Dicyemennea mcconnaugheyi, Furuya, 2018
|
publication ID |
https://doi.org/10.12782/specdiv.23.143 |
|
publication LSID |
lsid:zoobank.org:pub:82CD9349-810A-42F1-A602-343EBA1AE7A4 |
|
persistent identifier |
https://treatment.plazi.org/id/03D9344C-3754-FFCF-FEE1-AAD5FCD6D56D |
|
treatment provided by |
Felipe |
|
scientific name |
Dicyemennea mcconnaugheyi |
| status |
sp. nov. |
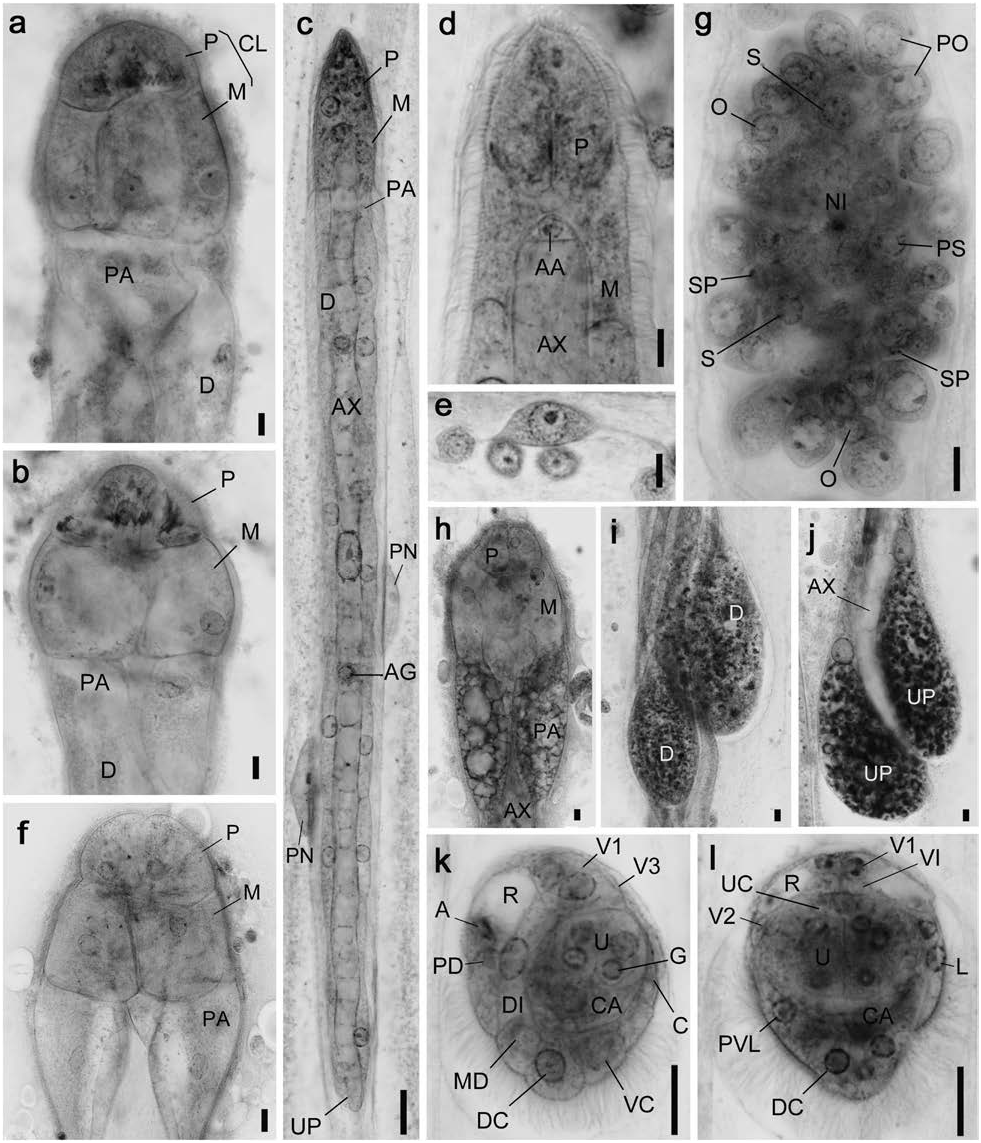
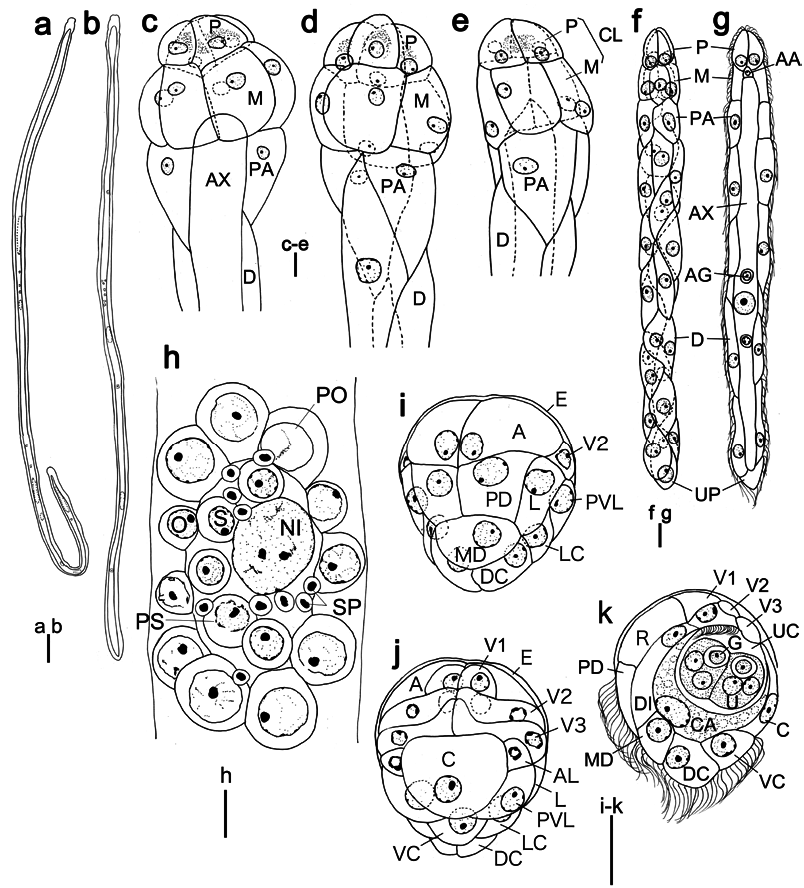
Genus Dicyemennea Whitman, 1883 View in CoL Dicyemennea mcconnaugheyi sp. nov. ( Figs 6 View Fig , 7 View Fig ; Tables 1, 3)
Diagnosis. Large dicyemid; body length up to 9,240 µm. Calotte shape conical. Vermiform stages with 34–37 peripheral cells: 4 propolars+5 metapolars+2 parapolars+23–26 trunk cells. Infusoriform embryos with 39 cells; refringent bodies solid; and 2 nuclei present in each urn cell.
Description. Nematogens ( Figs 6a, b, h–j View Fig , 7a, c, d View Fig ). Body length 4,000 –9,240 µm and width 70–90 µm; widest in region of diapolars; trunk width mostly uniform. Peripheral cell number 34–37 ( Table 3): 4 propolars+5 metapolars+2 parapolars+21–24 diapolars+2 uropolars. Calotte conical and round anteriorly; cilia on calotte about 4 µm long, oriented anteriorly. Propolar cells and their nuclei smaller than metapolar cells and their nuclei. Propolar cells occupying anterior 30–35% of calotte length when viewed laterally ( Figs 6a, b View Fig , 7c, d View Fig ). Cytoplasm of propolar and metapolar cells more darkly stained by hematoxylin than cytoplasm of other peripheral cells. Verruciform cells present in parapolar, diapolar, and uropolar cells ( Fig. 6h–j View Fig ). Axial cell cylindrical and rounded anteriorly; cell extending forward to middle of metapolar cells ( Fig. 7c View Fig ). About 20 vermiform embryos present in an axial cell of large individuals. Agametes occasionally fusiform in shape ( Fig. 6e View Fig ).
Vermiform embryos ( Figs 6c, d View Fig , 7f, g View Fig ). Full-grown vermiform embryo length 430–650 µm and width 20–35µm. Peripheral cell number 34–37 ( Table 3); trunk cells arranged in opposed pairs. Anterior end of calotte bluntly pointed. Axial cell rounded anteriorly; extending to middle of metapolar cells; nucleus usually located in middle or posterior half of axial cell. Anterior abortive axial cell present ( Figs 6d View Fig , 7g View Fig ). Axial cell of full-grown embryos often with 6 agametes.
Rhombogens ( Figs 6f View Fig , 7b, e View Fig ). Body similar in length but slightly stockier than nematogens, length 4,350 –8,700 µm, and width 72–115 µm. Peripheral cell number typically 34–37 ( Table 3). Calotte conical and round anteriorly. Verruciform cells present. Axial cell shape and anterior extent similar to nematogens. One to 3, rarely 6 infusorigens present in axial cell of each parent individual. Individuals with single infusorigen bearing larger infusorigens ( Fig. 6g View Fig ). About 170 infusoriform embryos present per axial cell of large individuals. Accessory nuclei occasionally present in trunk cells.
Infusorigens ( Figs 6g View Fig , 7h; n View Fig =20). Mature infusorigens largesized; composed of 59–264 (mode 117) external cells (oogonia and primary oocytes)+16–144 (mode 69) internal cells (spermatogonia, primary spermatocytes, and secondary spermatocytes)+14–112 (mode 56) spermatozoa. Mean diameters of fertilized eggs and spermatozoa 14.0 µm and 3.0µm, respectively. Axial cell round or ovoid, diameter 20–153µm.
Infusoriform embryos ( Figs. 6k, l View Fig , 7i–k; n View Fig =50). Full-grown embryos large, length 30.8±1.4µm (mean±SD, excluding cilia); length-width-height ratio 1.0:0.77: 0.77; shape ovoid, bluntly rounded to pointed posteriorly; cilia at posterior end 8 µm long. Refringent bodies present, solid, occupying anteri- or 30% of embryo length when viewed laterally ( Figs 6k View Fig , 7k View Fig ). Cilia projected from ventral internal cells into urn cavity ( Fig. 6j View Fig ). Capsule cells containing large granules ( Fig. 6j View Fig ). Mature embryos with 39 cells: 35 somatic+4 germinal cells. Somatic cells of several types present: external cells covering a large part of anterior and lateral surfaces of embryo (2 enveloping cells); external cells with cilia on external surfaces (2 paired dorsal cells+1 median dorsal cell+2 dorsal caudal cells+2 lateral caudal cells+1 ventral caudal cell+2 lateral cells+2 posteroventral lateral cells); external cells with refringent bodies (2 apical cells); external cells without cilia (1 couvercle cell+2 anterior lateral cells+2 first ventral cells+2 second ventral cells+2 third ventral cells); internal cells with cilia (2 ventral internal cells); and internal cells without cilia (2 dorsal internal cells+2 capsule cells+4 urn cells). Each urn cell containing germinal cell and 2 nuclei ( Fig. 7k View Fig ). All somatic nuclei pycnotic in mature infusoriform embryos.
Remarks. Dicyemennea mcconnaugheyi sp. nov. is the first species of the genus described from Octopus longispadiceus . This species is very similar to D. californica McConnaughey, 1941 , D. granularis McConnaughey, 1949 , and D. nouveli McConnaughey, 1959 , in the calotte shape of vermiform stages, peripheral cell numbers, and the cellular composition and cell number of infusoriform embryos ( McConnaughey 1941, 1949, 1959; Furuya et al. 2004; Furuya 2007, 2008). Eosinophilic granules in diapolar cells are characteristic of D. granularis ( McConnaughey 1949) . Dicyemennea mcconnaugheyi sp. nov. also has granules in the diapolar cells (verruciform). In this respect, D. granularis is the most similar species to D. mcconnaugheyi sp. nov. from among the above three species. However, D. mcconnaugheyi sp. nov. is distinguishable from D. granularis in the length of infusoriform embryos (nearly 31µm vs. 35µm) and maximum agamete number of vermiform embryos (6 vs. 2). Dicyemennea californica shares the most of characters with D. granularis except for lacking granules in diapolar cells, thus, D. mcconnaugheyi sp. nov. is also distinguishable from D. californica in the length of infusoriform embryos (nearly 31 µm vs. 35µm) and maximum agamete number of vermiform embryos (6 vs. 2).
Dicyemennea nouveli has been reported from the enteroctopodid octopus, Enteroctopus dofleini (Wülker, 1910) , in the northeastern Pacific Ocean (Monterey Bay, California), the northern region of the Sea of Japan ( Russia), and the northwestern Pacific Ocean (off northern and eastern areas of Shiriya Point, Aomori, Japan) ( McConnaughey 1959; Bogolepova-Dobrokhotova 1963; Furuya 2008). In Japanese waters, the habitat of O. longispadiceus overlaps with that of E. dofleini . However, D. mcconnaugheyi sp. nov. is distinguishable from D. nouveli in the maximum length of adult vermiform stages (9,240 vs. 12,000µm), the length of infusoriform embryos (nearly 31 µm vs. 40 µm), and maximum agamete number of vermiform embryos (6 vs. 4).
Etymology. The specific name mcconnaugheyi is in honor of Dr. B. H. McConnaughey who studied dicyemid taxonomy in the USA.
Taxonomic summary. Type material: a syntype slide (NSMT-Me-49) collected at 16 March 2015; additional syntypes on slide series No. OL3254 (5 slides) in the author’s collection.
Type locality: off Nou ( 37°09′N, 137°54′E), Niigata Prefecture, Honshu, the Sea of Japan, Japan, depth 200 m GoogleMaps .
Other material examined: slide series Nos . OL870, 871 (each 5 slides) collected off Iwase ( 36°48′N, 137°15′E), Toyama Bay , Toyama Prefecture, Honshu, Japan, depth 350 m, 6 March 2003; No GoogleMaps . OL2192 (5 slides) collected off Karo ( 35°47′N, 134°14′E), Tottori Prefecture, Honshu , the Sea of Japan, Japan, depth 200 m, 4 March 2009; No GoogleMaps . OL2367 (5 slides) collected off Ohda-shi ( 35°23′N, 132°19′E), Shimane Prefecture, Honshu , the Sea of Japan, Japan, depth 200 m, 28 January 2010 in the author’s collection GoogleMaps .
Host: symbiotype, Octopus longispadiceus ( Sasaki, 1917) (Mollusca: Cephalopoda: Octopoda ), female (mature), 89 mm ML (NSMT-Mo-85867).
Site : anterior ends (calottes) inserted into crypts of the renal appendages within the renal sacs.
Prevalence: in 34 of 510 specimens of hosts examined (6.1%).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.