Hystrix, Linnaeus, 1758
|
publication ID |
https://doi.org/10.5252/g2011n4a8 |
|
DOI |
https://doi.org/10.5281/zenodo.4608733 |
|
persistent identifier |
https://treatment.plazi.org/id/03DF8785-FFF9-4637-E505-FB3DFBFEF97B |
|
treatment provided by |
Felipe |
|
scientific name |
Hystrix |
| status |
|
Hystrix View in CoL sp.
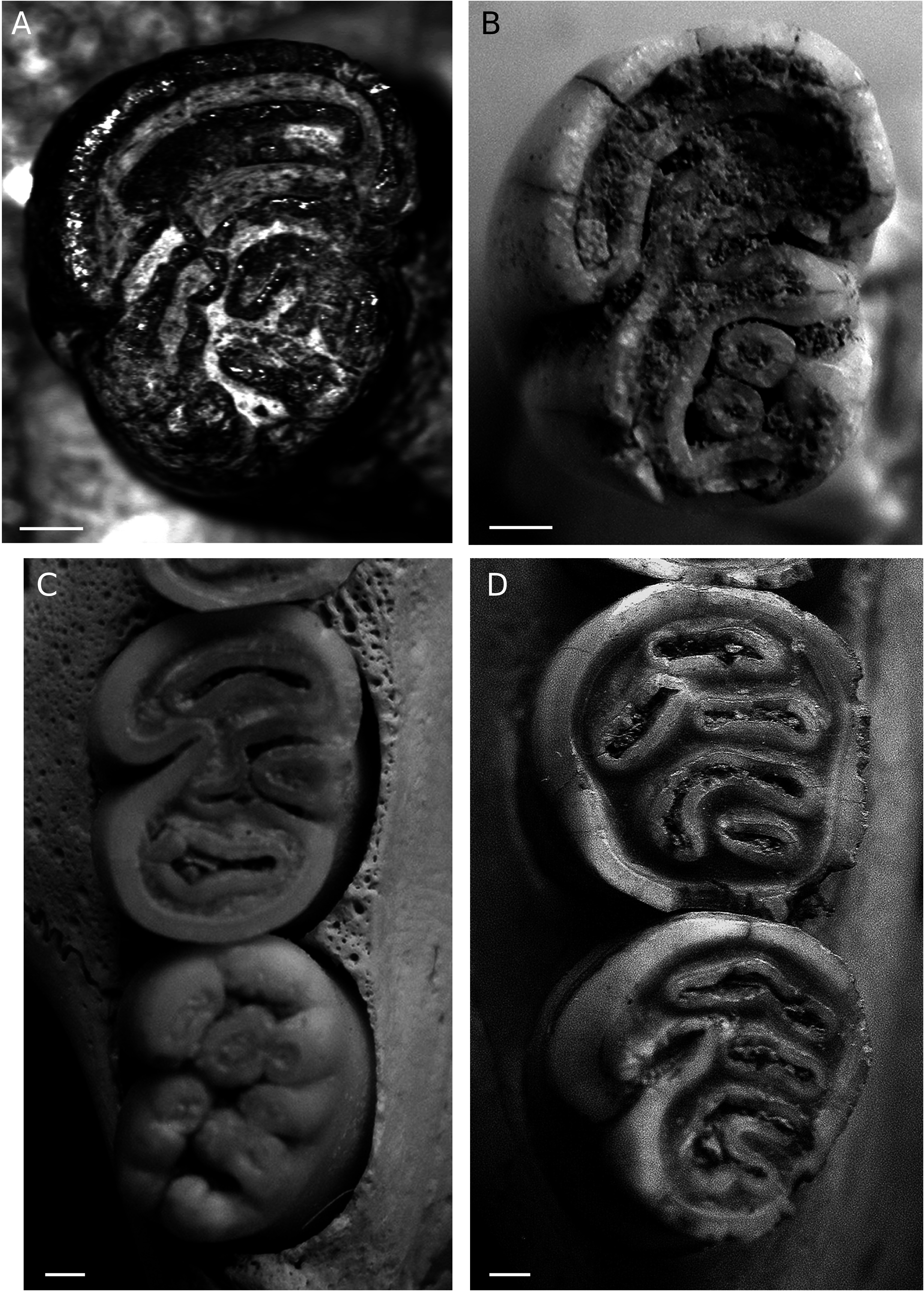
MATERIAL EXAMINED. — Isolated left upper M3 (HCRP- 1144).
DESCRIPTION
The molar is black with carbonate accretions. Some small breaks are observed in the crown and the intact enamel structure shows no signs of digestion. The crown is low with only one root and the molar is not very worn; some cusps are connected by lophs, and some cusps remain intact and are not individualized into small islands of enamel such as in aged specimens. The individual is probably a young adult ( Fig. 1 View FIG ; Table 1 View TABLE ). Due to its medium small size (smaller than in Xenohystrix Greenwood, 1955 and larger than in Atherurus Cuvier,1829 ) this molar can be attributed to Hystrix genus.
The molar displays a typical Hystrix upper M3 pattern by being longer than wide. The first loph is long and convex following the anterior margin of the molar, and it ends in the middle of the labial side of the molar. The lingual sinus is deep and oblique but there is the trace of a protocone. The labial sinus is transverse and joins the ectoloph. The ectoloph is longitudinally oriented and short, and though its end is not well visible, a small cusp (hypocone?) is seen at the back of the molar, which lacks a posteroloph. The labial side of the posterior part of the molar is comprised of two lophs: a small transverse loph upon which one can see two cusps (mesoloph with entocone?) and a distal short oblique loph with one cusp. This molar displays one oblique curved root.
DISCUSSION
Comparison and taxonomic affinities
Upper hystricid molars are not frequently preserved in the fossil record which makes a comparisons to other fossil species difficult. In modern specimens, the upper M3 may be absent in very young specimens or be an erupted germ displaying only isolated cusps. Very old specimens have very worn crowns and only islands of enamel persist. Consequently we agree with Van Weers (2005) that in this taxonomic group of rodents, the morphology of the cheek teeth may not be useful to distinguish between the species in the absence of comparisons between specimens at similar wear stages.
The Uraha molar is at an intermediate stage of wear, with lophs visible and connecting cusps, and it still has a relatively high crown. We did not found any upper M 3 in the fossil record corresponding to this wear stage but the comparison specimens are relatively close.
Two upper M3 fossils of Hystrix leakeyi and H.makapanensis have been recorded from Laetolil ( Denys 1987, 2011) and one M3 from the FLKN1 site at Olduvai attributed to H. makapanensis ( Sabatier 1979) , which are used here for comparisons despite they do not correspond to the same wear stages. Due to the absence of a comprehensive revision of the modern African Hystrix species, the museum specimen identifications remain doubtful and the comparisons can be made only at the genus level.
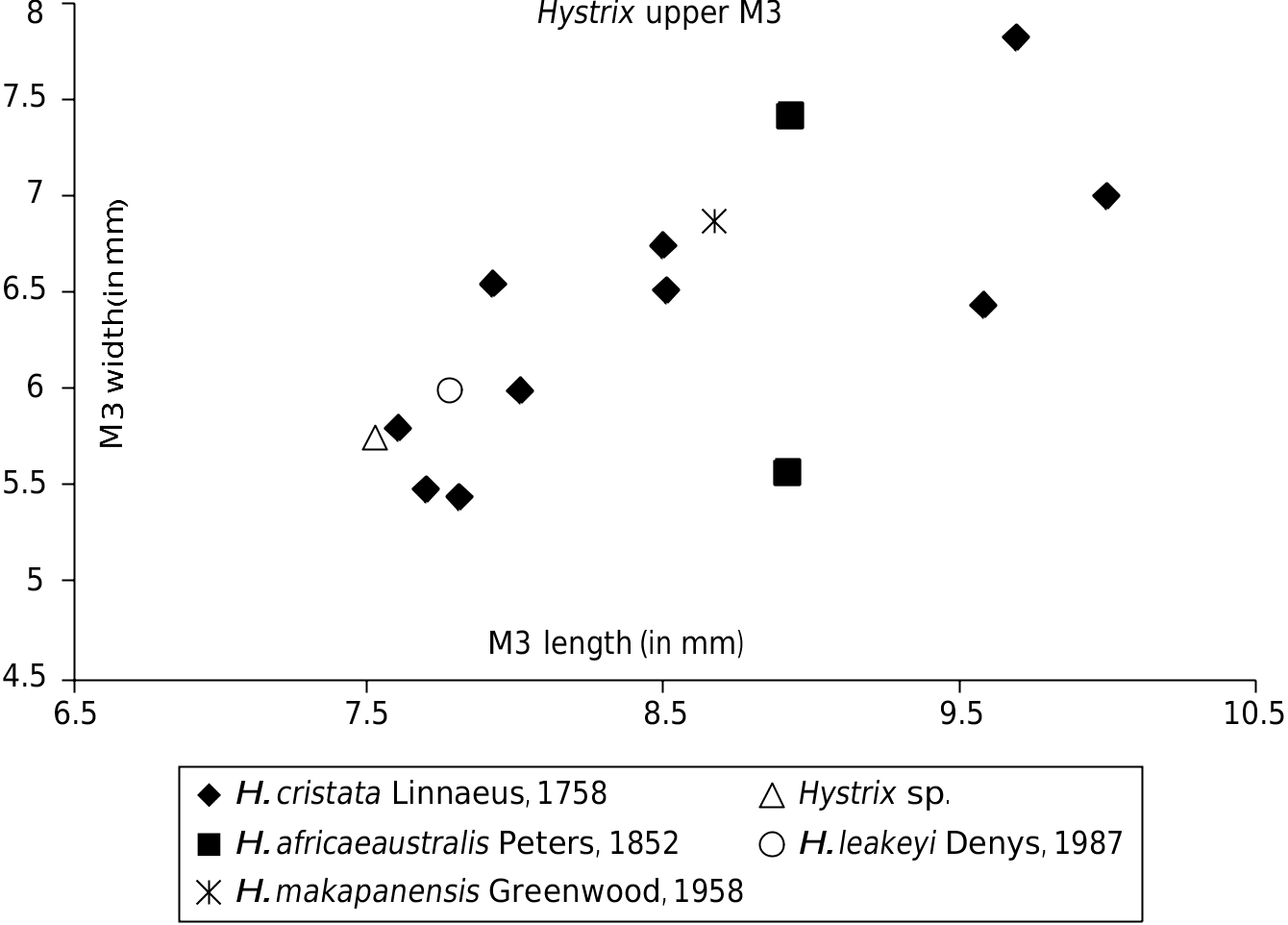
The Uraha specimen is smaller than H. leakeyi ( Fig. 2 View FIG ; Table 1 View TABLE ) and fits into the lower limit of variability of modern Hystrix species. It is about same size than the H. leakeyi upper M3 (75/468) but the Laetoli specimen is a germ and thus the two molars display different wear stages. In comparison to H. leakeyi , the labial sinus is less transverse and not as well related to the ectoloph ( Fig. 1 View FIG ).
In comparison to the upper M3 of H. makapanensis from Olduvai and Laetoli (00/3354), which are slightly more worn, the Uraha specimen is smaller in size. The Uraha molar has a smaller lingual sinus and a narrower distal loph. Moreover, the Uraha specimen harbors a hypocone that is more longitudinal and less visible and the distal labial cusps are more oblique. The Olduvai and Laetoli M3 H. makapanensis also have more lophs visible than the Uraha and Laetoli H. leakeyi specimens, which may be due either to a more advanced wear or to a more lophodont pattern.
Compared to modern Hystrix spp. of Africa, the Uraha specimen is slightly smaller ( Table 1 View TABLE ) and is more bunodont, contains fewer connected cusps, and the crown is not completely circled by an enamel ring posteriorly. The lingual sinus of the Uraha M3 is, however, deeper and persists further toward the base of the crown than in modern Hystrix spp., indicating a rather lower stage of hypsodonty in the genus at around 2.5 Ma. The modern Hystrix upper M3s have more transverse labial lophs when they are visible and the labial sinus is absent in all wear stages ( Fig. 1 View FIG ). There is a transversely long posteroloph at the back of the molar which is visible also in H. leakeyi and H. makapanensis , but not in the Hystrix sp. specimen from Uraha.
The Uraha specimen is close in size to a very young modern upper M3 germs and we do not have at the moment any equivalent fossil molar at a same stage to compare. Moreover, the variability related to age and sex in modern african Hystrix is still not well known. We can observe that the M3/3 are not visible on young specimens and they are always the smallest teeth as well as the latest to erupt. The African porcupine systematics must be revised and in the absence of such knowledge it is difficult to attribute the fossil species to a specific species on the basis of a unique specimen. However, our observations lead to the conclusions that the new Uraha specimen is a bunodont and small representative of the genus which is different in some respects from the modern Hystrix specimens and may be different from H. leakeyi and H. makapanensis . Due to an absence of knowledge of modern and fossil african Hystrix variability we cannot conclude upon size evolutionary trend. Van Weers (2005) in his revision of Eurasian Hystricids showed that all Miocene porcupines were low-crowned while high crowned specimens appear either during late Miocene or early Pliocene. If we assume similar evolutionary trend for tropical Africa, then the small size and bunodonty of the Uraha specimen could indicate an age earlier than 2.5 Ma. However, the scarcity of Hystricid upper molars fossil-record does not allow us to go further into phylogenetic, biochronological and biogeographical considerations for this specimen.
PALAEOENVIRONMENTAL
AND TAPHONOMIC IMPLICATIONS
Porcupines today are nocturnal herbivore generalists; they eat roots, bulbs, fruits and bark ( Kingdon 1974; De Graaff 1981). Hystrix lives in mostly non-desert habitats, including savanna woodlands, steppes and uplands. It is found sometimes along forest margins or galleries but avoids swamps and moist forest. This indicates that at around 2.5 to 2.33 Ma, the Uraha landscape was rather open. By the fact, based upon the proportions of bovids at Uraha, the habitat shared affinities of the fossil faunas of this locality fitted with the arid grasslands of Somali Masai region, which today is situated in the Zambezian phytochorion ( Sandrock et al. 2007). Unfortunately, we cannot confirm here if the Uraha porcupine has more affinities with eastern or southern populations of the fossil species, which prevent us to assign the Uraha localities into Somali-Masai
H. cristata Linnaeus, 1758 Hystrix sp.
H. africaeaustralis Peters, 1852 View in CoL H. leakeyi Denys, 1987
H. makapanensis Greenwood, 1958
or Zambezian vegetation zones and define the type of savannas were early Homo rudolfensis and Paranthropus boisei lived. Modern Hystrix live generally in hilly or rocky landscape and hide during the day in caves or natural crevices with narrow entrances ( De Graaff 1981). Hystrix is presently hunted by large felids (especially lion and leopard) and hyaenids, none of them being represented as fossils at Uraha. However porcupines are represented in low number of individuals in modern spotted hyaena and leopard dens studied ( De Ruyter & Berger Lee 2007; Pokines & Kerbis 2007). In the leopard den, De Ruyter & Berger Lee (2007) mentioned two skulls of juvenile Hystrix deposited in 1991 and not recovered at the second visit in 1998 for unknown reasons. Today Hystrix is considered as a valuable bushmeat in some parts of Africa ( Njiforthi 1996) and it could have also been hunted and consumed by early hominids.
Discovery of the Uraha Hystrix raises an interesting taphonomic problem since Schrenk et al. (1995) report that micromammals and their predators carnivores are virtually absent from the Chiwondo Beds, despite intensive screening of the sediment. Sandrock et al. (2007) also confirmed the low biodiversity and the ungulate-bias of the Chiwondo faunas. These authors suggested that such absences are likely the effects of destructive pre- and postdepositional taphonomic processes. Some taphonomic studies have noted numerous alterations and a rather complex taphonomic history for the Chiwondo Beds Malema site RC 11, equivalent in age to Uraha (Sandrock 1999; Sandrock et al. 1999, 2007). Geologically, the Uraha deposits are comprised of a ferruginous calcimorph palaeosol lying in siltsones to mudstones interbedded with lenticular sandstones. These deposits were assigned to a swamp to interchannel setting with no evidence of lacustrine condition ( Betzler & Ring 1995). Sandrock (1999) suggests that the loss of information in the fossil record at Malema site RC 11 resulted from fluviatile reworking and post-burial destruction due to rapid oxidation of organic components combined with the pedogenic process of ferrolysis. Alternating wet and dry conditions plus plant decomposition and iron oxidation may be a source of acidification of soils and of bone destruction. Acid soil generally leaves typical corrosion marks at the bone surface ( Andrews 1990) and enamel is first affected by low pH (Fernández-Jalvo & Andrews 1992). This could result in a strong destruction of small mammal teeth and bones. Moreover the study of Malema bone diagenesis reveals the existence of strong chemical modifications with a high cristallinity index and enrichment in Ca, P, Al, Si and depleted in S, Mg, Cl, Na ( Sandrock et al. 1999). The high rate of fragmentation and the existence of heavy weathering processes and crystallization indicate potential destructive effects for small mammal bones. However, taphonomic investigation at various sites in Africa and Europe have shown the existence of well preserved micromammal bones despite high diagenesis and complex taphonomic histories ( Dauphin et al. 1994; Denys et al. 1996).
Due to the selective preservation of large mammals at Uraha this suggests that other factors must account for the absence of small mammals.Generally, small mammals are concentrated in abundance by owls and small carnivores either in their regurgitation pellets or in their faeces ( Andrews 1990). But, even in the case of the most destructive predator category, there are always bones left behind and it is not realistic to imagine the presence of only one predator at this time in the surroundings. Examples of such accumulations are well known at Olduvai bed I (Fernández-Jalvo et al. 1998) and at Tighenif ( Dauphin et al. 1994) in open-air lacustrine environments. At Olduvai the alkaline conditions may have favoured exceptional preservation of all bone elements. But at Tighenif some levels show iron concentration and no signs of intense destruction of bones. In contrast, small mammals are nearly unknown from the fluviatile and deltaic deposits of East and West Turkana, except at Koobi Fora localities 103A, 130A and 131A, which, according to Behrensmeyer (1975), represent delta mudflats and delta margin settings. These deposits include rootcasts and coarse grained sands that fill the mudcracks and lack evidence of paleosoils. No small fossils have yet been found in the channels and river system and it seems that such types of high-energy and fluviatile deposits are poor environments for concentrating and preserving small mammals. Experiments by Fernández-Jalvo & Andrews (2003) also demonstrated the damage made to small mammal bones by water and sediment action. Skulls can be rapidly disintegrated (within 48 hours) and silts and clay sediment have a strong capacity to abrade bones. Rapid breakage (1 to 4 hours) was also reported by Andrews (1990) in tumbling experiments.
Small mammal bones experimentally exposed to a pH1 HCl solution and pronase were destroyed in 23 hours but acid attack alone was insufficient to reach total destruction ( Denys et al. 1995). Root growth and trampling in a densely covered vegetation landscape may alter bone by increasing breakage which could favour its destruction on long term ( Sanchez et al. 1997; Fernández-Jalvo et al. 1998). But, for small mammal assemblages there is not any evidence of the total destruction by roots nor by weathering ( Andrews 1990) because plenty fossil yielded rootmarks and display relatively advanced weathering stages. Another explanation for the absence of micromammals would be mechanical. After accumulation, the pellets or faeces of predators are removed by rain or dispersed during lacustrine episodes. According to Behrensmeyer (1975), bones display different behaviours to fluviatile transport and can be regrouped in different so-called Voorhies categories of dispersal in function of their shape and density. The dominance of some categories allows to extrapolate the presence of water transportation of some skeletal elements in a site. In Malema, Voorhies Group III elements are dominant indicating a removal of the lighter elements by water transportation. However, fluvial experiments by Korth (1979) have shown that even for small mammals the behaviour of bones is the same for small compared to large mammals, so micromammal bones of Voorhies Group categories III to V (including the denser identifiable teeth and molars) should have remained in situ. In contrast is the high energetic beach facies of the Chiwondo Beds Mwenerondo site, adjacent to Malema where most of the mammal bones are severely destroyed. Andrews (1990) and Korth (1979) have also shown that pellets can float a very long distancee (more than 500 m) before being degraded. This may explain not only the absence of small mammals at Uraha but all over Chiwondo beds in Malawi.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Hystrix
| Denys, Christiane, Sandrock, Oliver, Kullmer, Ottmar, Rozzi, Fernando Ramirez, Bromage, Timothy G. & Schrenk, Friedemann 2011 |
H. leakeyi
| Denys 1987 |
H. makapanensis
| Greenwood 1958 |
H. africaeaustralis
| Peters 1852 |