Neotrogla curvata, Lienhard & L. & Ferreira & Ch-, 2013
|
publication ID |
https://doi.org/ 10.5281/zenodo.5822926 |
|
publication LSID |
lsid:zoobank.org:pub:9C1BDED3-2AB1-46F5-AC47-5AB1F5C27676 |
|
DOI |
https://doi.org/10.5281/zenodo.7551711 |
|
persistent identifier |
https://treatment.plazi.org/id/9FBDF94C-88E8-4405-AB50-EF3C020717DD |
|
taxon LSID |
lsid:zoobank.org:act:9FBDF94C-88E8-4405-AB50-EF3C020717DD |
|
treatment provided by |
Carolina |
|
scientific name |
Neotrogla curvata |
| status |
sp. nov. |
Neotrogla curvata View in CoL View at ENA n. spec.
Figs 1-4 View FIG View FIG View FIG View FIG
HOLOTYPE: ISLA; ♀; Brazil (Bahia), São Félix do Coribe, cave PEA 380 (BA 042); 21.vii.2011; leg. Simone Soares Salgado.
PARATYPES: ISLA and MHNG; 43 (one of them allotype) and 5♀; same data as for holotype . – SEHU; 2♀ (one of them teneral) ; Brazil (Bahia), São Félix do Coribe , cave PEA 381 (BA 043); 18.vii.2011; leg. Simone Soares Salgado. – ISLA and MHNG; 13, 2♀, 2 nymphs (both with damaged abdomen) ; Brazil (Bahia), São Félix do Coribe , cave PEA 383 (BA 045); 18.vii.2011; leg. Simone Soares Salgado. – MHNG and SEHU; 13, 2♀ and 1 nymph (the latter lacking abdomen) ; Brazil (Bahia), Santa Maria da Vitória , cave PEA 343 (BA 003); 15.v.2011; leg. Simone Soares Salgado.
OTHER MATERIAL: Several additional females, males and nymphs ( ISLA and SEHU) were collected in the above mentioned caves or in three other caves situated in these municipalities (caves PEA 341, 342, 378; see Distribution and habitat, below), most of them in October 2012. Some of them were used for rearing, behavioural observations or micromorphological studies of pairs in copula.
DIAGNOSIS: Sclerotized area of anterior part of female subgenital plate with arms forming an almost straight transverse band, in middle not separated from posterior part of subgenital plate. Basal half of median lobe of posterior part of subgenital plate with a pair of small, hemispherical, hairy lobes and medially with a bifid, dark brown sclerotization; apical half of this lobe bare, ovally rounded. Female subgenital plate different in the three other species, in particular sclerotized area of anterior part broadly V-shaped (with opening of the V directed backwards) and medially separated from posterior part by an unpigmented area (see Lienhard et al., 2010: figs 1c, 5, 8d). In N. curvata n. spec. abdominal sternite anterior to subgenital plate medially with a rugose papillate area, this region smooth in the other species. Posterior sac of gynosome strongly ventrad-bent, having roughly the shape of a short and strongly bent sausage, bearing the opening of the spermathecal duct on the inner side of its broadly rounded distal end. Blister-like zone of gynosome with three strongly denticulate posteriad-directed lobes. Posterior part of gynosome (i. e. posterior sac and blister-like zone) shorter than its sclerotized anterior rod. In the other known species posterior part of gynosome longer than its anterior rod and posterior sac almost straight, only very slighty ventrad-bent in lateral view (see Lienhard et al., 2010: figs 2d, 2f, 6, 8c). The only characters of the male which could possibly be diagnostic are the slightly bilobed posterior thickening of the aedeagal arch and the relatively short but wide papillate channel of the endophallus (see Discussion, below).
