Orthotomus chaktomuk, Mahood & John & Eames & Oliveros & Moyle & Chamnan & Poole & Sheldon, 2013
|
publication ID |
https://doi.org/10.5281/zenodo.2764030 |
|
publication LSID |
urn:lsid:zoobank.org:pub:F1778491-B6EE-4225-95B2-2843B32CBA08 |
|
DOI |
https://doi.org/10.5281/zenodo.6145422 |
|
persistent identifier |
https://treatment.plazi.org/id/23E9A09C-AD9C-4346-A594-F187DAFB6013 |
|
taxon LSID |
lsid:zoobank.org:act:23E9A09C-AD9C-4346-A594-F187DAFB6013 |
|
treatment provided by |
Plazi |
|
scientific name |
Orthotomus chaktomuk |
| status |
sp. nov. |
Orthotomus chaktomuk View in CoL , sp. nov.
Cambodian Tailorbird
http://zoobank.org/ urn:lsid:zoobank.org:act:
Holotype and paratypes
Study skins deposited in NHMUK ( Table 1, Plate 1 View Plate1 , Plate 2 View Plate 2 a–c) were collected by JCE and SPM at Bateay District, Kompong Cham GoogleMaps
province, Cambodia ( 11°56’53.94”N 104°56’50.94”E), c. 43 km north of Phnom Penh GoogleMaps at c. 15 m elevation on 8 and 9 August 2012, and prepared by JCE. Tissue samples from the same individuals were deposited in Louisiana State University Museum of Natural Science, Baton Rouge, Louisiana, USA ( Table 1). Holotype ( NHMUK 2012.9.1 ): adult male; in active wing moult; one large testis (left testis 4.5 mm length, right testis 2 mm length). Paratypes aged by plumage: one adult male ( NHMUK 2012.9.2 ); one immature ma le ( NHMU K 2012.9.3 ) with unmoulted rectrices olive-green fringed ; one immature female ( NHMUK 2012.9.4 ) with some retained greater coverts fringed olive-green indicating immaturity, all other rectrices as adult; and one immature female ( NHMUK 2012.9.5 ) with unmoulted rectrices as immature male.
Diagnosis of species
Head: in male entirely rich cinnamon-rufous crown and contrasting white cheeks, very similar to O. atrogularis , differing from O. ruficeps and O. sepium in cheek colour ( Table 2, Plate 3 View Plate 3 a–c). Rufous of crown less extensive in female. Upperparts and wings: mid-grey in adult, superficially similar to those of O. sepium but lacking olive tones, strikingly different from O. atrogularis which is yellowishgreen ( Table 2, Plate 3a, c View Plate 3 ); tail with dark grey subterminal band and whitish tips when fresh. Underparts: pale grey ground colour
with profuse blackish throat-streaking in males (largely absent in females) with white drop-shaped marks, extensive mid-grey on flanks, and white vent; underparts of both sexes superficially similar to those of respective sexes of O. atrogularis owing to throat-streaking, but greyer on flanks and vent white, O. ruficeps and O. sepium lacking throat-streaking in both sexes ( Table 2, Plate 3b, c View Plate 3 ); further distinguished from other members of the genus by whitish-cinnamon thighs. Vocalisations: loud, lengthy, complex and highly varied. Very similar to O. atrogularis . Compared to O. atrogularis , phrases are given at a quicker pace and the gaps between phrases are shorter. Subjectively, these characteristics mean that the vocalisations of O. chaktomuk sound faster and more complicated than those of O. atrogularis .
Sexing and ageing
Based on field observations ( Plate 4 View Plate 4 a–k, Media Files SOM 1–3) and specimens ( Plate 2 View Plate 2 a–c), female O. chaktomuk can be distinguished from males by paler cinnamon-rufous on crown, which is restricted to forecrown and sides of mid-crown (in lateral view this appears as a short cinnamon-rufous supercilium), paler grey upperparts and wings and whitish underparts with usually faint dark streaking. The latter is usually evident only at the edges of the throat/upper breast, although some (possibly older birds) show stronger and more extensive streaking on throat and breast. Even in these extreme individuals, the degree of female streaking does not approach that in males ( Plate 4 View Plate 4 a–g). All three immature paratypes show shorter tails than adults ( Table 1). Immature birds possess bright yellowisholive fringing to the wing-feathers ( Plate 4h View Plate 4 ), which are moulted during August and replaced with grey adult-type feathers ( Plate 4 View Plate 4 i–j). Immatures are browner (slightly olive) above and paler below, with reduced streaking ( Plate 4 View Plate 4 h–j). Wing-feathers of subadults appear as in adults, except sometimes they retain yellowish-olivefringed greater coverts ( Plate 4k View Plate 4 ). Overall, subadults resemble adults, but are paler and less heavily marked below. In adults, there is individual variation in colour tone of grey feathering above and below, and intensity of throat-streaking (e.g. Media Files SOM 1–3). It is unknown if this is age-related.
