Aymaratherium, 2016
|
publication ID |
https://doi.org/10.1111/zoj.12429 |
|
persistent identifier |
https://treatment.plazi.org/id/03DDA010-FFA8-0941-FF7E-FB56FC1ECAEB |
|
treatment provided by |
Marcus |
|
scientific name |
Aymaratherium |
| status |
gen. nov. |
AYMARATHERIUM JEANI GEN. NOV., SP. NOV.
( FIGS 2, 4, 5 View Figure 5 , 7, APPENDIX S1)
Holotype. MNHN-Bol-V 008954 ( Fig. 2, Appendix S1), nearly complete right dentary, missing only posterior extremities of angular and condyloid processes, anterior extremity of the ‘spout’, and distal lophid of m3.
Etymology. In reference to the Aymara ( Aymar aru), a native ethnic group and language from the Andes, from where the specimens were recovered; and, the specific epithet for Jean Joinville Vacher (successively Director of the French Institute of Andean Studies – IFEA, Advisor of the Institute for Development Research – IRD in Bolivia, Advisor of Regional Cooperation for Andean Countries of the French Embassy during 2000/2012, and currently Assistant to General Executive Officer for Science of the IRD) for his friendship and constant support for palaeontological investigations over the years.
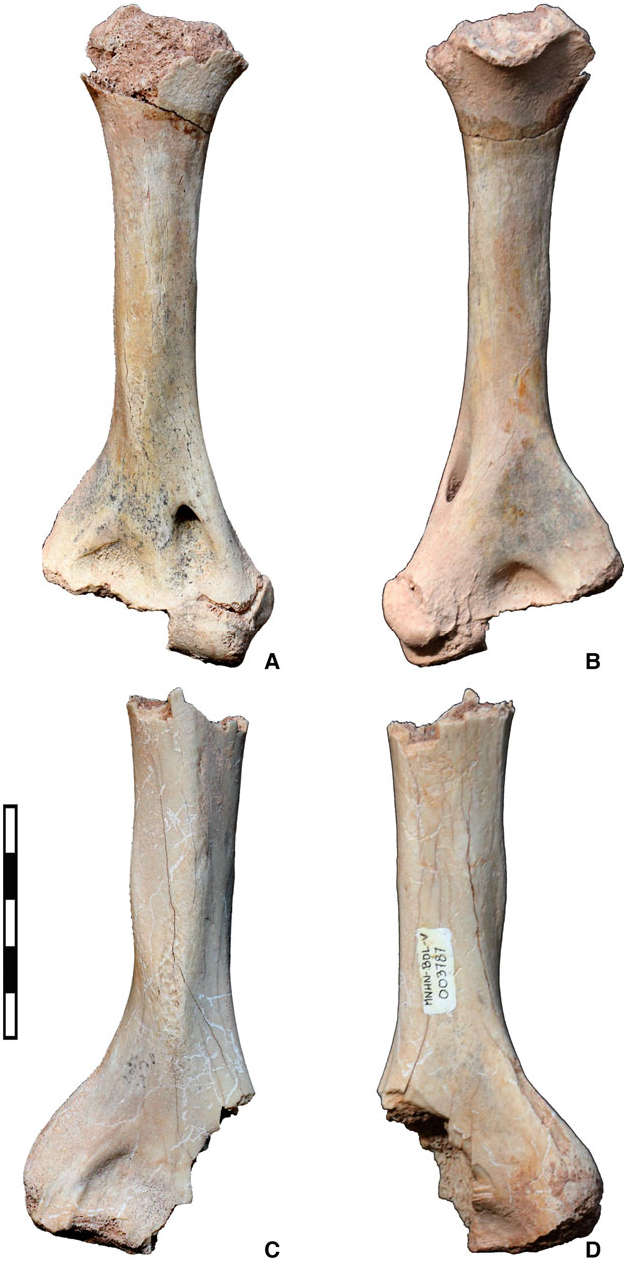
Hypodigm ( Figs 4, 5 View Figure 5 , 7, Appendix S1). Three humeri (MNHN-Bol-V 003787, 012874 and 012875), a complete astragalus (MNHN-Bol-V 012983) and a complete calcaneum (MNHN-Bol-V 003307).
Type locality and age. All specimens were discovered in the Montehermosan SALMA (late Miocene/early Pliocene) levels of the Pomata Ayte locality, 100 m above ‘Toba 76’ dated by St-Andre (1994: 83), using 40 K/ 40 Ar, as between 5.42 ƚ 0.6 and 5.97 ƚ 0.4 Ma.
Diagnosis. Small- to medium-sized sloth, similar in size to Thalassocnus and Nothrotherium ; lower dentition comprising a single small caniniform and three large molariform teeth; absence of diastema between c1 and m1; very high hypsodonty index (1.14), as in the most hypsodont specimens of Megatherium and Megalonyx ; deep buccinator fossa and extremely thin dorsal margin of the spout; c1 is distally curved and bears three isolated cuspids (mesiovestibular, distovestibular and distolingual); m1 – m3 mesiodistally compressed, m1 – m2 rectangular and m3 subtriangular (as for m 4 in Megatheriinae ); molariforms constituted by mesial and distal lophids perpendicular to the mesiodistal axis of the tooth row and separated by a deep transverse valley, open vestibularly and lingually as in Megatheriinae (open vestibularly only in other Nothrotheriidae and Megatherioidea of uncertain affinities such as Hiskatherium ); apicobasal grooves not present on m1 – m2, in contrast to other typical Nothrotheriidae ; shallow apicobasal grooves are present on distolingual and distovestibular surfaces of m3; the mesial lophid is shorter than the distal lophid in m1; in m2 and m3 the relationship is reversed; the angle between the dorsal edges of ascending and horizontal rami is close to 110°; the humerus is slender, with the greater and lesser tubercles well developed and strongly asymmetric with the greater tubercle larger than the lesser and the head medially inclined, in contrast to other Megatherioidea (except Diabolotherium ), which never have this combination; the deltopectoral shelf begins laterally, expands anterolaterally and is not well developed; the entepicondyle is rounded as in Thalassocnus , particularly T. natans , and poorly developed medially, in contrast to Pronothrotherium and Mionothropus ; the epicondyles are similar in size; in anterior view of the astragalus the discoid and odontoid facets meet at a right angle as in T. natans , the caput tali is positioned centrally and its dorsal border is located just below the discoid surface as in Thalassocnus ; the odontoid process is well defined, perpendicular to the main anteroposterior axis of the discoid facet and does not bear a strong plantar extension; the transverse width of the odontoid process is less than that of the discoid process, in contrast to Thalassocninae and Megatheriinae ; the calcaneum is massive and bulky and its general morphology is comparable to that of Thalassocninae (particularly T. natans ); the tuber calcanei is approximately symmetrical in dorsal view and slightly elongated posteriorly, rather than tapered, in contrast to other nothrotheriids; the ectal facet for the astragalus is oriented in an almost dorsoplantar plane (as in T. carolomartini ) and bordered by a dorsal groove; the sustentacular facet for the astragalus and cuboid facet are contiguous, as in Thalassocninae and Megatheriinae , and separated from the ectal facet by the deep sulcus calcanei; the dorsal portion of the tuber calcanei bears a tuberosity, as in Megatheriinae and Thalassocninae.
