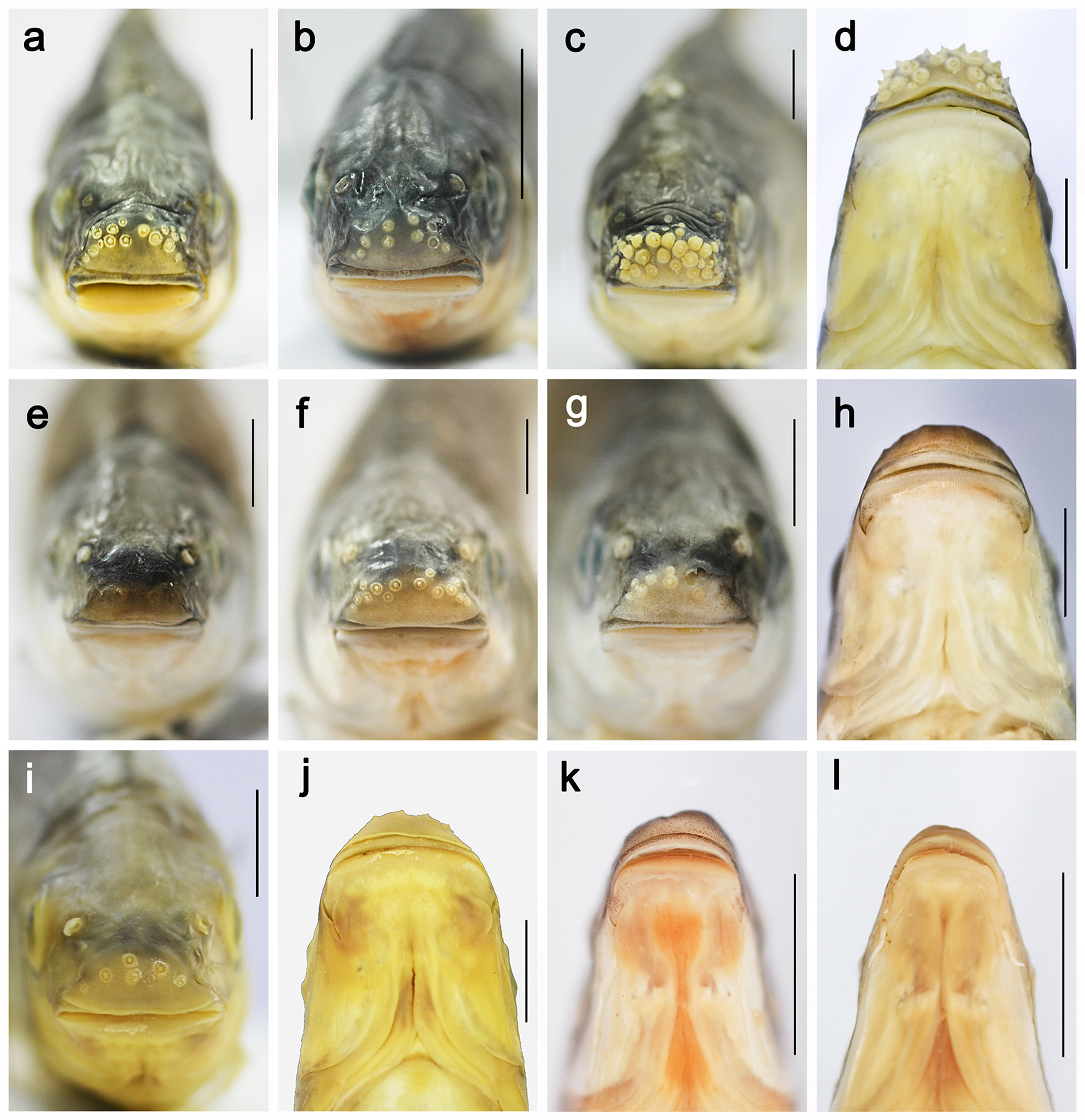
Diagnosis. Onychostoma dongnaiensis is distinguished from its congeners by a combination of (1) mouth wide (width 1.4−1.6 times in HW), (2) predorsal scales 14−15, (3) body depth high (3.1−3.4 times in SL), (4) eye diameter small (4.2−4.9 times in HL), (5) no barbels in adults and juveniles, (6) a strong serrated last simple ray of dorsal fin, and (7) dorsal-fin length high (3.8−4.5 times in SL) ( Figs. 5 View FIGURE 5 a, b, c; 3e, f, g, h).
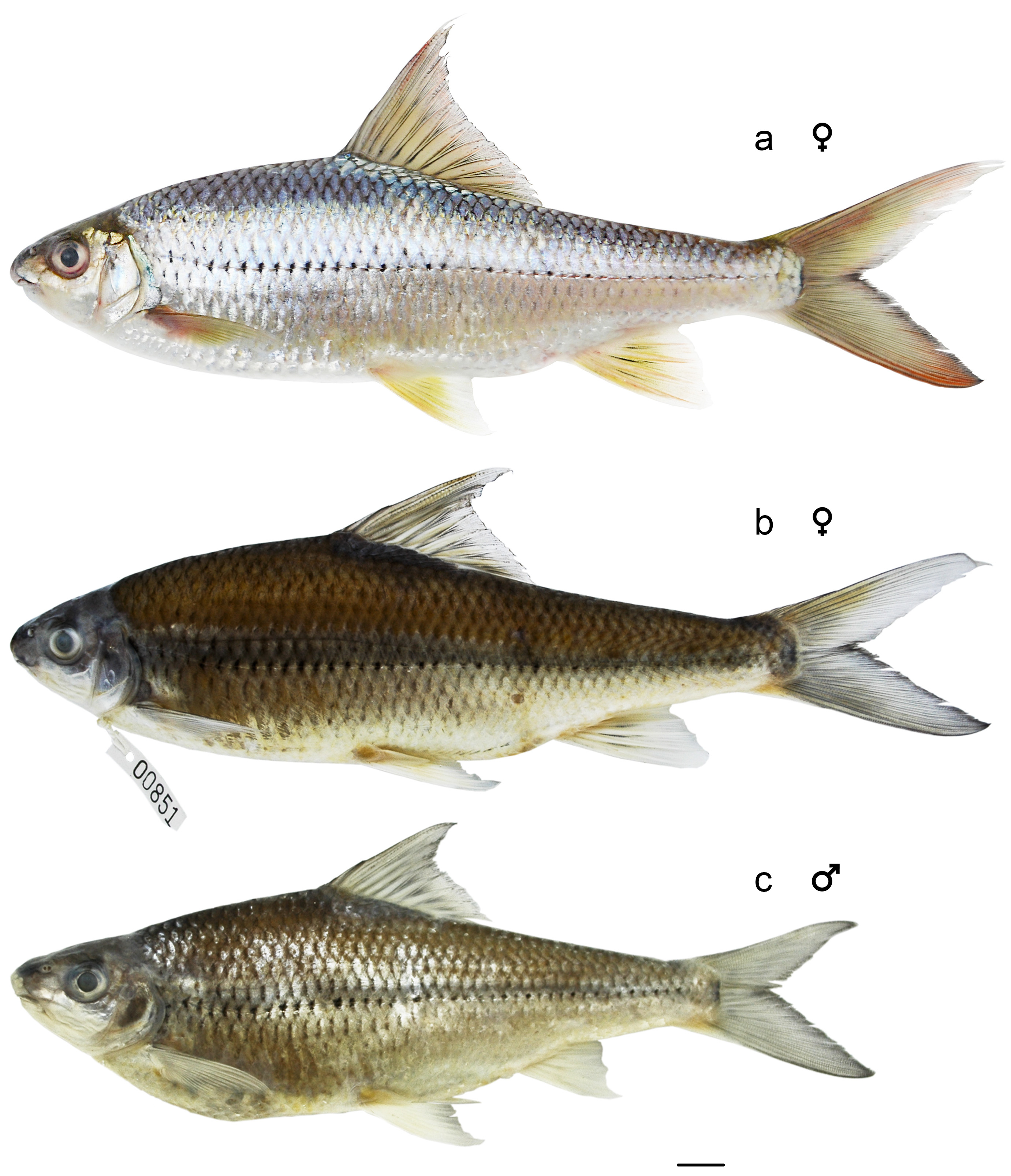
Description. General appearance shown in Figure 5 View FIGURE 5 ; meristic and morphometric data of seven adult type specimens given in Table 2 View TABLE 2 . Head longer than deep, dorsal profile strongly convex. Snout stout and rounded, longer than eye diameter in adults. Interorbital area slightly convex. Mouth subterminal. Maxillary reaching vertical of anterior margin of orbit. Upper lips thick, upper region covered by rostral fold (rostral cap) completely and exposed at corners. Epidermal tubercles on snout numerous but small to medium-sized and sparse in all type specimens except holotype, which has no tubercles ( Fig. 3 View FIGURE 3 e). Mouth width about 1.4−1.6 times in maximum HW; no barbels ( Fig. 3 View FIGURE 3 h).
Body high, moderately compressed; caudal peduncle slender, about 2.4−2.6 times longer than deep. Dorsal body profile convex, ventral profile rounded. Lateral line complete, 45−47 scales; 14−15 predorsal scales; 7/1/5 scales in transverse row anterior to pelvic-fin insertion.
Dorsal fin high with 4 simple and 8 branched rays; first simple ray as a tiny process, vestigial in a few specimens; last simple ray strongly serrated; dorsal-fin origin inserted slightly in front of vertical with pelvic-fin origin; distal margin anteriorly concave and posteriorly convex. Pectoral fin pointed with 1 simple and 16 branched rays. Pelvic fin pointed, with 1 simple and 8 branched rays; axillary scale present. Anus immediately in front of anal fin. Anal fin with 3 simple and 5 branched rays; short (17.8% SL) and small in male; long (20.0−21.7% SL) and large in female ( Fig. 5 View FIGURE 5 b, c). Caudal fin deeply forked with 9+8 principal rays, 8+7 being branched.
Colour in life. Head dark turquoise on back, light turquoise around orbital and on side, white on opercula and lower jaw. Body light turquoise on back, silver white on lateroventral surface, snowy white belly with a longitudinal black stripe running along lateral line. Scales silverly bluish between back and lateral line; lateral-line scales and row of scales just above lateral line in anterior half of body pigmented at center of scale bases. Fins hyaline. Dorsal fin dark turquoise at origin, bold on rays; distal margin concave anteriorly and convex posteriorly; reddish at distal part. Pectoral fin and pelvic fin yellowish green and reddish at first rays and distal parts. Anal fin yellowish green on rays, pinkish distally and hyaline on distal margin. Caudal fin near peduncle dark turquoise, yellowish to red on tips.
Colour in preservative. Similar to that of fresh condition except as noted below. Upper half of body including head brown. Lower half of body including head pale ivory colour except anterior half of body darker. Pigments at scale bases on almost whole body outstanding. All turquoise, yellowish, silverly bluish, reddish and snowy whitish colors disappeared ( Fig. 5 View FIGURE 5 b, c).
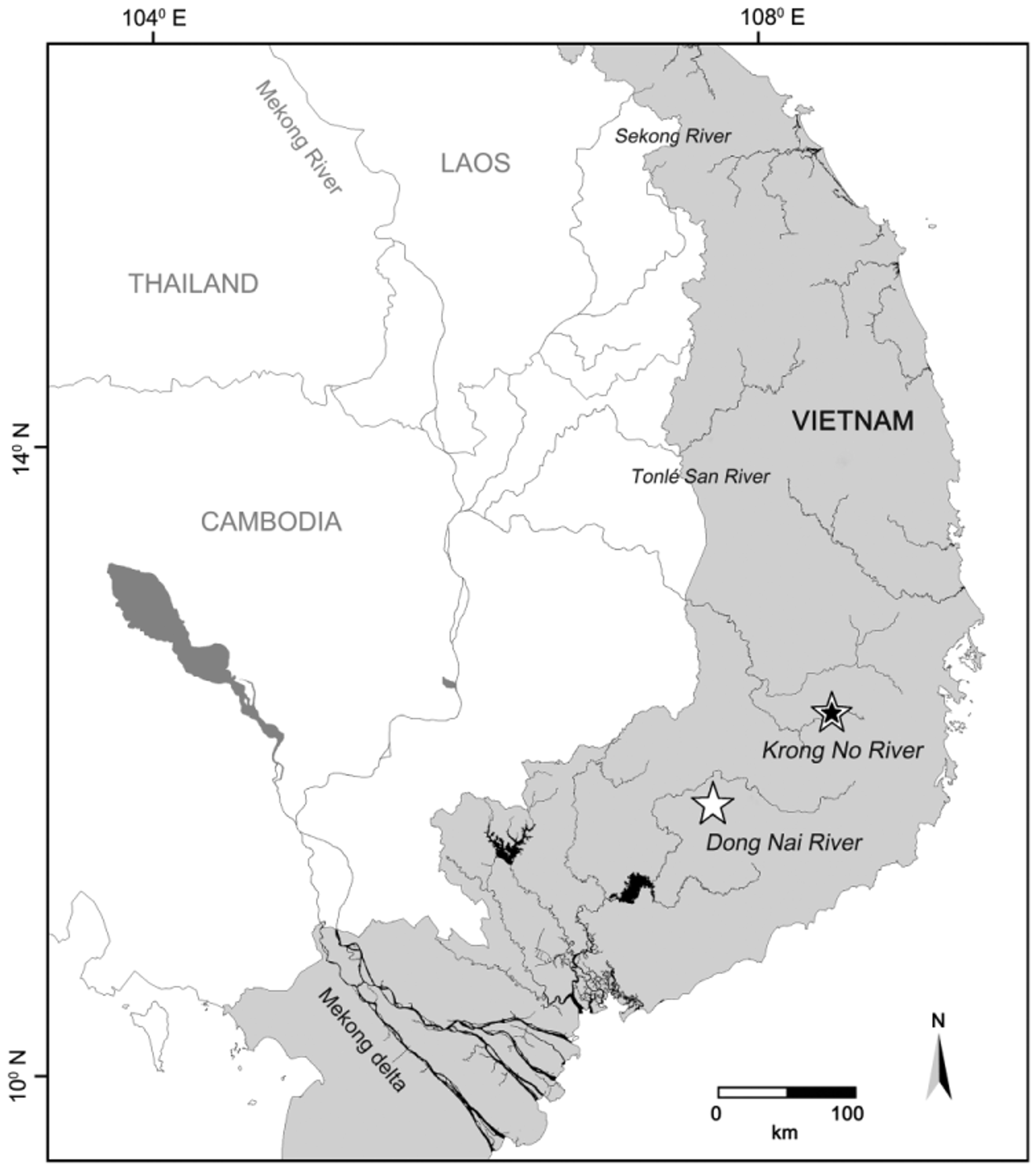
Ecology. All specimens of the new species were found in the Da Teh catchment of middle Dong Nai drainage in evergreen forest between 140−200 m ( Fig. 4 View FIGURE 4 d). Onychostoma dongnaiensis lives in clear water but some specimens were collected in silty water. Water conditions of 26.4−28.1°C, pH 7.02−7.6, DO 82.7−91.4%, conductivity 12−25 µS.cm -1 and flow velocity 0.12−0.77 m /s were recorded. The species occurred in swift currents over bedrock, large boulders and cobble substrates covered with periphyton ( Fig. 4 View FIGURE 4 d). This is similar to the habitat of O. krongnoensis . When feeding, O. dongnaiensis flips rocks and scrapes algae from them. Onychostoma dongnaiensis is found with Mystacoleucus obtusirostris , the bonylip barb Osteochilus vittatus , Poropuntius deauratus , Channa gachua , Yasuhikotakia morleti , and Acantopsis dialuzona .
Conservation status. The conservation status of this new species requires particular attention. It has been long recognized by the local Mạ minority people. It is likely to be restricted to relatively small stretches of low-elevation forest streams of the middle Dong Nai drainage and hence particularly vulnerable to threatening processes such as siltation and overfishing. Siltation (from road construction, deforestation, agriculture etc.) which covers rocks and stones is a particular threat to this species as it covers the algae growing on the stones ( Fig. 4 View FIGURE 4 d). This species feeds on the algae and animals living in the algae. Anthropogenic modification of stream morphology, logging, deforestation, agriculture and overfishing frequently occur in the Da Teh catchment. These activities impact the aquatic environment of this species so its populations are potentially threatened. Given the available information, we suggest the species should be considered Data Deficient following IUCN’s Red List categories (IUCN 2014, version 11).
Comparisons. In general, O. dongnaiensis most closely resembles O. leptura and O. gerlachi when compared with congeners in the Mekong basin and Red river basin. From detailed measurements and comparisons of specimens of both species, O. dongnaiensis differs from O. leptura in having scales in transverse row 7/1/5 vs. 7/1/ 4, predorsal scales 14−15 vs. 12−13, eye small with diameter 20.3−23.8 vs. 22.9−30.3% HL and serrated last simple dorsal ray vs. smooth last simple dorsal ray. Onychostoma dongnaiensis differs from O. gerlachi by having predorsal scales 14−15 vs. 12−15, caudal peduncle 23.2 vs. 21.2% SL, prepectoral length 18.7 −21.7 vs. 21.4−26.5% SL, eye small with diameter 20.3−23.8% vs. 27.4−35% HL. Onychostoma dongnaiensis differs from O. meridionale in having mouth 1.4−1.6 vs. 2.2−2.3 times in maximum HW ( Fig. 3 View FIGURE 3 h, k), the last simple dorsal ray strongly serrated vs. not serrated posteriorly, eye diameter 4.2−4.9 vs. 3.7−4.5 times in HL, and scales in transverse row 7/1/5 vs. 6.5/1/6.5. Onychostoma dongnaiensis differs from O. fusiforme in having mouth 1.4−1.6 vs. 2.0−2.2 times in maximum HW, caudal peduncle 2.2−2.6 vs. 3.0−3.6 times longer than deep, eye diameter 4.2−4.9 vs. 3.7−4.4 times in HL, and scales in transverse row 7/1/5 vs. 6.5/1/4−4.5.
Mai et al. (1992) described one specimen as the name Scaphidonichthys sp. from Phu Lap, Tan Phu, Dong Nai province near the Dong Nai River at elevation (11°27’17.23” N, 107°29’4.40” E, 155 m). We consider this species conspecific with O. dongnaiensis based on examination of its morphometrics ( Mai et al. 1992) similar to our O. dongnaiensis specimen (UNS00856) collected at the same locality.
Morphological data. Predorsal scales and eye diameter are considered reliable taxonomic characters, and to our knowledge, the short eye diameter (4.2−5.6 times in HL) in the two new species is extreme among all Onychostoma species from southeast Asia ( Taki 1975, Kottelat 1998, Lothongkham & Musikasinthorn 2005).
Onychostoma krongnoensis is most similar to O. dongnaiensis , but differs most dramatically in colour in life. Onychostoma krongnoensis is dark turquoise on the back and yellow to pinkish orange on lateroventral surface ( Fig. 2 View FIGURE 2 ), and O. dongnaiensis is light turquoise on the back, and bluish silver-white on the lateroventral surface ( Fig. 5 View FIGURE 5 ). Onychostoma krongnoensis also has a slightly deeper body (3.5−4.3 vs. 3.1−3.4 times in SL) and a slightly shorter caudal-peduncle (3.8−4.2 vs. 4.1−4.5 times in SL). The number of epidermal tubercles on the snout also may vary in the two species with more on O. krongnoensis , although seasonal and ontogenetic variation has not been studied.
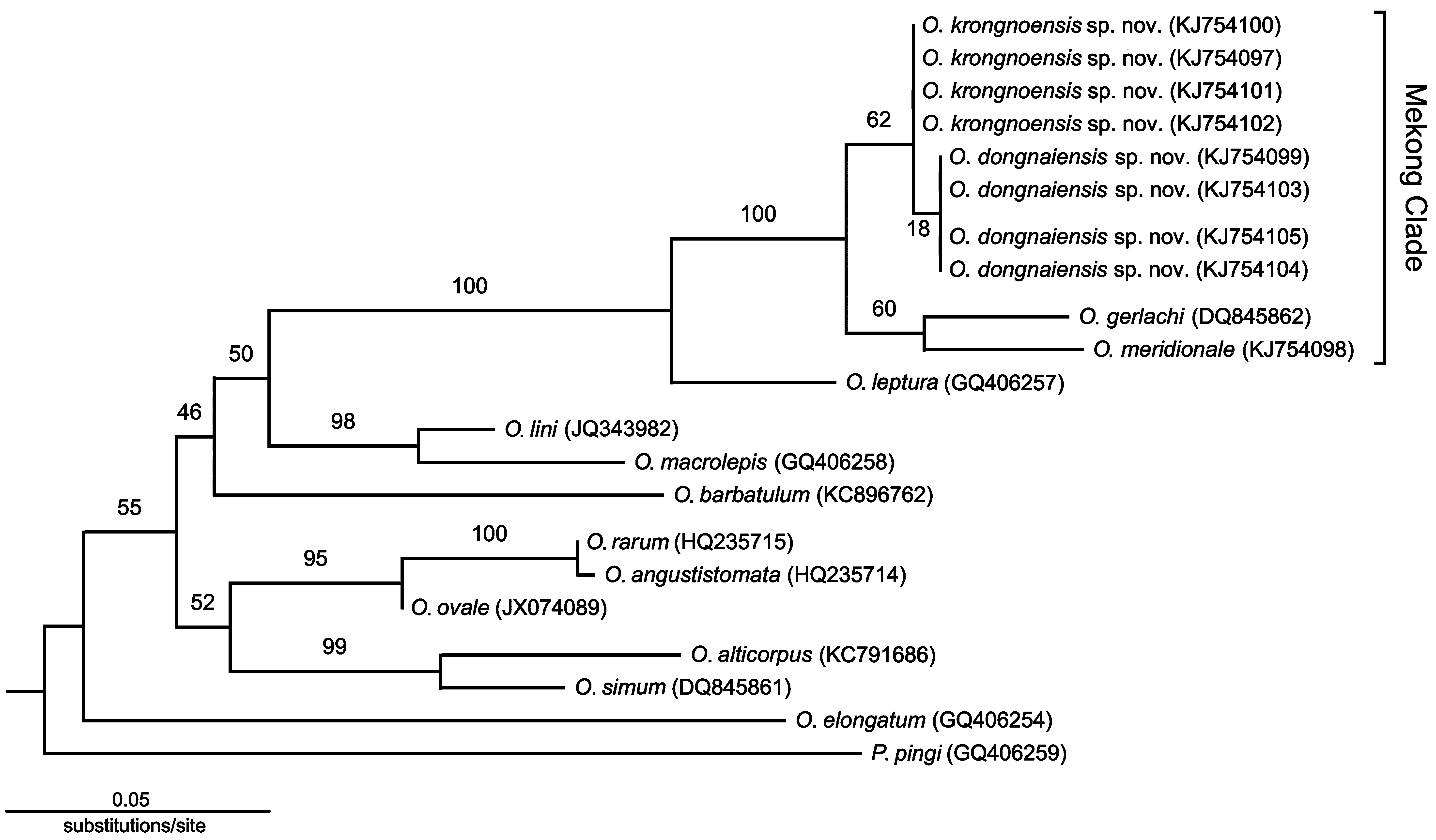
Molecular data. The newly collected specimens from the Krong No and Dong Nai rivers are embedded within a clade containing all Mekong Onychostoma species ( Fig. 6 View FIGURE 6 ). This clade receives 100% bootstrap support. Onychostoma meridionale was also embedded within this clade.
Our molecular phylogeny suggests that the Krong No and Dong Nai river specimens are most closely related to O. gerlachi , differing by 2.0% and 2.1%, respectively, to O. gerlachi , and>2.0% to all Onychostoma in the analysis. The Krong No and Dong Nai specimens are closely related to each other and differ by 0.2% sequence divergence, which is larger than the difference between O. angustistomata and O. rarum by 0.1% (Table 3). Onychostoma meridionale appears most closely related to O. gerlachi , but differs by 2.1% from this species and>2.0% from all other Onychostoma specimens in the analysis.
Table 3. Samples used in the phylogenetic analysis of Onychostoma with the outgroup Percocypris pingi and genetic distances between species.
1 2 3 4 5 6 7 8 9 10 11 12 13 14
Taiwan
KC791686 View Materials
China 0.045 HQ235714 View Materials
Taiwan 0.063 0.052 KC896762 View Materials
. O. dongnaiensis sp. nov. 0.063 0.066 0.070 KJ754099 View Materials
China: Rong’an, Guangxi Zhuang 0.072 0.068 0.065 0.084 Auto, Region
GQ406254 View Materials
China: Jinghong, Yunnan Prov. 0.067 0.064 0.076 0.021 0.089 DQ845862 View Materials
. O. krongnoensis sp. nov. 0.062 0.064 0.067 0.002 0.083 0.020 KJ754097 View Materials
China: Xilin, Guangxi Zhuang 0.060 0.058 0.063 0.030 0.080 0.033 0.028 Auto, Region
GQ406257 View Materials
. O. lini
China: Youyang, Chongqing 0.048 0.044 0.041 0.052 0.065 0.059 0.050 0.046 JQ343982 View Materials
10. O. macrolepis
China: Taian, Shandong Prov. 0.054 0.050 0.051 0.057 0.073 0.060 0.055 0.053 0.019 GQ406258 View Materials
11. O. meridionale 0.074 0.067 0.082 0.018 0.092 0.021 0.020 0.037 0.064 0.065 12. O. ovale
China: Tian’e, Guangxi Prov. 0.035 0.014 0.029 0.047 0.050 0.049 0.047 0.039 0.029 0.051 0.056 JX074089 View Materials
13. O. rarum
China 0.044 0.001 0.051 0.064 0.068 0.063 0.063 0.056 0.043 0.050 0.066 0.012 HQ235715 View Materials
14. O. simum
China: Hejiang, Sichuan Prov. 0.024 0.044 0.050 0.056 0.066 0.062 0.055 0.054 0.041 0.047 0.066 0.033 0.042 DQ845861 View Materials
China: Hejiang, Sichuan Prov. 0.073 0.067 0.074 0.089 0.085 0.089 0.087 0.076 0.070 0.072 0.095 0.052 0.066 0.070 GQ406259 View Materials
Discussion
With two new species described here, a total of 22 species of Onychostoma are now recognised, with five species occurring in the Mekong drainage. Onychostoma krongnoensis inhabits the Ea Krong No river drainage of the Langbiang Plateau. These mountain rivers are perennial, shallow water bodies characterized by low temperature, high turbulent current, and rocky substratum. As adaptions to strong water currents, O. krongnoensis has dense epidermal tubercles on the snout tip, a slender, streamlined body with an increased number of predorsal scales (mostly 17 scales), and a longer caudal peduncle compared to other species of Onychostoma .
Tubercles of cyprinids exhibit sexual dimorphism and ontogenetic variation in the size, shape and distribution ( Wiley & Collette, 1970). In contrast to other species of Onychostoma , in O. krongnoensis the epidermis of the snout possesses 2−3 irregular transverse rows of 13−43 tubercles in juveniles and adults of both sexes ( Fig. 3 View FIGURE 3 b). Variation in the surface of the snout in hill-stream fishes has been suggested to be an adaptation to life in torrential streams ( Hoshiyar et al. 2013). The snout epidermis of O. krongnoensis is subject to frictional stress as it comes into contact with water current, thus supporting this hypothesis. In contrast, species that inhabit low elevation streams with slower velocity, such as O. dongnaiensis , O. leptura , and O. uniforme , have fewer smaller tubercles on the snout (Hoang et al. pers.obs.). Breeding tubercles also occur on the anal-fin rays of males of O. krongnoensis and other stream cyprinids ( Witkowski & Rogowska 1991; Poncin et al. 2011).
Body shape affects movement in stream fishes ( Chuang et al. 2006) and may be a useful tool for predicting habitat preferences ( Stolbunov et al. 2011). For O. krongnoensis , the slender, streamlined body with a large number of predorsal scales (15−17) and long caudal peduncle may provide greater swimming ability and allow it to inhabit rapid rivers and streams at high elevations such as those in the Ea Krong No drainage. In contrast, O. dongnaiensis and O. leptura have a deeper body, fewer predorsal scales (12−15) and a short caudal peduncle, which may be more suitable features for slow, low elevation streams such as the middle of the Dong Nai and Red river drainages (Hoang et al. pers.obs.). Further studies on the capabilities and physiological mechanisms of O. krongnoensis that allow the species to live in such rapid streams would be interesting.
Pronounced sexual dimorphism in the anal-fin size has been observed for several species of Onychostoma (Hoang et al. pers. obs.), but has not been reported. This morphological character highlights the importance of making intraspecific comparisons separately for each sex. The functional role of the sexual dimorphism in O. krongnoensis and O. dongnaiensis is unknown.
Species diversity of Onychostoma in Mekong basin. Onychostoma krongnoensis and O. dongnaiensis are very similar to O. gerlachi , O. fusiforme and O. meridionale , and have probably been misidentified due to the similarities in their adult overall morphology and geographic distributions. Shan et al. (2000), Kottelat (2001, 2009), Lothongkham & Musikasinthorn (2005) and Kano et al. (2013) described O. gerlachi as a widespread species from the Pearl River, upper Red River to the Langcang River, Nam Ou, Se Banghiang River-Mekong basin, and the Nan River-Chao Phraya basin. Kottelat (1998), Xin et al. (2009) and Kano et al. (2013) recorded O. fusiforme as occurring from the Langcang River (Yunnan, China), Ing River, Kok River ( Thailand) to Nam Theun River ( Laos). Kottelat (1998, 2007, 2011), Kano et al. (2013) and our survey (2013) described and recorded O. meridionale from the Se Bangfai River, Sekong River ( Laos), Sesan River ( Cambodia), Sa Thay River-tributary of the Sesan River partly in the Vietnam Central Highlands to the Tonle Sap River ( Cambodia). Even amongst geographically close river basins such as the Sekong River, Sesan River and Sre Pok River in the middle Mekong basin, there are distinct species of Onychostoma present: O. krongnoensis in the Srepok River, O. meridionale in the Sekong and Sesan rivers.
Onychostoma krongnoensis and O. dongnaiensis are the first records of the genus in the upper Srepok River and middle Dong Nai River on the Langbiang Plateau, southern Vietnam. Among species of the Mekong group, O. gerlachi occurs the furthest north. This species could be the ancestral lineage of the Mekong clade. Despite the lack of O. fusiforme in our molecular data, phylogenetic analysis and geographical distribution suggest that this lineage may have migrated towards the southern Mekong basin. The geological event that separated the Dong Nai dranage from the Mekong drainage would have isolated O. dongnaiensis from O. krongnoensis . Detailed and comprehensive surveys to gain a better understanding of geographical distributions, and molecular phylogenetic analysis for Onychostoma in the Mekong basin are essential.
TABLE 2. Morphometric and meristic characters of Onychostoma dongnaiensis sp. nov. Range and mean include the holotype.
| Standard length (mm) | Holotype 172 | Range 15.5–19.6 | Mean±SD 17.1±1.4 | N 7 |
|---|---|---|---|---|
| Morphometrics | ||||
| % SL | ||||
| Head length | 19.8 | 19.4–21 | 20.3±0.7 | 7 |
| Depth of body | 29.2 | 29.2–32.7 | 30.8±1.1 | 7 |
| Body width at dorsal-fin origin | 16.0 | 12.7–16 | 14.5±1.3 | 7 |
| Predorsal length | 43.7 | 40.8–45.8 | 43.3±1.5 | 7 |
| Prepectoral length | 18.7 | 18.7–21.7 | 20.0±1.1 | 7 |
| Prepelvic length | 46.9 | 46.9–51.2 | 48.6±1.5 | 7 |
| Preanal length | 70.0 | 69.7–73.5 | 70.8±1.3 | 7 |
| Distance between pectoral- and pelvic-fin origins | 24.5 | 24.5–28.7 | 26.4±1.8 | 7 |
| Distance between pelvic- and anal-fin origins | 19.2 | 17.8–22.4 | 19.2±1.6 | 7 |
| Depth of caudal peduncle | 9.3 | 9.2–10 | 9.6±0.3 | 7 |
| Length of caudal peduncle | 23.6 | 22–24.3 | 23.2±0.7 | 7 |
| Length of dorsal fin | 23.3 | 22.3–26 | 23.5±1.2 | 7 |
| Length of dorsal-fin base | 16.3 | 15.6–17.6 | 16.7±0.8 | 7 |
| Length of pectoral fin | 18.1 | 17.8–19.4 | 18.3±0.6 | 7 |
| Length of pelvic fin | 19.2 | 17.4–19.2 | 18.4±0.6 | 7 |
| Length of anal fin | 21.0 | 17.8–21.7 | 20.6±1.3 | 7 |
| Length of anal-fin base | 8.7 | 7.7–11.1 | 9.2±1.3 | 7 |
| % HL | ||||
| Head depth | 88.2 | 71.4–89.5 | 81.1±6.6 | 7 |
| Head depth at eye | 67.6 | 57.1–70.3 | 64.3±5.0 | 7 |
| Maximum head width | 67.6 | 57.1–67.6 | 62.3±3.2 | 7 |
| Snout length | 35.3 | 33.3–36.8 | 34.7±1.3 | 7 |
| Interorbital width | 47.1 | 40.5–55.3 | 47.1±4.6 | 7 |
| Eye diameter | 20.6 | 20.3–23.8 | 21.7±1.5 | 7 |
| Mouth width | 41.8 | 33.3–44.7 | 40.5±3.6 | 7 |
| Counts | ||||
| Lateral-line scales | 47 | 44–47 | 46.1±1.2 | 7 |
| Scales between lateral line and origin of dorsal fin | 7 | 7 | 7.0±0.0 | 7 |
| Scales between lateral line and origin of pelvic fin | 5 | 5 | 5.0±0.0 | 7 |
| Scales between lateral line and origin of anal fin | 5 | 5 | 5.6±0.5 | 7 |
| Circumpeduncular scales | 16 | 16–16 | 16.0±0.0 | 7 |
| Predorsal scales | 15 | 14–15 | 14.7±0.5 | 7 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.