Description of species
The detailed description below was completed in the NHMUK based primarily on the prepared specimens ( Plates 1–2 View Plate1 View Plate 2 ), supplemented by information from individuals observed and photographed in the field ( Plate 4 View Plate 4 ). It refers to the holotype unless otherwise stated. Although moult of body feathers was almost complete when specimens were collected, all adult specimens retained a few head, throat or breast feathers in pin. The holotype and paratypes were in wing moult. Moult of wing feathers is complete by late August and followed immediately by moult and replacement of tail feathers, which were very worn in all specimens. Subjective colour assessments of plumage are, where possible, followed by a formal colour classification taken from Smithe (1975).
Head and face
Crown from forehead to nape, lores, and feathers on orbital ring and just behind eye rich cinnamon-rufous (136 Raw Sienna) (slightly richer-coloured in the adult male paratype); hindcrown slightly darker and more brownish (23 Raw Umber). Crown feathers in moult with newer feathers slightly richer rufous. On the immature female paratypes the crown is less richly coloured than that of the holotype (240 Kingfisher Rufous) and the cinnamon-rufous lores and feathering on the orbital ring and immediately behind the eye are replaced by rufous-buff (118 Warm Buff). The rufous crown feathering extends from the bill only as far back as the anterior of the mid-crown where dark-grey feathers predominate, imparting an overall greyish-brown colour (129 Dark Brownish Olive) to the hindcrown.
Five blackish rictal bristles per side, anterior two c. 3 mm, twice the length of posterior three. Ear-coverts, cheeks and moustachial stripe almost white contrasting strongly with crown and underparts; however, feathers have buff (124 Buff) tips imparting an off-white wash.Feathers of submoustachial stripe and malar stripe white with very dark grey (82 Blackish Neutral Gray) bases and sometimes tips and fringes; white predominates, giving an impression of white speckling on a blackish base and contrasting strongly with the whitish cheeks. The malar stripe on the immature paratypes is quite different to that of the adult male specimens. It is made up of white feathers with pale grey central portions (85 Light Neutral Gray) and therefore contrasts little with the cheeks.
Upperparts
Boundary between hindcrown and upper neck abrupt. Upper neck, mantle and rump concolorous mid-grey (84 Medium Neutral Gray), slightly blue-toned approaching 78 Plumbeous (all feathers fresh and body moult apparently completed). Feathers on mantle and particularly rump relatively long and filamentous.
Wings
Wings of all prepared specimens in active moult ( Table 1). On all specimens, fresh adult feathers are slightly darker grey (83 Dark Neutral Gray) than mantle, tinged very slightly brownish with mid-grey (84 Medium Neutral Gray) fringing (slightly broader on outer webs). Fresh primaries with off-white inner webs; worn adult rectrices buffy-brown (239 Ground Cinnamon) lacking fringing or pale webs. Underside of remiges dull silver-grey (84 Medium Neutral Gray). Underwing-coverts paler grey (85 Light Neutral Gray). Alula and axillaries contrasting white. Unmoulted rectrices of the immature male paratype and immature female paratypes differ strikingly from those of adult male specimens in being fringed bright olive-green (50 Yellowish Olive-Green).
Tail
Slightly rounded, outermost pair of rectrices 7 mm shorter than central pair. Tail of holotype very worn, buffy-brown (239 Ground Cinnamon), dorsal side slightly darker than ventral but heavily worn. Whitish-buff terminal tips just visible on all but central rectrices. Tail of immature female paratype (NHMUK 2012.9.4) less worn than that of the holotype (and other paratypes) and is dark greyish-brown (21 Fuscous) with broader whitish tips than those shown by other specimens. Field observations indicate that fresh tail feathers are mid-grey (similar in colouration to fresh wing feathers and therefore probably 83 Dark Neutral Gray or 84 Medium Neutral Gray) with a blackish-grey subterminal band (c. 1 cm wide) and whitish tips.
Underparts
The holotype shows white chin feathers with very dark grey (82 Blackish Neutral Gray) bases, tips and fringes, therefore darker overall than feathers on malar stripe, the latter overhanging those on throat. In the holotype, feathers of throat in an advanced stage of moult, some feathers in pin visible. Throat similar in colouration to chin although with much less white; feathers almost entirely solid dark grey (82 Blackish Neutral Gray) gradually becoming darker towards the breast (83 Dark Neutral Gray) with some white tips throughout. On the breast some dark grey feathers (83 Dark Neutral Gray) possess contrasting white rachis and base of barbs on distal two-thirds of feather, creating a pattern of whitish drops on a mid-grey background. On the edges of the breast, solid mid-grey (84 Medium Neutral Gray) feathers predominate. On the adult male paratype (NHMUK 2012.9.2), the whitish drop-shaped marks on the breast are better developed than on the holotype and extend onto the throat, perhaps because the darker fringes are more worn. Field observations indicate that there is variation in the extent and intensity of dark throat-streaking in males (Media files SOM 1–2). The boundary between breast and belly is gradual; feathers tend towards lighter grey on belly (86 Pale Neutral Grey) and flanks (85 Light Neutral Gray). Flank feathers are relatively long. Vent greyish-white (paler than 86 Pale Neutral Gray). Thighs whitish-cinnamon (6 Salmon).
The underparts of the immature male paratype (NHMUK 2012.9.3) differ from those of the adult male holotype in being paler with reduced dark grey on the throat and upper breast. There is an almost complete lack of dark tones on the throat, and the very dark grey (82 Blackish Neutral Gray) area on the throat is much smaller and barely extends onto the breast. On the throat and breast, feathers with white shaOEs and distal portions are more abundant than on the holotype, giving the throat a more speckled appearance. On the breast, solid white and pale grey feathers predominate such that the overall colour is whitish-grey (86 Pale Neutral Gray) rapidly grading to off-white on the belly. Flank feathers of the immature male are slightly whiter than those of the holotype. The underparts of the immature female paratypes are even paler than those of the immature male and almost completely lack dark tones. In those two specimens the chin and throat are white. Although there is a small area of mid-grey (84 Medium Neutral Gray) on the sides of the upper breast and the flank feathers are pale grey (86 Pale Neutral Gray or 85 Light Neutral Gray), the underparts are otherwise off-white.
Bare parts
Upper mandible dark horn, lower mandible pink horn, paler and pinker at base (more extensively pink on adult male paratype). Bill slender. Culmen decurved close to tip, not strongly carinated, tip very slightly hooked.Gonys convex.Tarsus and toes pinkish (slightly darker in adult male paratype, paler in immature male paratype); soles of the feet pale pink. Claws pale brownish pink, becoming paler towards tips. On female paratypes tarsi, feet, soles and lower mandibles are paler than those of the holotype. Iris orange-brown. Inside of mouth pale pink.
Description of vocalisations
For clarity we use the following terminology to describe vocalisations: note – a single song element; strophe – a continuous flow of notes, separated from other strophes by silent pauses; phrase – one or more strophes given in quick succession; and song – one or more phrases given in quick succession; strophe pace – number of notes per strophe/strophe length; phrase pace (for phrases with more than one strophe) – phrase duration/strophes per phrase. Note that recordings varied in length and quality, so only those with good quality strophes were analysed.
Male O. chaktomuk songs are lengthy, oOEen lasting more than one minute ( Figure S1 View Figure 1 o–s, S1u, Media Files SOM 1–6). They consist of multiple phrases repeated at intervals of 0.42–4.30 seconds, typically much shorter than the maximum interval (mean: 1.7 seconds). Phrases are made up of 2–5 strophes, which are given at 0.12–0.95 second intervals. Males occasionally switch to a different strophe type mid-way through a song, although not within the same phrase. Strophes are also sometimes given singly.Strophes are trilled, consist of 3–18 notes and typically last 0.17–0.49 seconds ( Table 3). Twelve distinct male strophe types are known, ranging from up, down or ‘overslurred’ (the latter referring to sequences of notes that rise and then fall) trills (oOEen with a louder initial or terminal note) to a mix of trilled notes and upslurs, downslurs or ‘overslurs’ ( Table 3, Figure S1 View Figure 1 a–l, Media Files SOM 1–6). Within strophe type, number of notes varies slightly ( Table 4).
Female O. chaktomuk vocalisations are typically emitted whilst the male is vocalising, but are sometimes given between male vocalisations ( Figure S1 View Figure 1 o–s, S1u, Media File S1–6). Females give a stereotyped trill at a higher frequency than male vocalisations (typically 5–16 notes lasting 0.24–0.84 seconds; Table 3, Figure S 1m View Figure 1 , Media File S1–6). Females, and exceptionally males, sometimes produce a nasal squeak consisting of a single note with harmonics ( Figure S1n View Figure 1 , Media File S6). This vocalisation is usually given singly ( Figure S1p View Figure 1 ), but occasionally more than one is repeated in quick succession; when many squeaks are given in sequence the first is usually longer than others ( Figure S 1t View Figure 1 , Media File S6).
Etymology
The specific epithet ‘ chaktomuk ’ is a Khmer word meaning ‘four faces’. It is used in reference to the low-lying area at which the Tonle Sap, Bassac and Mekong rivers come together to form an ‘X’ centred on Phnom Penh, itself historically known as ‘Krong Chaktomuk’ (literally ‘City of Four Faces’). Based on current knowledge, the global distribution of the new species is restricted to scrub within the dynamic floodplain created by the confluence of these waters. We use chaktomuk as a noun in apposition to the genus name, and it is thus invariable.
Nomenclatural acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature ( International Commission on Zoological Nomenclature 2012), and hence the new name contained herein is available under that Code from the electronic edition of this article. This published work and the nomenclatural act it contains have been registered in ZooBank, the online registration system for the International Commission of Zoological Nomenclature. The ZooBank Life Science Identifiers (LSIDs) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the digital repository BioTaxa (http://biotaxa.org).
ECOLOGY AND BEHAVIOUR
Habitat
All observations of O. chaktomuk were made on level ground in very dense humid evergreen scrub (multi-stemmed woody plants, 2–6 m tall), sometimes admixed with long grasses or trees ( Plate 5 View Plate 5 ), at elevations of 3–25 m above sea level. Trees occur exceptionally; where present they are typically scarce, the scrub forming a dense layer with occasional tree canopies emerging from it. Orthotomus chaktomuk has not been seen in forest (defined as a habitat where trees predominate) and is therefore assumed to be absent from it. At all locations where birds have been found, the scrub is located within a floodplain and experiences seasonal or permanent (artificial) flooding. The presence of seasonally flooded scrub in any location is probably typically transitory, since in the absence of disturbance by people, large ungulates or hydrological processes it would presumably revert to seasonally flooded forest.
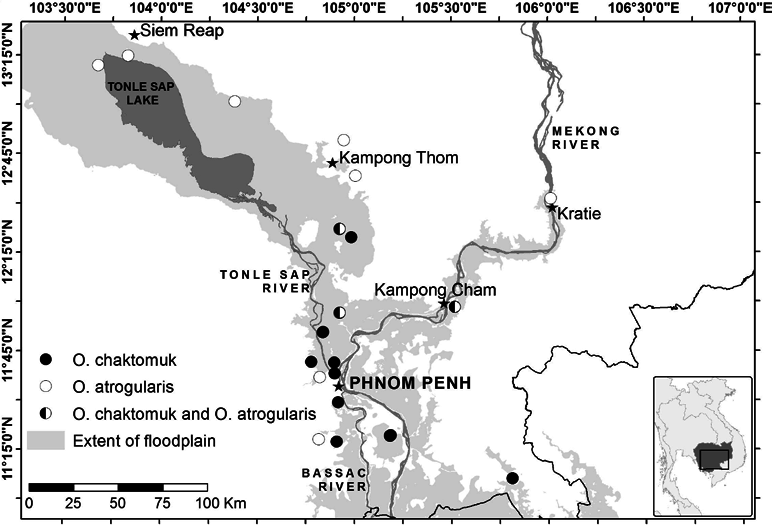
Orthotomus atrogularis is sometimes found in seasonally flooded scrub occupied by O. chaktomuk ( Figure 1 View Figure 1 , Table SOM 1). Where the two species are syntopic, O. atrogularis is much the rarer. Typically O. atrogularis is found in the edge and canopy of taller forest habitats, showing a preference for disturbed and secondary forest because these offer an abundance of vines ( Madge 2006, Wells 2007). In some parts of the Tonle Sap floodplain where O. chaktomuk is absent, O. atrogularis is common in seasonally flooded forest and scrub, presumably because this habitat also offers an abundance of edge surfaces. At the other end of the habitat continuum, O. sutorius replaces O. chaktomuk in open scrub and gardens, although at some locations the two species are syntopic, even vocalising from the same individual plants (SPM pers. obs.). Orthotomus chaktomuk possibly occupies a habitat intermediate between those of O. atrogularis and O. sutorius . However, as in other geographic areas where more than one lowland tailorbird species is present, habitat niches are difficult to define and distinguish. Ecological interactions and habitat associations of O. chaktomuk and other lowland tailorbirds are worthy of further research.
Birds sharing the habitat of O. chaktomuk include widespread species oOEen associated with gardens, e.g. Yellow-vented Bulbul Pycnonotus goiavier, Pied Fantail Rhipidura javanica, Oriental Magpie Robin Copsychus saularis , sometimes O. sutorius , and species usually associated with dense lowland humid evergreen scrub, including Striped Tit Babbler Macronous gularis , Yellow-bellied Prinia Prinia flaviventris, Plain Prinia Prinia inornata, Olivebacked Sunbird Cinnyris jugularis and O. atrogularis . From October to April, Palearctic migrants (e.g. Dusky Warbler Phylloscopus fuscatus and Siberian Rubythroat Luscinia calliope ) are abundant in this habitat. In locations where it occurs, O. chaktomuk oOEen appears to be one of the most abundant bird species.
Behaviour
Owing to the structural characteristics of its habitat, O. chaktomuk is rarely seen without the aid of playback of vocalisations, and thus data on ‘normal’ behaviour are few. Almost all encounters have been with what appear to be adult male–female pairs, or adult malefemale pairs with one subadult. Prior to moult, immature birds were seen singly, in male–female pairs of exclusively immature birds or in male–female pairs consisting of one immature and one adult bird. Birds usually stay within dense vegetation, where they glean and sally-glean from live and dead leaves of multi-stemmed bushes and occasionally vines, from ground-level to canopy. Orthotomus chaktomuk has not been observed foraging in trees. When vegetation is flooded, birds typically forage below the crown of the bush, on hanging branches just above the water. One individual that was lured out of dense scrub with the aid of playback foraged on long grass-stems, gleaning leaves of a vine that was growing amongst the grass. Individuals have been observed taking the following prey (once each): a small fly Diptera, a small spider Araneae, a small caterpillar Lepidoptera and a small katydid Tettigoniidae ; all were consumed immediately.
In response to playback, birds that have approached the observer have been seen to sing, usually in a duet, while perched (usually on or near the top of vegetation, including trees; Media Files SOM 1–3) and occasionally in song flight. Singing is sometimes accompanied by rapid downwards tail-wagging. Sometimes, while singing in duet, perched birds droop and shiver their wings. Immature males gave a simpler, less developed song than adults.
During March and April only males responded strongly to playback of vocalisations; females typically did not respond, or did so only briefly. Because this was in stark contrast to behaviour at other times of year it is thought to indicate that females were on the nest. Although there are no data on the timing of breeding of lowland tailorbirds in Cambodia, in Thailand O. atrogularis pairs with dependent young have been recorded from July to early September ( Round 2008).The nest and eggs of O. chaktomuk remain to be described.
Distribution
The distribution of O. chaktomuk is incompletely known. It is apparently constrained by the distribution of seasonally flooded dense scrub within the floodplain of the Tonle Sap, Mekong and Bassac rivers in Cambodia ( Figure 1 View Figure 1 ). However, based on current data it is absent from part of this floodplain. Searches at various locations in apparently suitable habitat in the Tonle Sap floodplain have thus far only found the species in the south-east (see Table SOM 1 for a list of all locations in the floodplain of the Mekong, Tonle Sap and Bassac rivers where searches for O. chaktomuk have been conducted). In the north of the Tonle Sap floodplain (where we have searched for and not found O. chaktomuk ), O. atrogularis is abundant in habitat that is superficially structurally similar to habitat in the south-east, and it is unclear how far north and west along the lakeshore the distribution of O. chaktomuk extends. There is no biogeographic reason why O. chaktomuk should be absent from parts of the Tonle Sap floodplain, and the causes of its absence are unknown; O. atrogularis is scarce or absent at sites where O. chaktomuk was recorded (Table SOM 1).