DESCRIPTION OF AYMARATHERIUM
MANDIBLE ( HOLOTYPE; FIG. 2, AND APPENDIX S1)
MNHN-Bol-V 008954 is a nearly complete right dentary ( Fig. 2). In occlusal view, the molariform teeth are mesially convex and the most mesial tooth closely approaches the dorsolateral margin of the mandible. Following Cartelle & De Iuliis (2006) for megatheriines and Pujos et al. (2011, 2014) for megatherioids, this latter characteristic suggests that this specimen probably belonged to an adult individual. The nearly complete dentary preserves four teeth, missing only the distal lophid of the last tooth. It preserves the horizontal ramus, missing only the tip of the spout. The dentary also lacks parts of the extremities of the coronoid and condyloid processes, and most of the angular process ( Fig. 2K, L). This specimen pertains to a small- to mediumsized megatherioid, similar in size to the nothrotheriids Thalassocnus , Lakukullus , Nothrotherium and Mionothropus , and the planopsines, larger than Hiskatherium , Xyophorus and Hapalops , and consider- ably smaller than large megatheriines such as Megatherium ( Fig. 3I, J) and Megathericulus ( Pujos et al., 2013) . The dentition of Aymaratherium jeani includes a caniniform followed by three molariform teeth, which is characteristic of basal tardigrade taxa such as Pseudoglyptodon and Octodontotherium (Tinguirirican and Deseadan SALMAs: Engelmann, 1987; McKenna, Wyss & Flynn, 2006; Pujos & De Iuliis, 2007; Pujos et al., 2012a), as well as the nothrotherids Nothropus , Mionothropus and Pronothrotherium , and several other Miocene basal megatherioid sloths (Santacrucian SALMA: e.g. Scott, 1903, 1904; De Iuliis et al., 2014).
Aymaratherium bears one of the highest HI values reported for a sloth (1.14), equivalent to that reported for some specimens of the Pampean megatheriine M. ( M.) americanum (1.14) and the North American giant megalonychid Megalonyx (1.17) ( Pujos et al., 2011). The HI is generally higher among Megatheriinae (0.77 – 1.14) and lower in Nothrotheriidae (0.40 – 1.06). In nothrotheriids that retain a plesiomorphic dental formula (i.e. the presence of a caniniform tooth), HI is considerably lower: between 0.43 in Pronothrotherium typicum and 0.86 in Mionothropus cartellei . In advanced nothrotheriids, which lack the caniniform, given the reduction of the length of the tooth row, the HI reaches higher values, and ranges between 0.52 in Nothrotherium maquinense to 1.05 in Thalassocnus antiquus (see Pujos et al., 2011: table 2 for further details and an overview of HI in sloths).
In lateral view, the ventral margin of the horizontal ramus is more convex than in other nothrotheriids, planopsines and Hapalops , and is thus similar to that of Megatherium and Huilabradys magdaleniensis from La Venta (Laventan SALMA: Villarroel, 1998). In MNHN-Bol-V 008954, the maximum convexity (and by extension the depth of the mandible) is located between m1 and m2 ( Fig. 2A, B, D, E) as in Thalassocnus antiquus and Planops martini . In Xyophorus bondesioi (MLP 32-IV-20-1) and Mionothropus cartellei , the maximum convexity of the mandible is located at the level of m2, between m2 and m 3 in the Megalonychidae View in CoL Eucholoeops and in Megatherium ( P.) tarijense , and at the level of m 3 in Prepoplanops ( Carlini, Brandoni & Dal Molin, 2013) . The deep buccinator fossa is located anterolaterally to c1 ( Fig. 2D – H). The posterior half of the external mental foramen is preserved ( Fig. 2A – H). This large foramen is located on the lateral margin of the spout, 25 mm in front of c1 and 10 mm below the dorsal margin of the dentary. Anteriorly to c1, the dorsal border of the spout is thinner than in most other sloths (2.2 mm), especially Lakukullus ( Pujos et al., 2014; Fig. 3E) and Megatheriinae , and comparable to the condition in Thalassocnus antiquus .
The anterior extremity of the spout is broken but it is possible to estimate its anterior extension. Based on the position of the external mental foramina and the depth of the anterior portion of the mandible, the spout of Aymaratherium extends far anteriorly, a condition somewhat similar to that observed in X. villarroeli UF 2668 , Mionothropus cartellei and Nothrotheriops shastensis , and in megatheriine sloths such as M. ( P.) tarijense (see De Iuliis, Pujos & Tito, 2009) and M. ( M.) altiplanicum ( St-Andre & De Iuliis, 2001) . In occlusal view, the lateral surface of the horizontal ramus is strongly convex, as in Mionothropus ( Fig. 3G) and in contrast to Diabolotherium (Pujos et al., 2007: fig. 6B, G, H); the medial surface is rectilinear as in most nothrotheriids. In anterior view, the lateral surface of the ramus is dorsoventrally rectilinear and the medial surface slightly concave.
The posterior external opening of the mandibular canal opens laterally. It is located below the tooth row, posterior to m3, at the base of the ascending ramus of the coronoid process ( Fig. 2D – H). In Thalassocnus natans (see de Muizon et al., 2004a), it is located at the same level as the tooth row and opens more anteriorly, whereas in Mionothropus cartellei ( De Iuliis et al., 2011; Fig. 3G, H), it opens laterally but at the level of m3. The angle between the ascending and horizontal rami is close to 110°, as in Nothrotherium maquinense , Nothrotheriops texanum , Xyophorus bondesioi , Pronothrotherium typicum , Hapalops and Planops martini . In most nothrotheriids, this value ranges between 100 and 115°, except in Mionothropus cartellei in which the posterior portion of the mandible is inclined posteriorly and this angle is close to 125° ( Fig. 3H). In Megatheriinae and Prepoplanops , the ascending ramus is generally more upright and the angle with the horizontal ramus lower, approximating 100° (see variation in megatheriines in De Iuliis, 1996). In relation to the strong hypsodonty of Aymaratherium , the posterior portion of the mandible (i.e. coronoid, condyloid and angular processes) is located dorsally relative to the horizontal ramus in comparison to its position in other nothrotheriids and planopsines. The anterodorsal margin of the ascending ramus is rough and corresponds to the insertion of the temporalis muscle ( Fig. 2C, F). Laterally, this muscle is located above the origin of the zygomaticomandibularis muscle and the insertion of the masseter pars superficialis muscle ( Fig. 2F; Naples, 1987; Naples & McAfee, 2012; Pujos et al., 2014). In Aymaratherium , these muscle attachments occupy the entire medial surface of the posterior portion of the dentary. Such expanded mandibular musculature is unusual in Nothrotheriidae , except Lakukullus ( Fig. 3E, F; Pujos et al., 2014), but much more common in Megatheriinae , such as M. ( P.) tarijense ( Naples & McAfee, 2012) .
The articular head of the condyloid process is partially missing but located above the tooth row and in a slightly more dorsal position than in Mionothropus cartellei ( Fig. 3H), Xyophorus sp. (UAFT V-000871, Croft et al., 2009), Planopsinae and Megatheriinae . The posterior extremity of the condyloid process lacks a ventral concavity, as it also occurs in most ground sloths. In posterior view, the base of the angular process inclines laterally. The ventral margin of the angular process is flat. The mandibular foramen is very large ( 10 mm), opening posterodorsally, and is located at the base of the medial side of the ascending ramus, just dorsal to the tooth row ( Fig. 2A, B, G, H). In Xyophorus , Eucholoeops and Hapalops , this foramen is located at the same level as or ventral to the tooth row. The medial side of the ascending ramus bears a long dorsal and a ventral insertion site for the temporalis and pterygoideus medius muscles, respectively ( Fig. 2C).
The lower dentition of Aymaratherium includes a caniniform and three molariforms ( Fig. 2). The c1 is posteriorly recurved, with its mesial surface convex and its distal slightly concave. It lies medial to the sagittal axis of the tooth row. In occlusal view, c1 is oval and slightly compressed mesiodistally ( Fig. 2K, L), rather than transversely, as occurs in Xyophorus villarroeli ( Fig. 3A, B) and Lakukullus anatisrostratus ( Fig. 3E), or circular as in Pronothrotherium typicum , Mionothropus cartellei and Prepoplanops boleadorensis . During the evolution of the nothrotheriids, several taxa such as Thalassocnus , Nothrotheriops and Nothrotherium have lost the most anterior tooth, whereas others (e.g. Pronothrotherium and Mionothropus ) have retained four lower teeth, including a caniniform first tooth. In some sloths such as Hiskatherium ( Fig. 3C), Diabolotherium and Megatheriinae ( Fig. 3I), four teeth are also present, with the anterior most molarized instead of being canine-shaped (Pujos et al., 2007, 2011).
In Aymaratherium , c1 is slightly larger than in some other nothrotheriids such as Mionothropus cartellei ( Fig. 3G, H), Xyophorus villarroeli ( Fig. 3A, B) and Pronothrotherium typicum ( Ameghino, 1907; FMNH P14467), Planopsinae ( Planops martini, FMNH P 13148, Hoffstetter, 1961), and most of the species of Hapalops (e.g. MACN A-6368). It is similar in robustness to that in Pseudortotherium australis (MLP 68-I-17-5, Scillato-Yane, 1981) and Lakukullus anatisrostratus ( Pujos et al., 2014; Fig. 3E, F), but smaller than that in Huilabradys magdaleniensis and in Megalonychidae View in CoL (e.g. Eucholoeops ingens, De Iuliis et al., 2014 ), and than that in some Mylodontidae that possess a robust caniniform.
It is worth nothing that the c1 of A. jeani differs from that of other Tardigrada in bearing three cuspids, a pattern usually recognized only in molariform teeth (e.g. Eucholoeops Bargo, Vizcaıno & Kay, 2009 ; Taxon Specimen Age and locality Reference(s)
Eucholoeops ingens MPM-PV 3401 Santacrucian SALMA of De Iuliis et al. (2014) Patagonia Argentina
Megatheriidae Planopsinae
Planops martini FMNH P 13148 Santacrucian SALMA of Hoffstetter (1961) Patagonia Argentina
Prepoplanops boleadorensis MLP 97-XI-3 – 1 [ holotype] Miocene [dubious age] of Carlini et al. (2013) Patagonia Argentina
Megatherium MNHN AYO 101 [ holotype] Montehermosan SALMA of St-Andre & De Iuliis
( Megatherium ) Bolivian altiplano (2001)
Megatherium MNHN-Bol-V 011564 Lujanian SALMA of Bolivian Pujos & Salas (2004);
( Pseudomegatherium) ( Fig. 3I, J), altiplano and Peru De Iuliis et al. (2009)
tarijense FMNH P 14216, and UNI 1
Nothrotheriidae Thalassocninae
Thalassocnus antiquus MUSM 228 Huayquerian SALMA of de Muizon et al. (2003) southern Peruvian coast
Thalassocnus natans MNHN.F.SAS 734 Montehermosan SALMA of de Muizon et al. (2003) southern Peruvian coast
Thalassocnus littoralis MNHN.F.SAS 1615 Montehermosan SALMA of McDonald & de Muizon southern Peruvian coast (2002); de Muizon et al. (2004b)
Thalassocnus yaucensis MUSM 434 Early Uquian SALMA of de Muizon et al. southern Peruvian coast (2004a)
Thalassocnus carolomartini SMNK PAL 3814 [ holotype] Chapadmalalan SALMA of McDonald & de Muizon and MNHN SAO 203 southern Peruvian coast (2002); de Muizon
et al. (2004b) Nothrotheriinae
Mionothropus cartellei LACM 11753 [ holotype] Huayquerian SALMA of De Iuliis et al. (2011) ( Fig. 3G, H) Brazil-Peruvian Amazon
Pronothrotherium typicum FMNH P 14467 and MACN Huayquerian SALMA of Ameghino (1907)
Pv 8140 [ holotype] Patagonia Argentina
Nothrotherium maquinense MCL 1020 Ensenadan SALMA of Brazil Cartelle & Fonseca (1983)
Nothrotheriops shastensis LACM 1801 – 7 Rancholabrean NALMA of Stock (1925)
USA
Lakukullus anatisrostratus MNHN-Bol-V 006601 Laventan SALMA of Bolivia Pujos et al. (2014) [ holotype] ( Fig. 3E, F)
Nothrotheriops texanum UF 86889 Irvingtonian NALMA of USA McDonald (1995) Xyophorus villarroeli MNHN ACH 43 [ holotype] Huayquerian SALMA of St-Andre (1996)
( Fig. 3A, B) and UF 2668 Achiri and Laventan
SALMA of Quebrada Honda,
Bolivia
Xyophorus bondesioi MLP 32-IV-20-1 [ holotype] Chasicoan SALMA of Scillato-Yane (1979),
or X. cf. bondesioi and UATF-V-000871 Argentina and Laventan Croft et al. (2009) SALMA of Bolivia
Nothropus priscus MACN Pv 975 [ holotype] Lujanian SALMA of Burmeister (1882) Argentina
* Hapalops will be considered at the generic level until intraspecific variation and revision of this genus is undertaken ( De Iuliis & Pujos, 2006).
Hiskatherium Pujos et al., 2011 ). These cusps, which are not homologous to ‘true’ cusps present in most other placentals, are in this case exclusively formed by wear during functional occlusion with the opposite upper tooth. Indeed, erupting teeth in sloths do not bear cusps but instead represent a rounded cap (see examples in Gervais, 1873; Naples, 1982, 1990; Cartelle & De Iuliis, 2006). The c1 of Aymaratherium bears a mesial central cuspid ( Fig. 2J, L), termed cuspid ‘C’, following the terminology suggested by Bargo et al. (2009). It is well defined and its extremity is rounded but not as prominent as in Mionothropus (which bears a single distal cuspid, Fig. 3H) and Megalonychinae , but more pronounced than in species of Xyophorus . Additionally, two distal cuspids are present in the c1 of A. jeani , one distolingually (cuspid ‘A’) and the other distovestibularly (cuspid ‘B’) on the margin of the occlusal surface ( Fig. 2A – C, J, L). A large concave wear facet occupies the central part of the occlusal surface. A diastema is not present between c1 and m1, in contrast to other Nothrotheriidae , Planopsinae and Hapalops .
The three molariform teeth (m1 – m3) are approximately rectangular and compressed mesiodistally. Similarly to c1, three cuspids also shaped by wear processes are present on each tooth, but located on two transverse lophids that are perpendicular to the mesiodistal axis of the tooth row, and separated by a deep transverse valley ( Fig. 2K, L). The general form of the molariform teeth of Aymaratherium combines several features in a distinct pattern for Tardigrada, although the condition is closely approached in the most basal megatheriine Megathericulus ( De Iuliis, Brandoni & Scillato Yane, 2008; Pujos et al., 2013). In occlusal view, m1 – m2 are very similar in shape, and mesiodistally compressed as in Megathericulus ; m3 is more triangular in section with a reduced distal lophid. A similar condition for the last lower molariform tooth occurs in Megatheriidae such as M. ( P.) tarijense (Ensenadan SALMA; Fig. 3I). The central transverse valley of m1 – m3 is open vestibularly and lingually as in Megatheriinae . In other Nothrotheriidae , the valley is open only vestibularly. The apicobasal grooves are missing in m1 – m2, in contrast to other Nothrotheriidae , Megatheriidae and Hapalops , and morphologically similar taxa.
The mesial lophid of m1 is slightly convex mesially and the distal lophid convex distally in occlusal view. The transverse width of the mesial lophid is less than that of the distal lophid. In occlusal view, the mesial lophid is concave mesially, and the distal lophid convex distally ( Fig. 2I). In lateral view, the distal lophid is higher than the mesial lophid. Cuspid ‘C’ lies at the centre of the mesial lophid, and cuspids ‘B’ and ‘A’ at the vestibular and lingual extremities of the distal lophid ( Fig. 2K, L). In medial view, the apicobasal height of the distal wear facet is reduced medially. In posterior view, the apicobasal height of the distal wear facet is constant. The maximal apicobasal height of the mesial and distal wear facets is 2.6 mm.
As in m1, m2 also bears two transverse lophids which are nearly straight and partially joined lingually, as the margin of the tooth is raised in this region. This condition is in sharp contrast to the typical condition observed in megatheriine molariform teeth (see De Iuliis, 1996). The transverse width of the distal lophid is slightly shorter than that of the mesial lophid; as in m1, the distal lophid is higher than the mesial lophid. In posterior view, the distal lophid is slightly concave at its lingual third. In m2, cuspid ‘C’ is at the centre of the mesial lophid, but cuspids ‘B’ and ‘A’ are located closer to the centre of the distal lophid ( Fig. 2L). In mesial and distal views, the apicobasal heights of the wear facets are constant. The maximal apicobasal height of the mesial and distal wear facets is 2.4 and 2.0 mm, respectively. In vestibular view, the central valley is deeper than in m1.
As in Hapalops and most Nothrotheriidae , such as Xyophorus ( Fig. 3A) and Lakukullus ( Fig. 3E), the m3 of Aymaratherium shows a distinct pattern. In Megatheriinae and Aymaratherium , the transverse diameter of the last lower molariform tooth is strongly reduced distally. The distal lophid of Aymaratherium is broken at the crown, but shallow and distovestibular apicobasal sulci are present ( Fig. 2K, L). In occlusal view, the mesial lophid is mesially convex and cuspid ‘C’ is reduced. The transverse valley is shallower than in m1 – m2. In mesial view the mesial lophid is convex apically and the apicobasal height of the mesial wear facet is reduced lingually (4.8 mm vestibularly, 2.8 mm at the centre and 2 mm lingually).
HUMERUS ( FIGS 4, 5 View Figure 5 AND APPENDIX S1)
Aymaratherium is represented by three humeri from three individuals of distinct ontogenetic stages: an adult (MNHN-Bol-V 012874; Fig. 4) on which the description is mainly based, a sub-adult (MNHN-Bol- V 012875; Fig. 5A, B View Figure 5 ) and a juvenile (MNHN-Bol-V 003787; Fig. 5C, D View Figure 5 ). The sutures are easily distinguishable between unfused epiphysis and diaphysis on non-adult specimens, and the juvenile humerus is somewhat more robust than the sub-adult humerus ( Fig. 5 View Figure 5 ). Although these humeri represent different ontogenetic stages, they present a common general pattern we consider as characterizing this new nothrotheriid taxon. The three humeri bear slight pathological abnormalities, mainly in the epiphysis and especially in the most complete specimen (MNHN-Bol-V 012874; Fig. 4). Such pathologies are relatively common in ground sloths ( McDonald, 1989) and consist of exostoses, which could possibly reflect injury repair.
In contrast to Mylodontidae , Megatheriinae and most Megalonychidae , the humeri of nothrotheriids are generally slender with a moderately developed deltopectoral shelf and a prominent entepicondyle. Humeri of modern tree-sloths Bradypus and Choloepus are disproportionately elongated and secondarily modified in relation to their suspensory posture and locomotion, features and habits that probably evolved convergently in Bradypodidae and Megalonychidae ( Nyakatura, 2012) . The humerus of Aymaratherium is gracile as in Mionothropus ( Fig. 6A), Pronothrotherium ( Fig. 6B) and Diabolotherium (NMR-PZ M4286 and MNHN CPN 9-1; Pujos et al., 2007). However, the general morphology of the humerus of the Bolivian nothrotheriid is more robust than that of Nothrotherium maquinense ( Fig. 6D), and less robust than that of Thalassocnus species (and especially T. natans ; Fig. 6C), megatheriid planopsines (e.g. Planops martini ; Fig. 6E), Hapalops sp. ( Fig. 6F) and megatheriines [e.g. Megatherium ( P.) tarijense ; Fig. 6G].
In Aymaratherium , the entire proximal epiphysis is modified in comparison with other Megatherioidea (except Diabolotherium ) by a combination of an asymmetry of the greater and lesser tubercles with a medial inclination of the head ( Fig. 4E, F). The greater tubercle is much more prominent and proximally extended than the lesser tubercle, and the latter is not well separated from the proximal epiphysis ( Fig. 4A, E – H). A similar asymmetry of the tubercles is generally present in Megalonychidae but never so pronounced, except in Eucholoeops (see Pujos et al., 2007: fig. 10). A similar condition is also present in Diabolotherium (see Pujos et al., 2007: fig. 10A – D), but in the latter taxon, the tubercles are extremely reduced. Among other Nothrotheriidae (e.g. Mionothropus and Thalassocnus ; Fig. 6A, C) and Planopsinae (e.g. Prepoplanops , see Carlini et al., 2013: fig. 5A, B, and Planops ; Fig. 6E), the greater and lesser tubercles display nearly the same proximal development and they are located at the same level. In Nothrotherium maquinense ( Fig. 6D), the two tubercles are strongly reduced. In Megatheriinae , including in early middle Miocene forms such as Megatheriops rectidens (MACN 2818) , the tubercles are extremely reduced ( Fig. 6D). In proximal view, the greater tubercle is located laterally and expands anteroposteriorly, whereas the lesser tubercle is located anteromedially and expands posteromedially to anterolaterally ( Fig. 4A, B). The articular surface of the head of the humerus is oval with its major axis oriented posteromedially to anterolaterally ( Fig. 4A, B).
In posterior view ( Fig. 4G, H), the head of the humerus is much more medially inclined than in other nothrotheriids (especially Thalassocnus ; Amson et al., 2015a: fig. 6C) and Megatheriidae . A similar but less marked condition is present in Diabolotherium (Pujos et al., 2007: fig. 10B, D). The bicipital groove is deep, anteroposteriorly elongated and pierced towards the greater tubercle by five large foramina. The presence of a well-developed bicipital groove is considered to be plesiomorphic for the clade Nothrotheriidae ( Amson et al., 2015a) , and is observed in basal nothrotheriids such as Mionothropus , Thalassocnus and Pronothrotherium . The groove is poorly developed in Nothrotheriops and almost absent in Nothrotherium . In posterior view, the neck of the humeral head of MNHN-Bol-V 012874 presents several osteological pathologies that are also present on the anterior surface of the greater tubercle ( Fig. 4E – H). In lateral view, the greater tubercle gives rise distally to a small crest ( Fig. 4I, J) that possibly represents the insertion of the teres minor muscle ( Amson et al., 2015a).
In Aymaratherium , the lateral and medial crests on the proximal third of the posterior diaphyseal surface mark the origin of the triceps muscle ( Fig. 4G, H). This insertion lies distal to the head of the humerus and extends proximomedially to posterolaterally. It widens slightly distally and is bordered laterally by the beginning of the deltoid crest. Two principal areas for muscle attachment are observable on the diaphysis of the humerus in Aymaratherium : proximoposteriorly for the triceps muscle and anteriorly – and anterolaterally – for the deltoid and pectoral muscles. According to De Iuliis (2003), the deltopectoral plate (or deltopectoral shelf following De Iuliis et al., 2011) begins proximally on the lateral face of the diaphysis, just behind the greater tubercle ( Fig. 4I, J). It continues distally, expanding anteriorly toward the central part of the diaphysis and then decreasing in width rapidly towards the distal epiphysis ( Fig. 4E, F). In Aymaratherium , as in most ‘ground sloths’, the deltopectoral shelf is formed by the deltoid (laterally) and better developed (anteriorly) pectoral crests, and it corresponds to the insertion area for the strong deltopectoral musculature. The brachiocephalicus crest identified in Thalassocnus species (except in T. antiquus ; Amson et al., 2015a), and generally located between the deltoid and pectoral crests, is not observable in Aymaratherium and most nothrotheriid sloths. The deltopectoral shelf extends through the entepicondylar ridge (= entepicondylar bar) in the direction of the entepicondyle (or medial epicondyle; Figs 4E, F, K, L and 5B View Figure 5 , 6A – F). The deltopectoral shelf is generally poorly developed in Nothrotheriinae ( Figs 4 – 6) in comparison with Thalassocninae ( Fig. 6C and Amson et al., 2015a), Mylodontidae , Megalonychidae View in CoL (see Pujos et al., 2007: fig. 10), Planopsinae (e.g. Planops ; Fig. 6E) and Hapalops ( Fig. 6F). In Pleistocene Megatheriinae , the deltoid and pectoral crests occur together on the centre of the anterior diaphyseal surface ( De Iuliis, 2003), and form a relatively reduced V-shaped deltopectoral shelf (e.g. Megatherium ; Fig. 6G), but in less derived megatheriines such as Megathericulus and Megatheriops , the general morphology of the humerus is more gracile, and the deltopectoral shelf resembles that of other sloths more closely. In Aymaratherium , the deltopectoral shelf is weakly developed especially anteriorly. The shelf is more developed than in Nothrotherium and Diabolotherium , somewhat less than in Mionothropus , and considerably less than in Nothrotheriops , Pronothrotherium and Thalassocnus ( Fig. 6A – D).
The distal epiphysis of MNHN-Bol-V 012874 expands transversely and bears pathological growths (exostoses) above the trochlea and capitulum on the anterior and posterior surfaces ( Fig. 4E – J). A large entepicondylar ridge connects the deltopectoral shelf to the entepicondyle, and delimits the entepicondylar foramen, which is oval, opens anteroposteriorly, and serves for the passage of the brachial artery and median nerve ( Fig. 4E, F, K, L). The foramen is not present in Diabolotherium , Megatheriinae , some Megalonychidae View in CoL (e.g. Parocnus and Mesocnus ) or Mylodontidae scelidotheriines (e.g. Catonyx cuvieri ), but is present in all Nothrotheriidae , Planospinae and Hapalops . The entepicondyle (or medial epicondyle) is rounded in outline, as in Thalassocnus natans ( Fig. 6C), and does not bear a marked medial extension as in Pronothrotherium , Mionothropus , Thalassocnus and the unnamed nothrotheriid from the middle Miocene of La Venta (UCMP 39949; Hirschfeld, 1985). In Aymaratherium , the ectepicondyle (or lateral epicondyle) is slightly larger than in Diabolotherium . The ectepicondyle is bordered anteriorly by the slightly sinuous lateral supracondylar line (= supinator crest, Fig. 4E – G). In Aymaratherium , the epicondyles are approximately equal in size, in contrast to other Nothrotheriidae , where the entepicondyle is larger than the ectepicondyle.
The trochlea is concave transversely, convex anteroposteriorly and slightly wider than the capitulum, as in Mionothropus and Thalassocnus ( Figs 4B, C and 6A – C). The distal edge of the trochlea is oriented proximolateral/distomedial, not transversely as in Diabolotherium . The capitulum is round and convex like most ‘ground sloths’, suggesting good pronation and supination, as in Diabolotherium (Pujos et al., 2007) . The radial fossa is short, particularly well marked, and located proximal to the capitulum ( Fig. 4E, F). We interpret this peculiar morphology as pathological. Indeed, in MNHN-Bol-V 003787 ( Fig. 5A, C View Figure 5 ), the radial fossa appears as a uniformly curved arc as in the holotype of Diabolotherium (NMR-PZ-M4286; Pujos et al., 2007). It is smaller and shallower in the sub-adult Aymaratherium than in Thalassocnus (especially T. natans and T. littoralis ; see Amson et al., 2015a: fig. 5). The distal epiphysis exhibits pathologies on the anterior surface, at the level of the proximal border of the trochlea and on the posterior surface surrounding the olecranon fossa, and between the entepicondyle and the trochlea ( Fig. 4E – H). Posteriorly, the olecranon fossa is deep.
The juvenile specimen, MNHN-Bol-V-003787 ( Fig. 5C, D View Figure 5 ), is similar but slightly more robust than MNHN-Bol-V 012874; the deltopectoral shelf exhibits a similar development ( Fig. 5C, D View Figure 5 ). The sub-adult specimen, MNHN-Bol-V 012875 ( Fig. 5A, B View Figure 5 ), is more slender than the adult specimen; the entepicondylar crest and entepicondyle are poorly developed and the deltopectoral shelf is barely visible. Except for the presence of an entepicondylar foramen, the humerus of Aymaratherium shares several similarities with Diabolotherium that are not present in other nothrotheriids (see Pujos et al., 2007).
ASTRAGALUS ( FIG. 7A – H, Q, AND APPENDIX S1)
In addition to the humerus and femur, the astragalus of Aymaratherium is well preserved. It is a tarsal element that offers considerable phylogenetic and morphofunctional information. MNHN-Bol-V 012983 is a complete right astragalus ( Fig. 7A – H); its plantar articular facets match almost perfectly with the corresponding facets of the calcaneum of a second individual, described below and also referred to Aymaratherium ( Fig. 7I – Q). This astragalus shows the typical megatherioid morphology with a wellmarked odontoid process ( Fig. 7A – D).
In the Aymaratherium astragalus, the discoid and odontoid facets of the trochlea tali meet approximately at a right angle (approximately 85°) in anterior view ( Fig. 7A, B). This angle is slightly wider in Thalassocnus antiquus , T. natans ( Fig. 8G), Pronothrotherium ( Fig. 8D) and Megatheriinae such as Megathericulus ( Fig. 8A), and considerably wider in Planopsinae, Nothrotheriini, Xyophorus ( Fig. 8K) and Hapalops ( Fig. 8N). The odontoid and discoid processes are convex transversely and anteroposteriorly. In dorsal view, the odontoid process is well defined, bulky and almost perpendicular to the anteroposterior main axis of the discoid facet, whereas in Xyophorus ( Fig. 8J), Hapalops ( Fig. 8M) and Diabolotherium (Pujos et al., 2007: fig. 14), it is poorly differentiated. In anterior view, the odontoid process of the Aymaratherium astragalus does not bear a strong plantar extension ( Fig. 7A, B) in contrast to the condition observed in astragali of other Megatheriidae ( Fig. 8A), Pronothrotherium ( Fig. 8D) and Thalassocninae ( Fig. 8G and Amson et al., 2015b). It is shallow, as in Megatheriinae . In anterior view, the transverse diameter of the odontoid process is shorter than that of the discoid process ( Fig. 7A, B); the opposite condition occurs in Thalas- socninae ( Amson et al., 2015b: fig. 24; Fig. 8G) and Megatheriinae (e.g. Megathericulus ; Fig. 8A). A fossa lies between the anterior extremity of the discoid process and the head ( Fig. 7E).
On the head of the astragalus the navicular facet is medially positioned, and the cuboid facet is lateral and ventral ( Fig. 7A, B) as in Megatheriinae ( Fig. 8A), Thalassocninae ( Fig. 8G), Nothrotheriini and Pronothrotherium ( Fig. 8D). In Diabolotherium , Xyophorus and Hapalops ( Fig. 8J, M), the navicular and cuboid facets extend medially to the extremity of the odontoid process. Two typical distinct astragalar patterns exist in Megatheriinae and Hapalops , whereas the condition in Planopsinae is approximately intermediate (e.g. Planops ; see Hoffstetter, 1961: fig. 12). In anterior view, the dorsal aspect of the head in Aymaratherium is located just below the discoid surface ( Fig. 7A, B) as in Thalassocnus , a condition intermediate between that observed in Megatheriinae , in which the head is more plantar (e.g. Megathericulus ; Fig. 8A), and Hapalops ( Fig. 8M), in which the head is more dorsolateral relative to the discoid surface. The cuboid facet, mainly plantar in position, is convex anteroposteriorly and transversely. It is bordered medially by the navicular facet and posteriorly by the sustentacular facet ( Fig. 7A, B, G, H). In contrast to Thalassocnus species (see Amson et al., 2015b: fig. 23C, D), in anterior view the cuboid facet does not extend onto the base of the discoid facet. The navicular facet comprises a deep and concave anterior cupula, and a reduced medioplantar portion that is convex dorsoplantarly and transversely as in all Megatherioidea and in the majority of sloths. The navicular facet constitutes the medial border of the head. In plantar view it is bordered laterally by the cuboid facet and contacts the sustentacular facet posterolaterally ( Fig. 7G, H). As in almost all sloths, the lateral surface of the astragalus of Aymaratherium is entirely occupied by the fibular facet. It is slightly convex anteroposteriorly and dorsoplantarly, triangular in shape, and its apex orientated posteriorly ( Fig. 7C, D). In MNHN- Bol-V 012983, a deep anteroposterior notch, possibly pathological, almost completely separates the fibular facet into dorsal and plantar portions ( Fig. 7C, D). In anterior view, the angle between the discoid and fibular facets is less than 90° ( Fig. 7A, B).
The astragalar plantar surface bears the cuboid and navicular facets, and the two calcaneal facets (ectal and sustentacular). The ectal facet is elongated and rectangular, extended anterolaterally to posteromedially, and concave along its main axis ( Fig. 7G, H) as in Megatheriidae , Nothrotheriini, Thalassocninae and Pronothrotherium ; in Xyophorus , Hapalops , Diabolotherium and some Megalonychidae , such as Pliometanastes prostitus (Pujos et al., 2007: fig. 15), the ectal facet is L-shaped. The sustentacular facet is smaller and concave anteroposteriorly. It extends anteriorly to the cuboid facet, and narrows posteriorly. The sulcus tali extends anterolaterally to posteromedially, and is narrower posteromedially.
CALCANEUM ( FIG. 7I – P, R, AND APPENDIX S1)
The complete right calcaneum MNHN-Bol-V 008954, referred to Aymaratherium ( Fig. 7I – P), resembles that of the thalassocnine lineage recently described by Amson et al. (2015b) and especially that of Thalassocnus antiquus . It is approximately intermediate between the slender and elongated calcaneum typical of nothrotheriids (e.g. Nothrotheriops , Nothrotherium , and Pronothrotherium ) and megalonychids (e.g. Pliometanastes and Megalonyx ), and that of megatheriines [e.g. Megatherium ( P.) tarijense and Eremotherium laurillardi ], in which the diaphysis is markedly robust and relatively short. The diaphyseal shaft or neck is oval in section ( Fig. 7I, O), as in Thalassocnus and Megatheriinae , in contrast to other Nothrotheriinae, Planopsinae, Hapalops and allied genera, in which the neck is generally dorsoplantarly compressed and flattened. The form of the tuber calcanei reflects the form of the neck, being rounded in the former group of taxa and flattened in the latter group.
The posterior end of the calcaneum, including the tuber calcanei, resembles that of megatheriines and Thalassocnus , in which the tuber is tapered posteriorly and elongated in the middle. In genera such as Hapalops , Prepoplanops and nothrotheriines, the tuber is, by contrast, broad and relatively short, and of approximately constant length (i.e. the distance between the epiphyseal line and end of the tuber is uniform across its width), as well as flattened (as noted above), thus imparting an axe-like appearance to this part of the calcaneum. In Aymaratheriun, the tuber resembles that of megatheriines and Thalassocnus , but is not drawn posteriorly to the same degree.
The posterolateral and posteromedial processes of the tuber calcanei are nearly symmetric in dorsal view, although the posterolateral process is slightly more prominent and projects to a greater degree than the posteromedial process ( Fig. 7I, J). In Nothrotherium and Pronothrotherium , only the proximal margin of the tuber calcanei contacts the ground ( Amson et al., 2015b), whereas in Thalassocnus , Aymaratherium ( Fig. 7K – N) and Megatheriinae (e.g. Megatherium and Eremotherium ), only the lateral and medial processes of the tuber calcanei contact the ground. As in species of Thalassocnus , the dorsal portion of the tuber calcanei bears a small tuberosity visible especially in lateral view ( Fig. 7K – N). Amson et al. (2015b) noted that this tuberosity is also present in megatheriines, such as Megatherium ( P.) americanum , and perhaps served for the insertion of the gastrocnemius muscle.
The arrangement of the astragalar and cuboidal articular facets in A. jeani ( Fig. 7O, P), at the anterior end of the calcaneum is similar to that in Thalassocnus carolomartini , with the major axis of the ectal facet for the astragalus orientated almost dorsoplantarly. In several species of Thalassocnus (i.e. T. natans and T. antiquus ), a medial torsion of the anterior epiphysis of the calcaneum results in the main axis of the ectal facet, extending mediodorsally to lateroplantarly (see Amson et al., 2015b). In Megatherium [e.g. M. ( P.) tarijense ], the main axis of the ectal facet is transverse and the sustentacular facet for the astragalus and the cuboidal facet are located on the plantar portion of the anterior epiphysis ( De Iuliis, 1996). In Aymaratherium , the ectal facet is the largest articular facet and the sustentacular the smallest one. In anterior view, the ectal facet for the astragalus is oval and occupies the dorsolateral portion of the anterior epiphysis; it is convex dorsoplantarly and transversely ( Fig. 7O, P). The ectal facet is bordered dorsally by a transverse groove that is absent in other Nothrotheriidae . As usual in sloths (but not in all Mylodontidae ), the ectal facet is separated from the sustentacular and cuboidal facets by a deep sulcus calcanei. The sustentacular and cuboidal facets are not separated as in Planopsinae (e.g. Prepoplanops ), Nothrotheriini, Diabolotherium , Hapalops and allied genera, but contiguous as in Thalassocnus (and especially T. carolomartini ) and Megatheriinae . In Aymaratherium , the sustentacular facet is oval, transversely concave, and its main axis is dorsoplantar. The cuboid facet is plantar to the sustentacular facet. It is transversely and dorsoplantarly concave, and extends lateroplantarly on the anterior surface of the calcaneum.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Aymaratherium
| Pujos, Francois, Iuliis, Gerardo De, Quispe, Bernardino Mamani, Adnet, Sylvain, Flores, Ruben Andrade, Billet, Guillaume, Fernandez-Monescillo, Marcos, Marivaux, Laurent, Munch, Philippe, Pramparo, Mercedes B. & Antoine, Pierre-Olivier 2016 |
Xyophorus villarroeli
| UF 2668 |
Aymaratherium
| JEANI 2016 |
Aymaratherium
| JEANI 2016 |
A. jeani
| Pujos & Iuliis & Quispe & Adnet & Flores & Billet & Fernandez-Monescillo & Marivaux & Munch & Pramparo & Antoine 2016 |
Aymaratherium
| JEANI 2016 |
A. jeani
| Pujos & Iuliis & Quispe & Adnet & Flores & Billet & Fernandez-Monescillo & Marivaux & Munch & Pramparo & Antoine 2016 |
Aymaratherium
| JEANI 2016 |
Aymaratherium
| JEANI 2016 |
Aymaratherium
| JEANI 2016 |
Aymaratherium
| JEANI 2016 |
Aymaratherium
| JEANI 2016 |
Aymaratherium
| JEANI 2016 |
Aymaratherium
| JEANI 2016 |
Aymaratherium
| JEANI 2016 |
Eucholoeops ingens
| De Iuliis 2014 |
Hiskatherium
| Pujos 2011 |
Eucholoeops
| Bargo, Vizcaino & Kay 2009 |
Eucholoeops Bargo, Vizcaıno & Kay, 2009
| Bargo, Vizcaino & Kay 2009 |
Huilabradys magdaleniensis
| Villarroel 1998 |
Huilabradys magdaleniensis
| Villarroel 1998 |
Thalassocnus
| de Muizon & McDonald 1995 |
Thalassocnus
| de Muizon & McDonald 1995 |
Megatheriops
| Ameghino & Kraglievich 1921 |
Nothrotheriidae
| AMEGHINO 1920 |
Nothrotheriidae
| AMEGHINO 1920 |
Nothrotheriidae
| AMEGHINO 1920 |
Nothrotheriidae
| AMEGHINO 1920 |
Pronothrotherium
| Ameghino 1907 |
Pronothrotherium
| Ameghino 1907 |
Megalonychinae
| Trouessart 1904 |
Nothrotherium
| Lydekker 1889 |
Hapalops
| Ameghino 1887 |
Hapalops
| Ameghino 1887 |
Xyophorus
| Ameghino 1887 |
Hapalops
| Ameghino 1887 |
Hapalops
| Ameghino 1887 |
Planops
| Ameghino 1887 |
Hapalops
| Ameghino 1887 |
Hapalops
| Ameghino 1887 |
Megatheriinae
| Gill 1872 |
Megatheriinae
| Gill 1872 |
Megatheriinae
| Gill 1872 |
Megatheriinae
| Gill 1872 |
Megatheriinae
| Gill 1872 |
Catonyx cuvieri
| McDonald 1839 |
Nothrotherium maquinense
| MCL 1020 |