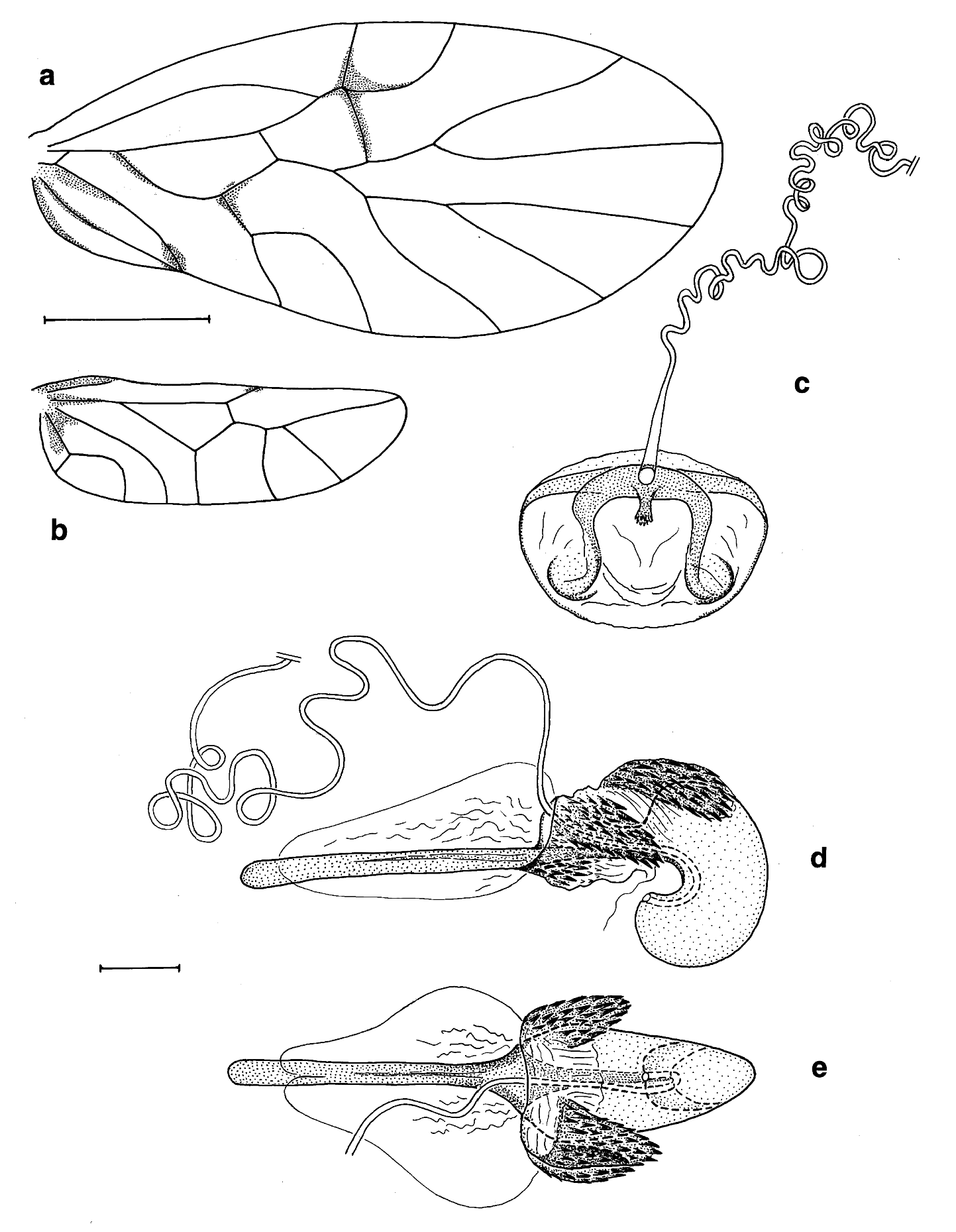
DESCRIPTION: Habitus of adults as in Fig. 4a View FIG . Colouration and general morphology of males, females and nymphs as described for the type species Neotrogla brasiliensis (see Lienhard et al., 2010). In forewing ( Fig. 1a View FIG ) fused portion of Rs and M longer than basal portion of Rs, unpigmented area of pterostigma very slightly opaque. In hindwing ( Fig. 1b View FIG ) M2 slightly concave (bent towards wing base) and R1 distally with a tiny patch of dark pigmentation. Nymphs dorsally with short glandular hairs bearing a minute globular thickening at their tip, similar in shape to the glandular hairs known in some troctomorph psocids (see Lienhard, 1998: fig. 45g). Such hairs also present in nymphs of N. brasiliensis , though not mentioned in the original description.
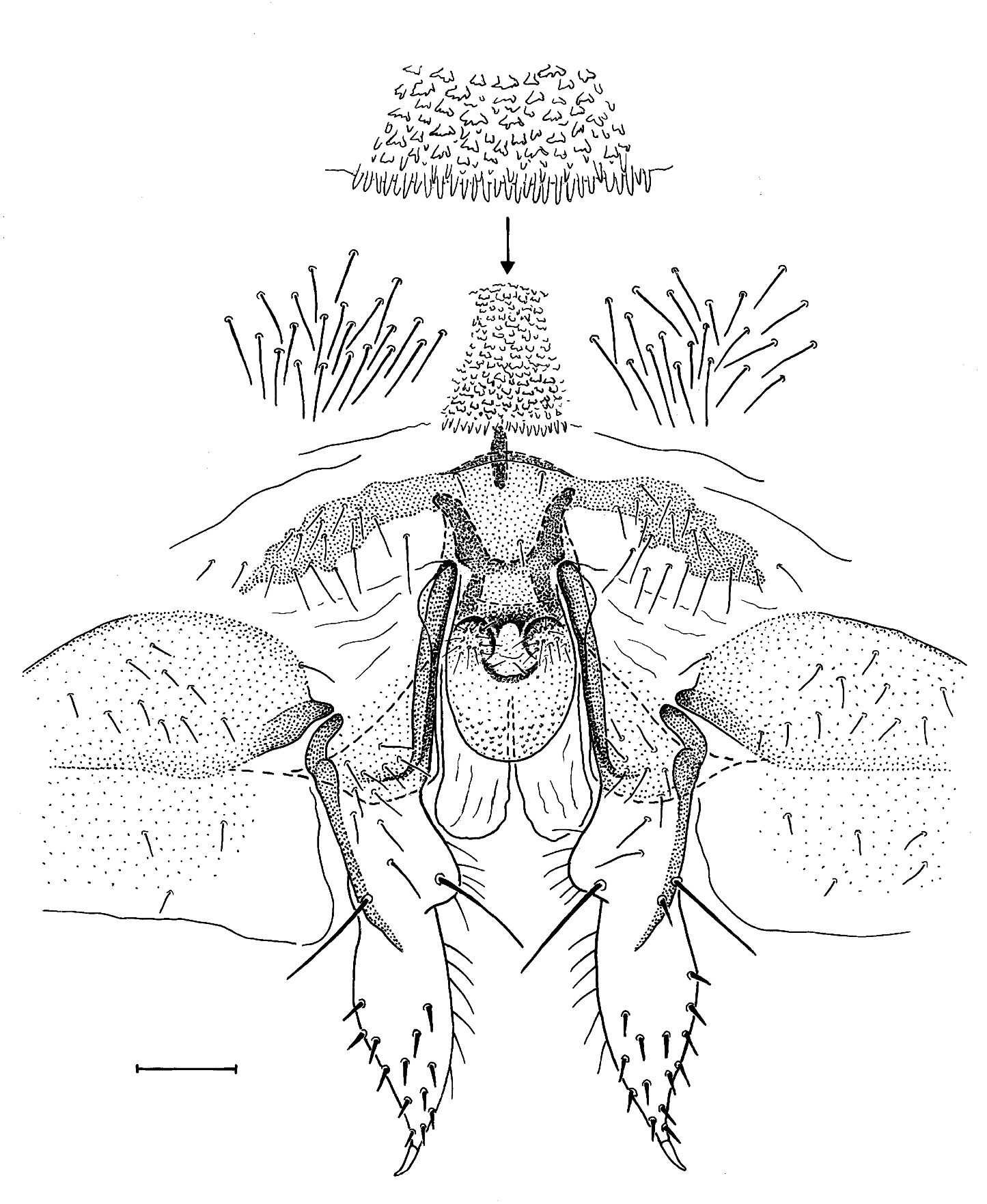
Female terminalia: Epiproct and paraproct as described for N. brasiliensis by Lienhard et al. (2010). Subgenital plate, ovipositor valvulae and ventrolateral parts of clunium as shown in Fig. 2 View FIG . Sclerotized area of anterior part of female subgenital plate with arms forming an almost straight transverse band, in middle not separated by an unpigmented area from posterior part of subgenital plate, on anterior margin medially with a short sclerotized longitudinal rod. Abdominal sternite anterior to subgenital plate medially with a rugose area bearing numerous hyaline papillae of irregular shape, mostly more or less lobate (see detail of Fig. 2 View FIG ). Posterior part of subgenital plate with an ovally rounded median lobe, its apical half bare, its basal half on each side with a small, hemispherical, hairy lobe (clearly visible as a proeminence in non-dissected terminalia, in lateral view), this zone medially with a characteristically bifid, dark brown sclerotization (well visible in non-dissected terminalia, in ventral view). Just dorsally of posterior lobe of subgenital plate, and basally covered by the latter, a pair of longitudinal membranous bulges. The foliaceous external gonapophysis with 13-17 short spine-like setae on ventral surface of apical half and a claw-like apical spine (dense dorsal pilosity not shown in Fig. 2 View FIG , only some internal dorsomarginal setae figured). Gynosome as shown in Fig. 1d, e View FIG (length 670-680 µm, holotype and one paratype examined). Its slightly sclerotized posterior sac strongly ventrad-bent, having roughly the shape of a short and strongly bent sausage, bearing the opening of the spermathecal duct (spermapore) on the inner side of its broadly rounded distal end. In resting position tip of gynosome situated dorsally of posterior lobe of subgenital plate (analogous to the situation shown for N. brasiliensis in Lienhard et al., 2010: fig. 2d). Blister-like zone of gynosome with three posteriad-directed denticulate lobes (i. e. one asymmetrical dorsal lobe directed to the left and a pair of slightly ventrad-directed lateral lobes) and a pair of denticulate ventro-lateral areas (left area visible in Fig. 1d View FIG ). Posterior part of gynosome (i. e. posterior sac and blister-like zone) shorter than its sclerotized anterior rod. Spermathecal duct long and irregularly curled (complete length shown in Fig. 1c, d View FIG combined). Wall of spermathecal sac thin, bearing numerous small pores and a plate with slightly sclerotized wrinkles at origin of spermathecal duct ( Fig. 1c View FIG ). Spermatophores not observed.
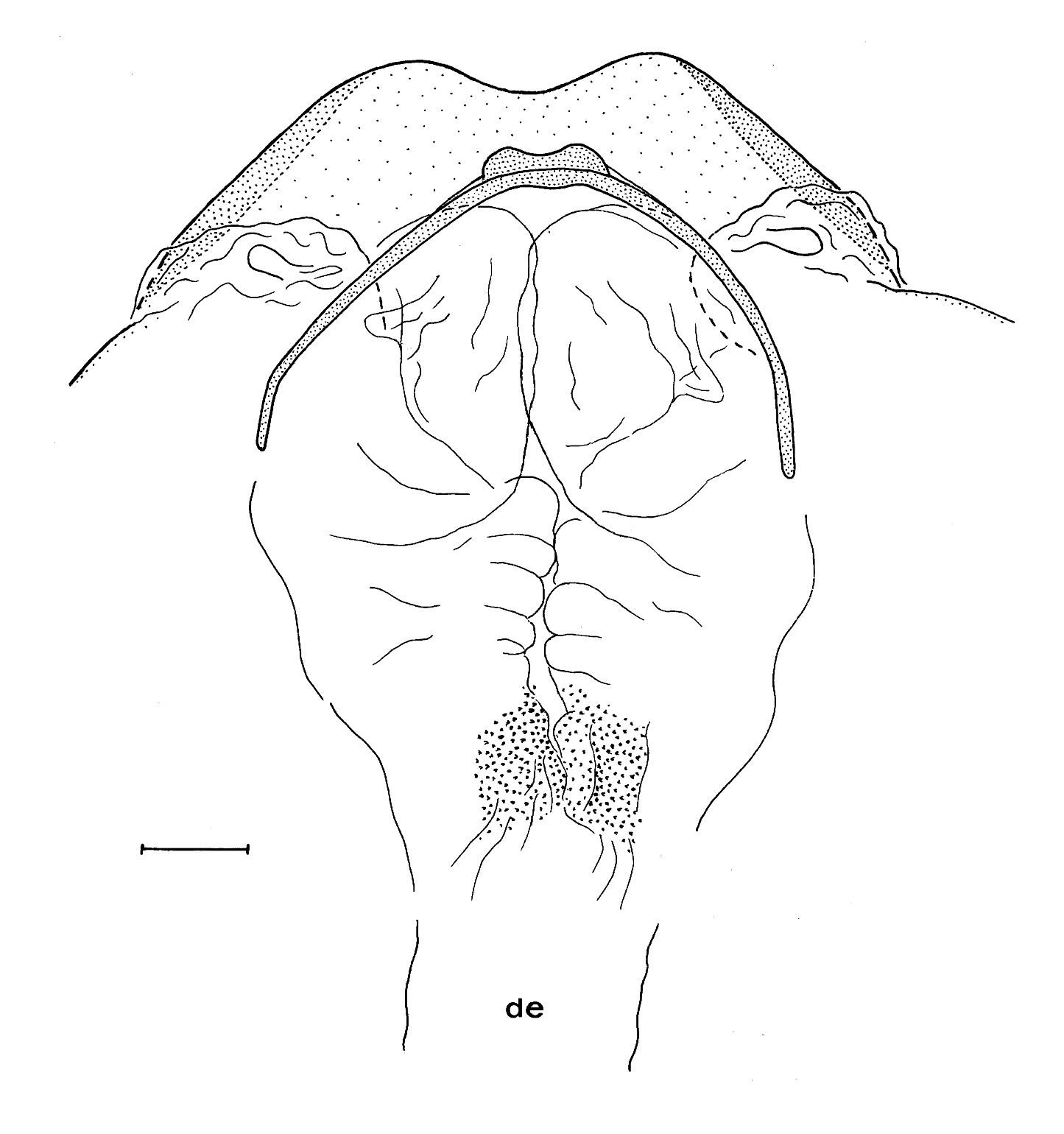
Male terminalia: Epiproct and paraproct as described for N. brasiliensis (see Lienhard et al., 2010), hypandrium with 8th sternite distinctly sclerotized, its hind margin slightly bilobate. Phallosome as in Fig. 3 View FIG ; aedeagal arch apically with a slightly bilobed and weakly rugose thickening; on each side of the aedeagal arch a folded membranous bulge situated dorsally of the postero-lateral hypandrial margin. Endophallus on each side with medially bulging membranous structures; in its anterior half, near the distal end of the ejaculatory duct ( Fig. 3 View FIG : de, ductus ejaculatorius) a relatively wide and short longitudinal membranous channel bearing small sclerotized papillae.
MEASUREMENTS: Female holotype: BL = 3.3 mm; FW = 4.2 mm; HW = 2.2 mm; F = 1080 µm; T = 1620 µm; t1= 707 µm; t2 = 150 µm; t3 = 180 µm; Ant (damaged); IO/ D = 2.0. – Male allotype: BL = 3.5 mm; FW = 4.4 mm; HW = 2.2 mm; F = 1130 µm; T = 1720 µm; t1= 740 µm; t2 = 146 µm; t3 = 183 µm; Ant (damaged; see Discussion, below); IO/D = 2.2.
ETYMOLOGY: The specific epithet (curvatus, -a, -um) refers to the curved posterior part of the gynosome.
DISTRIBUTION AND HABITAT: At present this species is known from seven caves situated in the municipalities of Santa Maria da Vitória (caves PEA 341, 342, 343) and São Félix do Coribe (caves PEA 378, 380, 381, 383) in Bahia State, Brazil. The limestones of the area are part of the Bambuí speleological province (upper Proterozoic). The caves of both municipalities are predominantly small, their length rarely exceeding 250 m. In Santa Maria da Vitória the caves are very small (with a length of 35 m cave PEA 341 is the biggest in this area). In São Félix do Coribe the caves are bigger and more complex, some of them being labyrinthic (cave PEA 383 has almost 300 m). Three other caves in this area were also sampled, but no Neotrogla specimens were found in them. The average temperature in each cave was different during the sampling period (May to July 2011); the highest values were observed in cave PEA 380 (26.27±0.39ºC), the lowest values in cave PEA 378 (21.14±0.78ºC). The average moisture in each cave was also different, with the highest values measured in cave PEA 383 (79.33±6.71% RH) and the lowest in cave PEA 342 (54.80±7.55% RH). Although the caves in which the specimens were observed are slightly different, all of them represent dry oligotrophic systems. The main resource in all caves is bat guano, especially from the insectivorous species Carollia perspicillata (Linnaeus) and Peropteryx macrotis (Wagner) and from the carnivorous species Chrotopterus auritus (Peters) , but some piles of faeces of the rodent Kerodon rupestris (Wied-Neuwied), popularly known as mocó, may also be found in some areas of the caves. The main vegetation type outside the caves is pasture, with some fragmented areas of Caatinga formation. The environment outside the caves is dry and the degree of human impact is quite variable. Furthermore, some caves in which specimens were found are influenced by human activities, as cave PEA 380 (type locality), the entrance of which ( Fig. 4e View FIG ) is used by local residents as a shelter while fishing in the river in front of the cave ( Fig. 4d View FIG ).
BIOLOGY: N. curvata was observed on different substrata inside the caves, mostly in deeper zones, rarely also near the entrance (e. g. in cave PEA 380, the type locality). This distribution pattern is quite different from that of the other three Neotrogla species, which clearly prefer areas close to cave entrance (personal observations by RLF). Adults of N. curvata usually were observed on the cave walls, mostly sheltered in small crevices. In contrast to this, all nymphs were observed on the cave floor, walking on the dry soil or on rocky debris. The nymphs were particularly abundant near organic resources as bat guano ( Fig. 4b View FIG ). Due to the presence of glandular hairs on their dorsal side, especially on abdominal tergites, nymphs were more or less well camouflaged by adherent dust particles. The presence of glandular hairs could also be confirmed in nymphs of N. brasiliensis and N. aurora. Nymphal glandular hairs have never before been observed in the suborder Trogiomorpha (see Lienhard, 1998: 25). Adults and nymphs are probably preyed upon by spiders of the genera Loxosceles Heineken & Lowe (Sicariidae) and Theridion Walkenaer (Theridiidae) , which are frequently encountered in the same caves. A freshly moulted teneral adult was observed being eaten by Theridion sp. in cave PEA 380 in October 2012 ( Fig. 4c View FIG ).
| MHNG |
Museum d'Histoire Naturelle |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.