Macrobothriotaenia ficta (Meggitt, 1931) Freze, 1965
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3640.3.12 |
|
publication LSID |
lsid:zoobank.org:pub:525F222F-057A-4921-9C0D-A8CFD49DA988 |
|
DOI |
https://doi.org/10.5281/zenodo.6165229 |
|
persistent identifier |
https://treatment.plazi.org/id/03E1485B-2858-4724-FF33-D0236576FEC3 |
|
treatment provided by |
Plazi |
|
scientific name |
Macrobothriotaenia ficta (Meggitt, 1931) Freze, 1965 |
| status |
|
Redescription of Macrobothriotaenia ficta (Meggitt, 1931) Freze, 1965
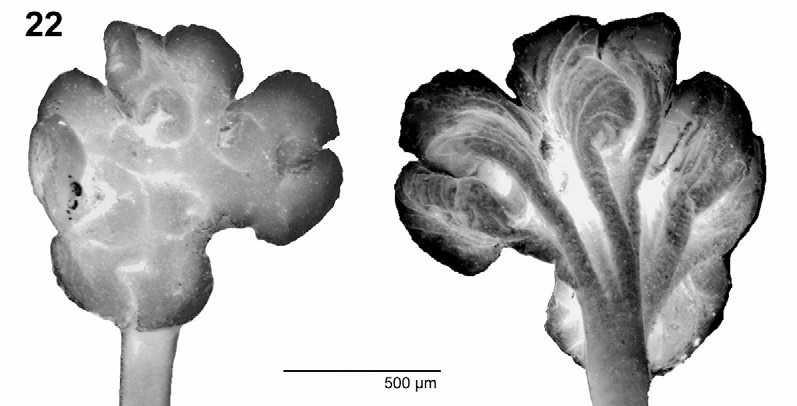
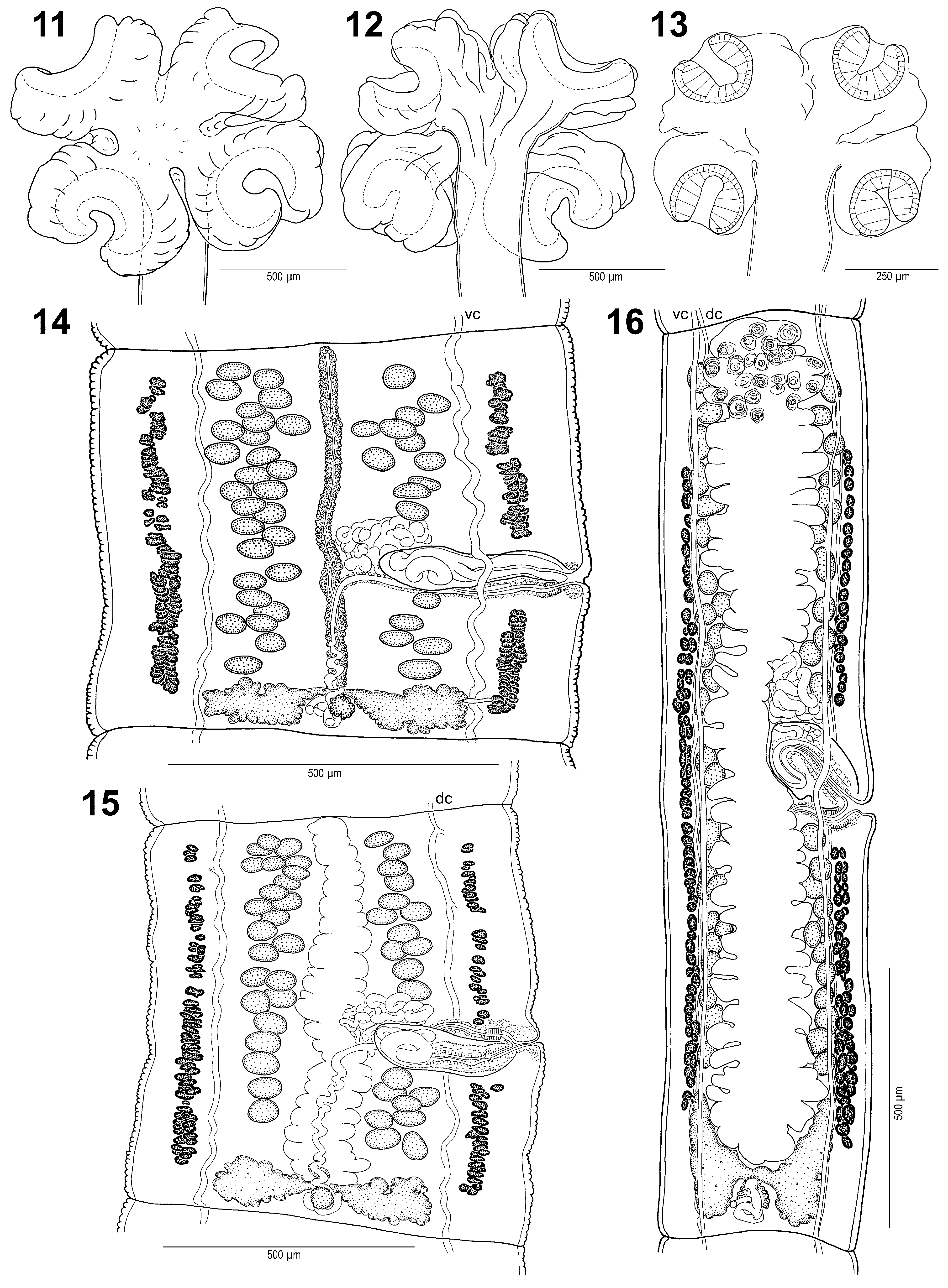
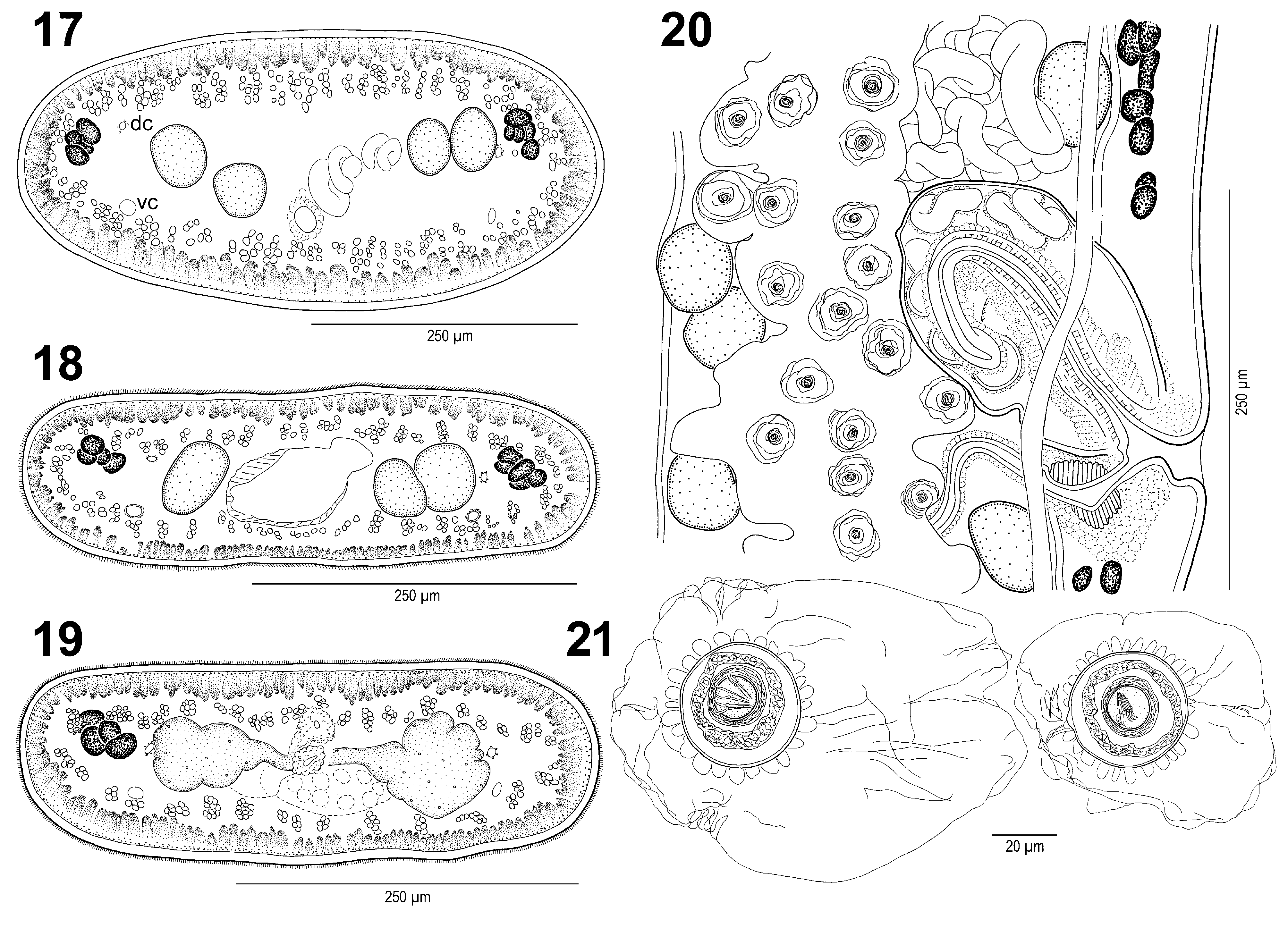
( Figs. 1–22 View FIGURES 1 – 10 View FIGURES 11 – 16 View FIGURES 17 – 21 View FIGURE 22 )
Syns: Crepidobothrium fictum Meggitt, 1931 ; Proteocephalus fictus (Meggitt, 1931) Hughes, Baker & Dawson, 1941
Type and only host: Xenopeltis unicolor Reinwardt in Boie, 1827 (Ophidia: Xenopeltidae ).
Type locality: Rangoon (also Yangon), Burma (also Republic of the Union of Myanmar).
Type material: BMNH 1932.3.3.33.
Site of infection: Intestine.
Prevalence: 17% (n = 6) in Hanoi, North Vietnam, 21.x.2010; other data not available.
Intensity of infection: one specimen found in X. unicolor from Hanoi, North Vietnam, 21. x. 2012; other data not available.
Distribution: Indomalayan Region (South East Asia – Burma / Myanmar, Thailand, Vietnam).
Re-description (based on 1 mature, newly collected specimen from Vietnam – MHNG-PLAT 75454, IPCAS C-584 and 2 mature specimens from Thailand – BMNH 1976.4.13.14–15; measurements from the original description by Meggitt [1931] in square brackets): Proteocephalidae, Proteocephalinae. Testes , ovary, vitelline follicles and uterus medullary. Strobila acraspedote, with first immature proglottides much wider than long, last immature proglottides rectangular to slightly longer than wide, mature proglottides rectangular to longer than wide, pregravid and pregravid proglottides much longer than wide. Total length 51–62 mm (n = 3) [50 mm], maximum width 680 (n = 19) [800].
Proliferative zone (neck) narrow, 600–900 long and 165–420 wide (n = 3). Strobila consists of 132–220 proglottides: 102–183 immature (up to appearance of spermatozoa in vas deferens), 11–33 mature (up to appearance of eggs in uterus), 3–5 pregravid (up to appearance of hooks in oncospheres) and 13 gravid.
Scolex highly modified, short and wide, 525–1290 long and 720–1685 wide (n = 4) [790–1000 wide, 590–830 dorso-ventrally], much wider than neck (n = 4), formed by 4 lobes (peduncles), each 400–775 wide, separated from each other by deep grooves ( Figs. 1, 2 View FIGURES 1 – 10 , 11–13 View FIGURES 11 – 16 , 22 View FIGURE 22 ). Each lobe contains unilocular, pincer-shaped sucker, 290–420 in diameter (n = 8), situated within cavity of each lobe, opening outside posterolaterally ( Figs. 1–3 View FIGURES 1 – 10 , 11–13 View FIGURES 11 – 16 ). Apical organ not observed, probably absent. Apical surface of scolex and upper non-adherent surface of suckers covered with long capilliform filitriches ( Figs. 5, 6 View FIGURES 1 – 10 ). Luminal surface of suckers covered with capilliform filitriches and few gladiate spinitriches ( Fig. 7 View FIGURES 1 – 10 ). Non-adherent surface of suckers covered with long capilliform filitriches and few gladiate spinitriches ( Fig. 8 View FIGURES 1 – 10 ). Posterior part of suckers and proliferation zone covered with long gladiate spinitriches ( Figs. 9, 10 View FIGURES 1 – 10 ).
Longitudinal internal musculature well developed, formed by wide bundles of muscle fibres ( Figs. 17–19 View FIGURES 17 – 21 ). Ventral osmoregulatory canals thin-walled, wide, with secondary canals ( Fig. 15 View FIGURES 11 – 16 ); dorsal osmoregulatory canal thickwalled ( Fig. 17–19 View FIGURES 17 – 21 ); canals situated between testes and vitelline follicles, sometimes overlapping them ( Fig. 20 View FIGURES 17 – 21 ).
Testes medullary, oval, 45–65 long and 30–55 wide (n = 19) [43–59 in diameter], relatively few, 43–63 in number (x = 51, n = 30, CV = 10%) [51–61], in 1 layer, forming 3 well separated fields separated by uterine stem and terminal genitalia ( Figs. 14–16 View FIGURES 11 – 16 ): aporal field composed of 20–31 testes (n = 30) [28–31, but illustrated with as many as 35 on aporal side – Fig. 6 View FIGURES 1 – 10 in Meggitt 1931], preporal field with 10–21 testes and postporal fields with 5–14 testes, both on poral side [23–30 on poral side]. Testes present also in last gravid proglottides ( Fig. 16 View FIGURES 11 – 16 ).
Vas deferens strongly coiled, voluminous, reaching midline of proglottides, but not crossing it aporally ( Figs 14, 15 View FIGURES 11 – 16 ). Cirrus-sac elongate, thin-walled, long, 410–680 long [21–23 (?); see remarks below] and 75–130 [12–17? see remarks below] wide; length/width ratio 32–68% (n = 17); cirrus-sac may reach midline of proglottides, length of cirrus-sac representing 34–48% of width of proglottides (x = 40%, n = 19) [33%]. Cirrus muscular, unarmed, occupies 1/2 to 4/5 of cirrus-sac. Ejaculatory duct coiled ( Fig. 20 View FIGURES 17 – 21 ).
Genital pore irregularly alternating, postequatorial, situated at 44–62% (x = 52%, n = 16, CV = 9%) of proglottis length from anterior margin. Genital atrium narrow and deep ( Fig. 14–16 View FIGURES 11 – 16 ).
Ovary bilobed, medullary, follicular, relatively short, length of ovary representing 10–19% of length of proglottides (n = 30). Mehlis’ gland about 40–50 in diameter, representing 6–12% of proglottis width. Relative ovarian size (percentage of ovarian size in relation to that of entire proglottis – see de Chambrier et al. 2012 for details) 4.1% of proglottis size.
Proximal (preovarian) part of vaginal canal directed anteriorly, slightly sinuous, turned almost perpendicularly to proglottis axis at level of genital pore ( Figs. 14, 15 View FIGURES 11 – 16 ); distal (terminal) part of vagina near genital pore with thickened wall, surrounded by large, elongate vaginal sphincter; 30–40 long and 25–30 wide ( Fig. 20 View FIGURES 17 – 21 ). Vagina posterior in 63% (n = 43) to cirrus-sac, anterior in 37%.
Vitelline follicles medullary, few in number, small, 20–30 long and 10–20 wide, form 2 narrow bands on dorsal side of lateral margins ( Figs. 14–19 View FIGURES 11 – 16 View FIGURES 17 – 21 ); band on poral sides separated by terminal genitalia to preporal and postporal group ( Figs. 14–16 View FIGURES 11 – 16 , 20 View FIGURES 17 – 21 ). Bands slightly widening towards posterior margin of proglottides, with more follicles posteriorly, never reaching their anterior and posterior margins (postporal follicles reach anterior margin of ovary or its middle part); length of bands represents only 63–91% and 72–92% of length of proglottides on poral and aporal side, respectively (n = 13; Figs. 14–16 View FIGURES 11 – 16 ).
Primordium of uterine stem medullary, already present in immature proglottides. Uterus medullary, with development of type 1 as defined by de Chambrier et al. (2004). Briefly, uterine stem present as undifferentiated longitudinal median concentration of chromophilic cells in immature proglottides. In mature proglottides, uterine stem straight, occupying almost entire length of proglottides ( Fig. 14 View FIGURES 11 – 16 ), reaching anterior margin, but not posterior margin, never crossing posteriorly ovarian isthmus. Lumen appears in first mature proglottides. Diverticula (lateral branches) formed when eggs appear in uterus. In pregravid proglottides, eggs mature quickly. Pregravid and gravid proglottides with 26–37 diverticula on each side (n = 12) [31–35]; diverticula occupy most space (63–70 % of proglottis width) in last gravid proglottides ( Fig. 16 View FIGURES 11 – 16 ).
Eggs spherical, with three-layered embryophore and rounded digitiform projections on its external surface. Hyaline outer envelope (collapsed in whole mounts) 80–120 in diameter (n = 8); thick, round embryophore consisting of 3 layers; outer layer 33–39 (n = 8) in diameter, thicker than nuclei-containing envelope; oncospheres spherical, 12–14 wide (n =8), with 6 hooklets 8–11 (n = 14) long ( Fig. 21 View FIGURES 17 – 21 ).
Remarks: The original description of Macrobothriotaenia ficta (as Crepidobothrium fictum ) was brief and contained only basic information on its morphology and very few measurements (Meggitt 1931). The scolex, the morphology of which distinguishes M. ficta from all other proteocephalidean tapeworms, was described just: “Scolex ... provided with four suckers sunk within four spherical lobes nearly constricted off from the true scolex”. Although Meggitt (1931) admitted in the description of C. fictum that “This type of the scolex approximates more to the Phyllobothrium (....) type than to the Crepidobothrium ”, he placed his new species in the latter genus. The original description contained only 2 relatively simple figures of the scolex and a (probably mature) proglottis, the latter with an incorrect scale bar (0.05 mm instead of 0.5 mm). Gravid proglottides, terminal genitalia (cirrus-sac and the distal part of the vaginal canal), eggs and cross sections were not illustrated. However, both figures of Meggitt (1931) provided the information sufficient for characterization of the new species, which markedly differs from other proteocephalidean cestodes in its scolex morphology (Rego 1994). Based on the peculiar shape of the scolex, with suckers lying on four protruding parts of the scolex, Freze (1965) proposed a new genus, Macrobothriotaenia , to accommodate Meggitt’s (1931) species.
In addition, M. ficta possesses other distinctive morphological characteristics, such as the dorsal position of the vitelline follicles, eggs with a three-layered embryophore covered with short, rounded projections, the postequatorial position of the genital pore, the extraordinarily long cirrus-sac, which can reach up to the midline of the proglottides, the fairly low number of testes (less than 65), etc. Based on the new information provided in this paper, the generic diagnosis of Macrobothriotaenia is amended:
Macrobothriotaenia Freze, 1965 : Proteocephalidae, Proteocephalinae. Worms of medium size (5–6 cm long); strobila acraspedote, proglottides variable in shape, from short and wide to rectangular to much longer than wide in mature, pregravid and gravid proglottides. Scolex highly modified, much wider than neck, formed by four lobes (peduncles), separated by deep grooves, bearing unilocular, pincer-shaped suckers opening outside posterolaterally. Internal longitudinal musculature well developed. Testes medullary, few in number, in 3 separate lateral fields (aporal, preporal and postporal), in 1 layer, present also in last gravid proglottides. Cirrus-sac elongate, large, may reach mid-line of proglottis. Vagina posterior to cirrus-sac, occasionally anterior. Vaginal sphincter present. Genital pores irregularly alternating, postequatorial. Ovary medullary, follicular, short. Vitelline follicles not numerous, forming two narrow lateral bands on dorsal side of proglottides, not reaching to their anterior and posterior margins; bands slightly widening posteriorly; poral band interrupted by terminal genitalia (cirrus-sac and vagina). Formation of uterus of type 1 (see de Chambrier et al. 2004). Eggs with three-layered embryophore and short, rounded projections on its external surface. Parasites of snakes of the family Xenopeltidae in South East Asia. Type and only species Macrobothriotaenia ficta (Meggitt, 1931) Freze, 1965 .
Molecular data and phylogeny
New sequences for proteocephalidean taxa used in this study were deposited in GenBank under accession Nos. KC785980 View Materials – KC786025 View Materials (see Table 1 for full details). Six gene sequences from four taxa were not characterized as a result of PCR failure or weak products unsuitable for sequencing; all other sequences (46 for 13 taxa) were aligned by gene and concatenated by taxon. The full alignment was comprised of 5423 positions (40% lsrDNA, 32% ssrDNA, 17% rrnL, 11% cox1), of which 442 positions across the alignment could not be unambiguously aligned and were excluded prior to analysis. A multi-gene phylogenetic analysis places M. ficta in a clade of Ophiotaenia spp., where O. lapata Rambeloson, Ranaivoson & de Chambrier, 2012 from Madagascar forms the sister group to ( M. ficta — Vietnam, O. bungari de Chambrier, Binh & Scholz, 2012 — Vietnam, Ophiotaenia sp. from Compsophis — Madagascar (( Ophiotaenia sp. from Antaresia maculosa— Australia, Ophiotaenia sp. from Notechis scutatus— Australia) ( O. gallardi [Johnston, 1911] — Australia, O. longmani — Australia))) ( Fig. 23). Within the ingroup, Crepidobothrium gerrardii (Baird, 1860) forms the earliest diverging taxon, followed by Thaumasioscolex didelphidis Cañeda-Guzmán , de Chambrier & Scholz, 2001 ( Fig. 23), the latter forming the sister group to the above mentioned clade.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |