Sciurus ignitus ( Gray, 1867 )
|
publication ID |
https://doi.org/ 10.1644/915.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03EE8787-110D-D547-FEE7-FC2CFAEC1897 |
|
treatment provided by |
Carolina |
|
scientific name |
Sciurus ignitus ( Gray, 1867 ) |
| status |
|
Sciurus ignitus ( Gray, 1867) View in CoL
Bolivian Squirrel
Macroxus ignitus Gray, 1867:429 . Type locality ‘‘ Bolivia;’’ restricted to ‘‘probably near Yungas, upper Rio Beni,’’ by Allen (1915:204).
Macroxus leucogaster Gray, 1867:430 . Type localities ‘‘ South America , Bolivia, Santa Cruz de la Sierra’ ’ and ‘‘ Brazil .’’
Macroxus irroratus Gray, 1867:431 . Type locality ‘‘ Brazil, Upper Ucayali;’’ restricted to ‘‘from the Ucayali River, probably from near Sarayacu,’’ by Thomas (1899:40).
Sc [iurus]. aestuans Burmeister, 1869:456 View in CoL . Type locality ‘‘Brasilia y la Bolivia vecina. (Sa Cruz de la Sierra).’’
Sciurus aestuans cuscinus Thomas, 1899:40 . Type locality ‘‘ Ocabamba , Cuzco,’’ Peru (see ‘‘Nomenclatural Notes’’).
Sciurus cuscinus: Thomas, 1902:129 . Name combination.
Sciurus cuscinus ochrescens Thomas, 1914:362 . Type locality ‘‘ Bolivia, in upper parts of Beni and Mamoré Rivers. Type from Astillero , 678 W., 168 S. Alt. 2700 m.’’
Leptosciurus ignitus ignitus: Allen, 1915:204 . Name combination (see ‘‘Nomenclatural Notes’’).
Leptosciurus ignitus irroratus: Allen, 1915:206 . Name combination.
Leptosciurus leucogaster: Allen, 1915:207 . Name combination.
Sciurus ignitus: Osgood, 1916:204 View in CoL . First use of current name combination.
Sciurus irroratus ochrescens: Osgood, 1916:204 . Name combination.
Sciurus (Mesociurus) argentinius Thomas, 1921:609 . Type locality ‘‘Higuerilla, 2000 m, in the Department of Valle Grande , about 10 km. east of the Zenta range and 20 km. from the town of Tilcara,’’ Jujuy Province, Argentina.
Sciurus boliviensis Osgood, 1921:39 . Replacement name for Macroxus leucogaster Gray, 1867 (see ‘‘Nomenclatural Notes’’).
Guerlinguetus rufus Moojen, 1942:14 . Type locality ‘‘S. João (cabeceiras do Aripuanã) M. Grosso;’’ restricted to ‘‘ Brasil, en las sierras del noroeste de Mato-Grosso. Localidad típica: São Joao, en las Fuentes del río Aripuana, estado de Matto [Mato]-Grosso,’’ by Cabrera (1961:371). Preoccupied by Sciurus rufus Kerr, 1792 .
Sciurus cabrerai Moojen, 1958:50–51 . Replacement name for Guerlinguetus rufus Moojen, 1942 (see ‘‘Nomenclatural Notes’’).
Sciurus aestuans argentius Anderson, 1985:12 . Incorrect subsequent spelling of Sciurus aestuans argentinius Anderson, 1985 .
CONTEXT AND CONTENT. Order Rodentia View in CoL , suborder Sciuromorpha View in CoL , family Sciuridae View in CoL , subfamily Sciurinae View in CoL , tribe Sciurini View in CoL , genus Sciurus View in CoL , subgenus Guerlinguetus . Dental morphology and molecular evidence support the high-level grouping of sciurids into superfamily Sciuroidea (e.g., Montgelard et al. 2002; Marivaux et al. 2004) and provide some evidence that suborder Sciuromorpha View in CoL comprises polyphyletic units ( Marivaux et al. 2004); however, order Rodentia View in CoL , suborder Sciuromorpha View in CoL is still the most commonly recognized taxonomy (e.g., Thorington and Hoffmann 2005). The genus Sciurus View in CoL contains 28 species (Thorington and Hoffmann 2005). Eight species are recognized within Guerlinguetus : Sciurus aestuans View in CoL , S. gilvigularis View in CoL , S. granatensis View in CoL , S. ignitus View in CoL , S. pucheranii View in CoL , S. richmondi View in CoL , S. sanborni View in CoL , and S. stramineus View in CoL ( Honacki et al. 1982; Thorington and Hoffmann 2005). Five subspecies of S. ignitus View in CoL are currently recognized ( Cabrera 1961; Anderson 1997; Thorington et al. 2012):
S. i. argentinius Thomas, 1921:609 . See above.
S. i. boliviensis Osgood, 1921:39 . See above, aestuans Burmeister and leucogaster Gray are synonyms.
S. i. cabrerai Moojen, 1958:50–51 . See above, rufus Moojen is a synonym.
S. i. ignitus ( Gray, 1867:429) . See above, ochrescens Thomas is a synonym.
S. i. irroratus ( Gray, 1867:431) . See above, cuscinus Thomas is a synonym.
NOMENCLATURAL NOTES. The assignment of South American squirrel specimens to species has been difficult due to variable pelage color patterns, poor representation of certain taxa, and a lack of sufficient documentation or familiarity with the type localities. Assignment to genus and species has changed many times for S. ignitus following successive reviews of New World tree squirrels (e.g., Gray 1867; Thomas 1899, 1902, 1914; Allen 1915; Cabrera 1961) and remains unresolved due to lack of sufficient molecular data (Amori et al. 2011). Assignment of subspecies also warrants further study because currently recognized subspecies are based primarily on geographic locations rather than observable differences or genetic study ( Anderson 1997).
Gray (1867) identified New World tree squirrels possessing postauricular ear tufts as belonging to the genus Macroxus and separated specimens from the currently accepted range of S. ignitus into 3 species of Macroxus : Macroxus ignitus , Macroxus irroratus , and Macroxus leucogaster . Thomas (1899:40) attributed Gray’s (1867) Macroxus irroratus to the Sciurus aestuans group (as per Allen, 1878), naming a new subspecies (S. a. cuscinus ) and suggesting that the type locality of this form was ‘‘probably from near Sarayacu’’ on the Ucayali River, in Peru. In 1902, Thomas named a specimen collected near Cochabamba, Bolivia, as Sciurus cuscinus , however, apparently later ( Thomas 1914:363) decided that the true S. cuscinus type ‘‘occurs in the upper parts of the Ucayali and Madre de Dios Rivers’’ in Peru, which aligns with Gray’s (1867) M. irroratus type locality. Thomas (1914) then named a specimen taken from Astillero, Bolivia, as S. c. ochrescens. Allen’s (1915:199) review of the South American Sciuridae created a genus, Leptosciurus , based on the Sciurus pucheranii group, to which Allen states ‘‘ Sciurus ignitus (¼ ‘‘ cuscinus ’’)’’ must be associated based on pelage color and texture, tooth structure, number of mammae, and tail length. Here, Allen included Gray’s (1867) ignitus , leucogaster , and irroratus types as synonymous with Thomas’s (1914) cuscinus group. Osgood (1916) referred to a specimen from Roquefalda, Bolivia, as Sciurus ignitus , adhering strictly to the original descriptions of Gray’s (1867) ignitus and preferring to keep Thomas’s (1914) cuscinus group separate; Osgood named a 2nd specimen from the Chaparé River ( Bolivia) as S. irroratus ochrescens . Sanborn (1951), Moojen (1958), Cabrera (1961), Anderson (1997), and Thorington et al. (2012) applied the genus Sciurus (instead of Leptosciurus ) with various types subsumed into the ignitus group, with 3 subspecies occurring in Bolivia ( ignitus , irroratus , and boliviensis , and also possibly argentinius ), 2 in Peru ( irroratus and ignitus ), 1 in Argentina ( argentinius ), and 2 in Brazil ( cabrerai and irroratus ). S. i. cabrerai is based on only 1 specimen, in poor condition without an associated skull. However, Cabrera (1961) decided to recognize this subspecies, because the original description is similar to characteristics of the ignitus group. Sciurus boliviensis ( Osgood, 1921) is a replacement name for Macroxus leucogaster ( Gray, 1867) and no type locality is given. However, the subspecies S. i. boliviensis was restricted by Sanborn (1951:18–19) to ‘‘Sarayacu, on the Ucayli River south to Santa Cruz de la Sierra, Bolvia. In Peru specimens have been taken in Chanchamayo, Junin; Pucallpa, Loreto; Agua Caliente and Pozuzo, Huanuco; Ocabamba, Hacienda Cadena, Quincemil, and Huajyumbe, Cuzco.’’ Anderson (1997) identified Sciurus aestuans Burmeister, 1869 , as a synonym of S. i. boliviensis , presumably based on the stated type locality, which is out of the known range for S. aestuans .
The genus name Sciurus is from Greek skiouros, from skia meaning shadow and oura meaning tail. The specific epithet ignitus means to ignite or make red hot in Latin ( Gotch 1996). Common names include antara ( Thomas 1902); la ardilla colorada (Pinazo and Gasparri 2003); ardilla gris (Schulte-Herbrüggen and Rossiter 2003); ardilla ígnia ( Pacheco et al. 2009); ardilla roja ( Mares et al. 1989; Emmons and Feer 1999); Bolivian squirrel (Emmons and Feer 1999; Wilson and Cole 2000); masi chico ( Fernández et al. 1963); nuecero (Cassini and Guichón 2009); kapampi (Matses—Fleck and Voss 2006); kapa nakush, mentsud (Matis), tsanka kapa, kasu (Marubo), and kapa kudu (Katukina—Dienst and Fleck 2009); and ouatipuru roxo (Portuguese— Calouro 1999).
DIAGNOSIS
Microsciurus (dwarf squirrels) are smaller in size (mean body mass ¼ 60.0– 120 g; length of hind foot ¼ 32.0–43.0 mm) compared to S. ignitus (mean body mass ¼ 183.0–242.0 g [ Emmons 1984; Anderson 1997; Emmons and Feer 1999; Peres 2001; Haugaasen and Peres 2005; Hayssen 2008]; length of hind foot ¼ 41.0–52.0 mm [ Anderson 1997; Eisenberg and Redford 1999; Emmons and Feer 1999]). In Microsciurus , the ears (the auricle does not extend above the crown) and tail (length of tail ¼ 92.0–150.0 mm) are shorter compared to S. ignitus (ear length ¼ 20.0–30.0 mm; tail length ¼ 150.0–230.0 mm [ Anderson 1997; Eisenberg and Redford 1999; Emmons and Feer 1999]). The distribution of Sanborn’s squirrel ( S. sanborni ) overlaps with that of S. ignitus in the department of Madre de Dios, Peru, near the border with Bolivia ( Thorington et al. 2012), but it is somewhat smaller in size (length of hind foot ¼ 44.0–50.0 mm; length of head and body 152.0–175.0 mm; length of tail 164.0–184.0 mm) compared to S. ignitus (length of head and body ¼ 140.0–220.0 mm [ Anderson 1997; Emmons and Feer 1999]). Feet of S. sanborni are paler in color, upperparts sharply contrast pale underparts including the inner thighs, and it has a bright buff eye-ring (Emmons and Feer 1999; Thorington et al. 2012) compared to the pale buffyochraceous eye-ring and less sharply contrasting inguinal region in S. ignitus (Emmons and Feer 1999; Cassini and Guichón 2009). The Junin red squirrel ( S. pyrrhinus ) overlaps with S. ignitus along the eastern slopes of the Andes in Peru. S. pyrrhinus is larger in size (length of head and body ¼ 240–280 mm; length of tail ¼ 208–240 mm) compared to S. ignitus , with dark red dorsal pelage, sometimes with flecks of white, and a tail that is orangish red at the tip and more chestnut to black toward the base ( Thorington et al. 2012), compared to the darker olivaceous dorsal pelage flecked with yellow and black, and tail flecked with yellow and red in S. ignitus ( Gray 1867) . The Guianan squirrel ( S. aestuans ) is somewhat smaller in size (mean body mass ¼ 159–218 g; length of hind foot ¼ 42–50 mm) and darker in color compared to S. ignitus , but likely indistinguishable in the field (Emmons and Feer 1999); however, the range of S. aestuans does not appear to overlap with that of S. ignitus in Brazil, which is restricted to the eastern Amazon Basin ( Thorington et al. 2012). Pallas’s squirrel ( Callosciurus erythraeus ), introduced to Argentina from Southeast Asia, could potentially overlap with S. ignitus in northern Argentina where a new center of invasion for C. erythraeus has been identified in Córdoba Province (Cassini and Guichón 2009; Lurz et al. 2013). Pallas’s squirrel is larger (measurements from specimens from Argentina [mean – SD] include: body mass ¼ 260.1 – 36.7 g; length of head and body ¼ 201.2 – 12.8 mm; length of tail ¼ 187.7 – 17.2 mm), has smaller ears (length of ear ¼ 16.1 – 2.3 mm), and fewer mammae (2 pairs) compared to S. ignitus (3 pairs) and can be distinguished by a lack of an eye-ring, reddish venter (venter color can vary from dark maroon to creamy buff) and olive agouti-brown dorsum (Cassini and Guichón 2009; Lurz et al. 2013).
GENERAL CHARACTERS
The fur of Sciurus ignitus is short and soft, often dark olive, punctuated with black and yellow ( Fig. 1 View Fig ; Gray 1867). The underparts of the chin, throat, and chest are well furred, buff to white colored, and washed with pale buff ( Gray 1867; Emmons and Feer 1999). The pale color of chest is usually grizzled with gray posteriorly to grayish inner thighs and inguinal region that is not sharply contrasting with the sides. The venter and inner thighs also may be whitish (Emmons and Feer 1999). The inner sides of the limbs are reddish to yellow gray ( Gray 1867). In Bolivia the venter ranges from reddish or ochraceous to white ( Anderson 1997). The upperparts and slender tail are uniform brownolivaceous with finely grizzled hairs tipped with yellow ( Fig. 2 View Fig ; Gray 1867; Emmons and Feer 1999). The tail is grizzled at the base, black in the proximal ventral surface, and hairs on the dorsal surface are tipped with yellow and red often with 2 black rings; the long, outer hairs of the tail often have long red-bay tips ( Gray 1867). An indistinct pale buffy-ochraceous eye-ring is present (Emmons and Feer 1999; Cassini and Guichón 2009). The feet are the same color as the upperparts and tail. The ears extend above the crown of the head and postauricular buff- or bay-colored patches are prominent ( Fig. 1 View Fig ; Gray 1867; Emmons and Feer 1999). Although color variations have been used for resolving various proposed subspecies (e.g., Thomas 1914; Sanborn 1951), the differences are not clearly geographic and do not pertain to subspecies in a consistent manner, particularly in Bolivia ( Anderson 1997).
Body mass can range from 183.0 to 242.0 g ( Emmons 1984; Anderson 1997; Emmons and Feer 1999; Peres 2001; Haugaasen and Peres 2005; Hayssen 2008). Total length of adult S. ignitus varies from 315.0 to 450.0 mm ( Anderson 1997). Head and body length of adult S. ignitus ranges from 140.0 to 220.0 mm ( Anderson 1997; Emmons and Feer 1999) and the mean is less in adult females compared to males (female ¼ 184.8 mm; male ¼ 191.0 mm— Hayssen 2008). Tail length in S. ignitus ranges from 150.0–230.0 mm ( Anderson 1997; Eisenberg and Redford 1999; Emmons and Feer 1999) with mean tail lengths of adult males and females similar (female ¼ 191.9 mm; male ¼ 190.8 mm — Hayssen 2008). Hind-foot length in S. ignitus ranges from 41.0 to 52.0 mm and ear length varies from 20.0 to 30.0 mm ( Anderson 1997; Eisenberg and Redford 1999; Emmons and Feer 1999).
Cranial features ( Fig. 3 View Fig ) include 1 complete and 1 incomplete septa in the auditory bullae (Cassini and Guichón 2009). Mean cranial measurements of 10 S. ignitus deposited in natural history museums in Argentina (mm – SD, range in parentheses) included: maximum cranial length, 50.1 – 1.1 (48.1–52.1); zygomatic breadth, 28.9 – 0.8 (27.3–29.7); length of palate, 21.9 – 0.8 (20.0–22.7); length of diastema, 12.9 – 0.4 (12.3–13.6); interorbital breadth, 16.9 – 0.7 (16.0–18.4); length of nasals, 14.0 – 0.7 (12.6–15.0); length of maxillary toothrow, 8.4 – 0.4 (8.1– 9.5—Cassini and Guichón 2009). Four specimens of S. i. irroratus from Inca Mines, Peru, had the following mean measurements (mm, range in parentheses): occipitonasal length, 45.0 (44.5–46.0); zygomatic breadth, 26.5 (26.0– 27.0); interorbital breadth, 14.5 (13.0–15.0); breadth of braincase, 20.9 (20.5–21.0); length of nasals, 12.6 (12.0– 13.3); length of diastema, 11.2 (10.2–11.5); length of maxillary toothrow, 6.9 (6.5–7.0— Allen 1915). One adult female skull from Bolivia had the following measurements (mm): occipitonasal length, 48.0; zygomatic breadth, 28.0; Argentina ( Barquez et al. 2006). The range of S. ignitus does not extend beyond 258S ( Ojeda et al. 2008). No fossils of S. ignitus are known.
interorbital breadth, 15.3; postorbital breadth, 17.0; breadth of braincase, 21.0; length of nasals, 14.0; length of diastema, 12.0; and length of maxillary toothrow, 8.0 ( Allen 1915). In Bolivia, 9 males and 2 females had the following measurements (mm, range): occipitonasal length, 43.7–50.8; interorbital breadth, 12.8–16.5; postorbital breadth, 15.7–17.9; breadth of braincase, 18.1–20.2; maxillary toothrow length, 7.2–8.6; dental span, 10.6–11.8 ( Anderson 1997).
DISTRIBUTION
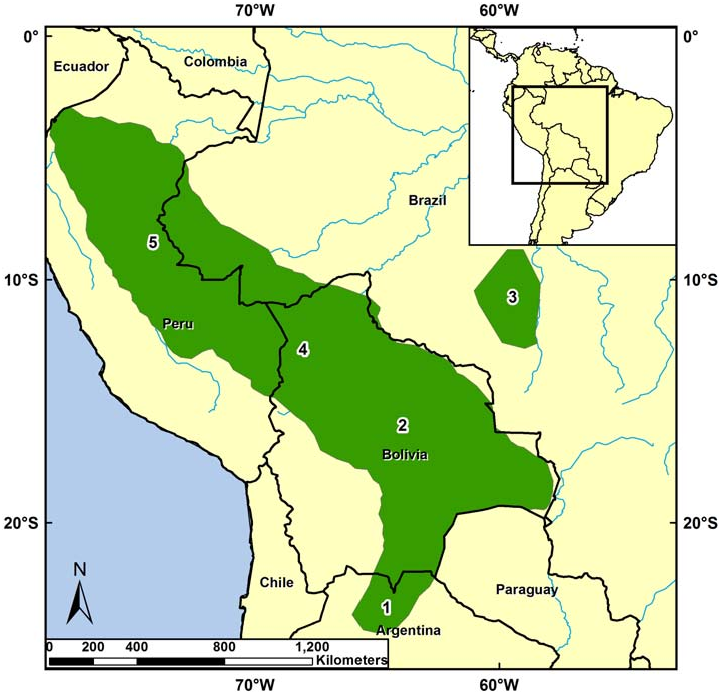
Sciurus ignitus occurs in South America along the eastern Andes cordillera in Peru, Bolivia, and extreme northern Argentina and eastward into the Amazon Basin of western Brazil and eastern Peru including Cuzco Amazónico, Manu, Balta, Cordillera de Vilcabamba, and Cocha Cashu ( Fig. 4 View Fig ; Emmons 1984; Mares et al. 1989; Emmons and Feer 1999; Medellín 1994; Woodman et al. 1996; Alonso et al. 2001; Lim and Engstrom 2005; Thorington and Hoffmann 2005). It occurs between 300 and 600 m in the Manu Biosphere Reserve in Peru ( Solari et al. 2006). S. ignitus also occurs in the Bosque Experimental Elías Menses, Santa Cruz, Bolivia (Acosta and Aguanta 2006), Parque Nacional da Serra do Divisor, Brazil ( Calouro 1999), western Mato Grosso, Brazil ( Moojen 1942; Cabrera 1961), and Finca las Capillas, Jujuy Province,
FORM AND FUNCTION
Sciurus ignitus has plantigrade locomotion. It has 3 pairs of mammae ( Allen 1915; Emmons and Feer 1999). The dental formula for S. ignitus is i 1/1, c 0/0, p 1/1, m 3/3, total 20 ( Allen 1915; Emmons and Feer 1999).
The baculum is small with dextral chirality and a prominent dorsal crest ( Didier 1955). The baculum hatchet is smaller than in other South American sciurid species and is as high as it is wide with a margin twisted 3 times ( Didier 1955). The proximal extremity of the baculum is narrow and has an irregular posterior outline. Mean baculum measurements (mm, parenthetical ranges) from 7 Peruvian specimens were: length, 7.6 (7.3–8.0); height of proximal extremity, 2.0 (1.8–2.0); height of hatchet, 1.9 (1.8–2.0); mean width, 0.9 (0.8–1.0— Didier 1955).
Average lever arm lengths, pertinent to closing mechanics of the mandible, measured (mm) as line distances from the posterior of the condyle to the following points: tip of coronoid process to proximal end of temporalis insertion, 7.7; base of coronoid process to distal end of temporalis insertion, 15.9; posterolateral corner of angular process to posterior end of the superficial masseter insertion, 9.8; medioventral corner of angular process to ventral end of superficial masseter insertion, 14.7; anterior end of the anterior deep masseter insertion, 21.4; tip of the incisor, 33.0; distal end of the 1st molar, 19.4 (Swiderski and Zelditch 2010).
ECOLOGY
The habitat preferences and ecology of Sciurus ignitus are poorly known ( Amori et al. 2008). S. ignitus occupies the understory and subcanopy of disturbed and mature lowland and montane rain forest (Emmons and Feer 1999) from 200- m-elevation lowland tropical rain forest to the transitional zone between dry and humid tropical forest ( Woodman et al. 1996; Haugaasen and Peres 2005) up to 2,700 m in elevation ( Allen 1915). S. ignitus is relatively common along riversides in dense, liana vegetation and uses the understory and subcanopy forest strata (Haugaasen and Peres 2005). In Peru, S. ignitus occurs in Yungas, Selva Baja, and Sabana de Palmera vegetation associations ( Pacheco et al. 2009) and the species is relatively abundant in the Cordillera de Vilcabamba, which consists primarily of premontane moist tropical forest and moist tropical forest ( Alonso et al. 2001). In Peru’s Madre de Dios Basin, S. ignitus is a common arboreal mammal in lowland rain forest ( Woodman et al. 1991), and is frequently observed along the banks of the Las Piedras River (Hammer and Tatum-Hume 2003; Schulte- Herbrüggen and Rossiter 2003). In the Madre de Dios Basin along the Las Piedras River, S. ignitus was observed along a 2.8-km survey transect leading up to a colpa (area used by many species for geophagy, or soil consumption) but was not observed in the colpa consuming soil (Hammer and Tatum-Hume 2003). Mean abundance of S. ignitus was lower (0.17 animals/ 10 km surveyed) in the vicinity of active illegal logging camps along the Las Piedras River, compared to abandoned logging camps inactive for at least 3 years (0.23 animals/ 10 km surveyed), with a 36.8% increase in observed abundance at inactive sites. In both cases, S. ignitus was less abundant than S. pyrrhinus (120% and 240% less abundant at active and inactive logging camps, respectively [Schulte-Herbrüggen and Rossiter 2003]).
Sciurus ignitus is diurnal, usually solitary, and constructs round drey nests of green leaves and twigs (Emmons and Feer 1999). These nests are hidden in subcanopies of palm or dense vine tangles about 6–10 m off the ground (Emmons and Feer 1999). Two females collected in August in Bolivia each had 2 embryos ( Anderson 1997). A juvenile was captured in an arboreal trap during the dry season (June– July— Woodman et al. 1995).
Sciurus ignitus is an omnivorous seed predator and frugivore (Emmons and Feer 1999, Haugaasen and Peres 2005) and primarily feeds on nuts, mushrooms, fruits, and insects (Emmons and Feer 1999). S. ignitus may play an important role in seed dispersal and regeneration of Juglans australis following selective logging (Pinazo and Gasparri 2003). S. ignitus is known to feed on cocoa and corn in Peru ( Aguilar et al. 1977), suggesting it may be considered an agricultural pest.
Hoplopleura sciuricola is a sucking lice species that is a common parasite of S. ignitus ( Ferris 1921; Durden and Musser 1994; Smith et al. 2008). The parasitic mite Bdellonyssus vitzthumi ( Macronyssidae — Fonseca 1959) and the flea Polygenis bohlsi bohlsi ( Smit 1987) also are ectoparasites of S. ignitus .
GENETICS
Chromosomal data for Sciurus ignitus are deficient. The majority of arboreal squirrels in the genus Sciurus possess the same diploid number of chromosomes (2n ¼ 40— Fagundes et al. 2003); however, some chromosomal variation occurs in South American arboreal squirrels (e.g., red-tailed squirrel [ Sciurus granatensis ], 2n ¼ 42 [Nadler and Hoffmann 1970]; Sciurus aestuans ingrami , 2n ¼ 40; and southern Amazon red squirrel [ Sciurus spadiceus ], 2n ¼ 40 [ Fagundes et al. 2003]). Molecular studies show the S. ignitus taxon is problematic and likely represents a species complex (Amori et al. 2011). Phylogenetic analysis of 2 nuclear genes, proto-oncogene c-myc and recombination activating gene 1 (RAG1), indicates that S. ignitus is most closely related to the Guayaquil squirrel ( Sciurus stramineus ) and the Amazon dwarf squirrel ( Microsciurus flaviventer — Steppan et al. 2004).
CONSERVATION
Sciurus ignitus has been assigned to the ‘‘Data Deficient’’ category by the International Union for Conservation of Nature and Natural Resources Red List of Threatened Species (Amori et al. 2011). S. ignitus is not a preferred target of hunters and is most often ignored (Peres and Lake 2003; Schulte-Herbrüggen and Rossiter 2003; Fleck and Voss 2006); however, this species is sometimes maintained as a pet by local residents (Dienst and Fleck 2009).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sciurus ignitus ( Gray, 1867 )
| Merrick, Melissa J., Ketcham, Shari L. & Koprowski Abstract, John L. 2014 |
Sciurus aestuans argentius
| ANDERSON, S 1985: 12 |
Sciurus cabrerai
| MOOJEN, J 1958: 51 |
Guerlinguetus rufus
| CABRERA, A 1961: 371 |
| MOOJEN, J 1942: 14 |
Sciurus (Mesociurus) argentinius
| THOMAS, O 1921: 609 |
Sciurus boliviensis
| OSGOOD, W 1921: 39 |
Sciurus ignitus: Osgood, 1916:204
| OSGOOD, W 1916: 204 |
Sciurus irroratus ochrescens:
| OSGOOD, W 1916: 204 |
Leptosciurus ignitus ignitus:
| ALLEN, J 1915: 204 |
Leptosciurus ignitus irroratus:
| ALLEN, J 1915: 206 |
Leptosciurus leucogaster:
| ALLEN, J 1915: 207 |
Sciurus cuscinus ochrescens
| THOMAS, O 1914: 362 |
Sciurus cuscinus: Thomas, 1902:129
| THOMAS, O 1902: 129 |
Sciurus aestuans cuscinus
| THOMAS, O 1899: 40 |
Macroxus ignitus
| ALLEN, J 1915: 204 |
| GRAY, J 1867: 429 |
Macroxus leucogaster
| GRAY, J 1867: 430 |
Macroxus irroratus
| THOMAS, O 1899: 40 |
| GRAY, J 1867: 431 |