Orthotomus chaktomuk was not found in seemingly appropriate small seasonally flooded scrub patches at the northern limit of the Mekong floodplain ( 12°36’27.52”N 106°01’36.06”E) in Kratie province (Table SOM 1, J. A. Eaton verbally 2012). Satellite data indicate that there is little, if any, suitable habitat for O. chaktomuk in the Mekong floodplain in Vietnam and it is currently unrecorded there (although no specific searches have been conducted). As might be expected, we have located only O. atrogularis in scrub habitats outside of the Mekong, Tonle Sap and Bassac floodplain (where these records were within 10 km of superficially suitable habitat for O. chaktomuk they are mapped on Figure 1 View Figure 1 ). Based on current knowledge of its range, the distribution of O. chaktomuk covers less than c. 10,000 km 2 ( Figure 1 View Figure 1 ); it therefore can be considered a restricted-range species ( sensu Stattersfield et al. 1998).
Conservation
Orthotomus chaktomuk is restricted in distribution. Suitable habitat is patchy outside of the Tonle Sap floodplain and in the latter its distribution is poorly understood. Trends in loss, degradation and fragmentation of floodplain scrub are poorly documented and subject to considerable local variation (e.g. Packman et al. 2013). However, most floodplain scrub in Cambodia occupies land suitable for rice cultivation and could be further threatened by changes in ongoing burning, fuel-wood collection, cattle grazing (all of which potentially have a dual role because they also serve to slow succession) and the spread of the invasive plant Mimosa pigra . Ironically, O. chaktomuk might now be dependent on human activity to keep suitable scrubby habitat from becoming forest, since other anthropogenic impacts—eradication of wild ungulates, replacement of domestic animals by machines, water flow/level control, and changes in agricultural practices such as fallows and cyclical abandonment—have greatly curtailed processes that maintained the scrub. The species occurs in one protected area, Baray Bengal Florican Conservation Area, although at that site habitat is managed to maximise the area of grassland. It has already been lost from one site (Kraing Check) where birds were netted in 2009: visits in late 2012 found no birds and all suitable habitat had been converted to aquaculture ponds.
We believe that O. chaktomuk should be classified as Near Threatened on the IUCN Red List because it approaches the thresholds for Vulnerable under criteria B1a+bi,ii,iii,iv ( IUCN 2001). Its Extent of Occurrence is 9,385 km 2 and thus below the threshold for Vulnerable (< 20,000 km 2; criterion B1). Although most locations where it occurs are small and isolated it has been found in the Tonle Sap floodplain where there is a large area of apparently suitable habitat (although it apparently does not occupy all of it). Because of this, its habitat cannot be considered severely fragmented (subcriterion a). Nonetheless it is inferred to be undergoing a continuing decline (subcriterion b) in (i) extent of occurrence, (ii) area of occupancy, (iii) area, extent and/or quality of habitat, and (iv) number of locations or subpopulations. Its Area of Occupancy has not yet been evaluated owing to uncertainty regarding both the distribution of suitable habitat and its distribution within apparently suitable habitat. Notwithstanding this assessment, if the species is found to be more widely distributed in the Tonle Sap floodplain, then it would warrant downlisting to Least Concern.
Ongoing habitat loss is likely to be exacerbated by the impacts of hydropower development on the Mekong and its tributaries. Models of the effects of hydropower dams predict changes in the duration and size of the annual flood-pulse that will lead to a reduction in the extent of seasonally flooded habitats ( Arias et al. 2012). Dam construction will also reduce fish populations (the primary protein source in rural Cambodia), cause changes in flood regime and lead to water shortages in the floodplain ( Orr et al. 2012). These changes will probably lead to additional loss of floodplain scrub owing to expansion of agricultural land for rice production, fish ponds and grazing land for cattle. Construction has started on one mainstream lower Mekong dam (Xayaburi, in northern Lao PDR) and numerous tributary dams and ‘pre-construction’ works are thought to have begun on another (Don Sahong) in the far south of Lao PDR ( International Rivers 2012); nine more mainstream dams are planned ( Mekong River Commission 2011).
| NHMU |
NHMU |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |