Cambarus (Cambarus) appalachiensis, Loughman, Zachary J., Welsh, Stuart A. & Thoma, Roger F., 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4243.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:6771949E-C3B2-4A3F-ABFD-FA3D90454E9A |
|
DOI |
https://doi.org/10.5281/zenodo.5630136 |
|
persistent identifier |
https://treatment.plazi.org/id/03D787EE-8F22-3569-FF13-FADDFDFB5C3E |
|
treatment provided by |
Plazi |
|
scientific name |
Cambarus (Cambarus) appalachiensis |
| status |
sp. nov. |
Cambarus (Cambarus) appalachiensis View in CoL new species
Diagnosis. Body and eyes pigmented. Rostrum broad, moderately excavated, and deflected anteriorly, margins thickened, parallel though rarely sub-parallel; converging to acumen abruptly at terminance of rostral margins. Acumen distinctly triangular with prominent dorsally deflected spiniform tubercle at terminus. Areola 2.2–5.1 (= 3.4, n = 98, σ = 0.6) times as long as wide with 5–8 (usually 7) punctations across narrowest point. No cervical spines; 4–7 ( = 6.1, SE = 0.8) tubercles present. Mandibular, branchiostegal, and orbital regions of carapace with well-developed tubercles. Postorbital ridges short; pronounced, spiniform, dorsally deflected cephalic tubercle present in juveniles and subadults; adult postorbital ridge terminating in reduced to prominent dorsally deflected cephalic tubercle. Suborbital angle acute. Total carapace length (TCL) 1.8–2.1 ( = 1.9, n = 98, SE = 0.1) times longer than width. Form I and II males possessing hook on ischium of third pereopod only; hook gently curved at apex, overarching basioischial joint in form I males, not reaching basioischial joint in form II males; hooks not opposed by tubercle on basis. Mesial surface of chela nearly always possesses a single mesial row of tubercles; but rarely (3% of individuals; n = 98) an incomplete second row of tubercles also present; mesial most row with 6–10 ( = 7.9, n = 98, SE = 0.5) tubercles, second incomplete row when present with 2–4 (= 2.2, n = 3, SE = 0.6) tubercles. Ventral surface of chelae with 1–3 ( = 1.9, n = 98, SE = 0.7) subpalmar tubercles near the dactyl/ propodus junction. Dorsomedian ridge of fixed finger of propodus pronounced. Lateral impression at base of fixed finger shallow (61% of individuals) or absent (39% of individuals). Dactyl and fixed finger each with sharp corneous tip. Form I male palm length 64.3–77.1% ( = 69.9%, n = 32, SE = 2.8%) of palm width, form I male palm length 27.1–31.3% ( = 29.6%, n = 32, SE = 1.2%) of total propodus length; female dactyl length 55.2– 64.1% ( = 61.5%, n = 35, SE = 2.2%) of total propodus length. First pleopod of form I male with short terminal elements. Central projection not tapering distally; recurved>90° to main shaft, with distinct subapical notch. Mesial process directed 90° to shaft, bent cephalolaterally; inflated proximally, tapering to a point distinctly caudal to slighty cephalic to terminance of central projection and not projecting beyond the edge of the margin of gonopod shaft. Annulus ventralis immovable; distinctly asymmetrical caudally; cephalic portion with median trough leading to strongly sculptured central fossa; exaggerated “S” bend in sinus terminating at caudal edge.
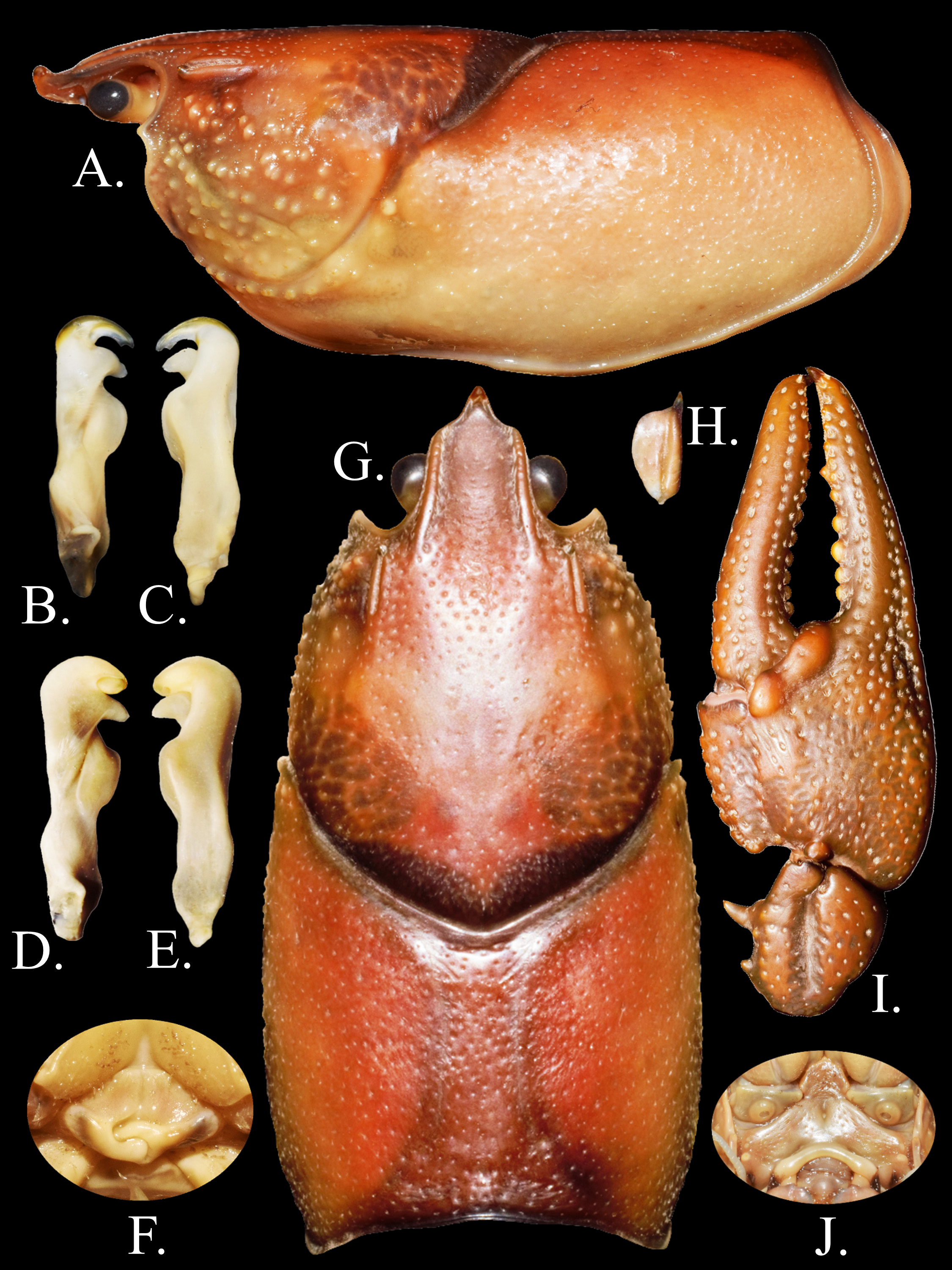
Description of holotypic male, form I. ( Fig. 2 View FIGURE 2 A–C, G–J; Table 1 View TABLE 1 ). Body somewhat compressed dorsoventrally ( Fig. 2 View FIGURE 2 A); thoracic section of carapace slightly wider than abdomen. Carapace depth less than carapace width at caudodorsal margin of cervical groove. Total carapace length 38.1 mm; post orbital carapace length 32.1 mm. Areola 2.8 times longer than wide, with 7 punctations across narrowest part ( Fig. 2 View FIGURE 2 G); length of areola 34.9% of TCL (41.4% of postorbital carapace length (PCL). Rostrum weakly excavated, more so anteriorly than posteriorly; margins thickened and parallel; acumen margins noticeably thinner than rostral margin forming distinct 90 0 break from rostrum margins, continuous to base of acumen; floor of rostrum with numerous punctations. Rostrum 1.7 times longer than wide. Acumen distinctly triangular, ending in dorsally deflected corneous tip ( Fig. 2 View FIGURE 2 A). Postorbital ridges well developed, terminating in dorsally deflected spiniform tubercles. Suborbital angle acute, with tubercle ( Fig. 2 View FIGURE 2 A). Cervical spine absent; several weakly to moderately developed tubercles present. Mandibular, branchiostegal, and orbital regions of carapace punctated with well-developed tubercles; greatest tubercle density in hepatic region. Abdomen slightly longer than carapace, pleura rounded cephaloventrally, angled caudoventrally. Cephalic section of telson with 2 large spines in each caudolateral corner. Proximal podomere of uropod with distal spine on mesial lobe; mesial ramus of uropod with median ridge ending distally in distomedian spine not overreaching margin of ramus; laterodistal spine pronounced. Distal margin of proximal segment of lateral ramus of right uropod having 14 immovable, small spines and 1 lateral, large, movable spine. Cephalomedian lobe of epistome subtriangular, zygoma moderately arched ( Fig. 2 View FIGURE 2 J); cephalolateral margins thickened, forming sharp angle at junction with endostyle; main body possessing prominent cephalomedian fovea. Antennal scale broadest anteriorly; lateral margin thickened, terminating in large corneous spine; ( Fig. 2 View FIGURE 2 H). Right antennal scale 5.4 mm long, 2.5 mm wide. Tip of right antenna reaching middle of telson when appressed. Mesial surface of right chela with one well-formed row of 8 tubercles ( Fig. 3 View FIGURE 3 K). Palm length 68.1 % of palm width; depth of palm 9.6 mm. Ventral surface of palm lacking subpalmar tubercles. Dorsal longitudinal ridge of dactyl developed and possessing moderate sized tubercles ( Fig. 2 View FIGURE 2 I); dactyl terminating in large corneous spine. Dorsomedian ridge of fixed finger of propodus weakly developed. Weakly developed lateral impression at the junction of fixed finger and palm; numerous setiferous punctations present. Dactyl and fixed finger with sharp, corneous tip. All measurements and counts from right chela. Carpus with prominent dorsal furrow ( Fig. 2 View FIGURE 2 I) and 2 weak dorsomesial tubercles; rest of surface with setiferous punctations; mesial margin with large, procurved spine at about midlength, and one reduced proximal tubercle. Distodorsal surface of merus with 9 spiniform tubercles; ventrolateral ridge with 2 small spines and one large, corneous distal spine; ventromesial ridge with two welldeveloped spines. Carapace depth less than width. Hook on ischium of third pereopod only; hook gently curved at apex, overarching basioischial joint, not opposed by tubercle on basis. Form I gonopod as described in diagnosis ( Fig. 2 View FIGURE 2 B–C); tip reaching anterior margin of fourth caudomesial boss when abdomen flexed.
Description of allotypic female. ( Fig. 2 View FIGURE 2 F, Table 1 View TABLE 1 ).––Differing from holotype in following respects; carapace height less than carapace width (16.9 and 21.1 mm, respectively); TCL 39.6 mm, PCL 33.5 mm. Areola length 35.1% of TCL (41.4% of PCL), 3.0 times as long as wide. Posterior portion of rostrum more excavated than anterior portion; rostrum 1.7 times longer than wide. Abdomen length 41.1 mm. Mesial surface of chelae with single row of 8 tubercles. Palm length (10.0 mm) 62.5% of palm width (16.0 mm); depth of palm 9.2 mm. All measurements and counts from right chela. Antennal scale 5.7 mm long, 2.3 mm wide. Annulus ventralis as described in diagnosis ( Fig. 2 View FIGURE 2 F); width of postannular sclerite half total width of annulus ventralis; first pleopods uniramous, reaching central region of annulus ventralis when abdomen flexed.
Description of morphotypic male, form II. ( Fig. 2 View FIGURE 2 D–E, Table 1 View TABLE 1 ). Differing from holotype in the following respects: Carapace height less than carapace width (19.0 and 24.3 mm respectively); TCL 44.0 mm and PCL 37.8 mm. Areola length 36.8% of TCL (42.8% of PCL), 3.6 times longer than wide. Rostrum margins parallel to base of acumen and thickened; rostrum ventrally deflected and excavated; rostrum 1.6 times as long as wide. Abdomen 43.0 mm long. Single mesial row of tubercles on palm of chela with 7 tubercles. Palm length (13.3 mm) 65.5% of palm width (20.3 mm). All measurements and counts from right chela. Antennal scale 6.6 mm long, 2.9 mm wide. Gonopods reaching anterior margin of 4th pereopod caudomesial boss. Central projection curved 90° to shaft, with complete apex; rounded ( Fig. 2 View FIGURE 2 D–E). Mesial process tapered, bulbous, directed caudolaterally. Hook on ischium of third pereopod small, not reaching basioischial joint.
Size. Form I male (n = 32) TCL ranges in size from 30.1–48.6 mm (PCL 25.4–41.0 mm) with a mean TCL of 37.4 mm. Form II male (n = 31) mean TCL is 36.6 mm and ranges in size from 24.9–46.9 mm (PCL 20.7–40.0 mm). Female (n = 35) TCL mean is 37.0 mm and ranges from 11.1–45.0 mm (PCL 8.5–38.1 mm). The largest specimen examined was a form I male with TCL of 48.6 mm (PCL 41.0 mm).
Color. Cambarus appalachiensis ( Fig. 3 View FIGURE 3 ) carapace ground color is chestnut brown to pink to orange-brown; posterior margin of carapace dark brown to black. Hepatic and antennal region of carapace punctuated with cream, olive, or tan tubercles. Postorbital ridge orange, red-brown, to light. Rostrum margins and acumen cream to orange, orange-brown, red-brown or tan. Cephalic section of carapace immediately anterior to and including cervical groove is black, forming weak saddle; mandibular abductor scars mottled, ranging from light-brown, brown, to dark-brown. Lateral margin of antennal scale olive to light brown; body of antennal scale brown to cream. Antennal flagellum and antennules green-brown, with olivaceous hue; dorsal surface of lamellae tan to brown; ventral surface light-green to olivaceous. Dorsal surface of chelae variable ranging from green, green-brown, olivaceous, orange, light-brown, or brown; occasionally with mesial row of tubercles cream, tan or orange. Denticles on opposable surfaces of fingers yellow, white, or tan. Ventral surface of chelae cream or tan. Dorsal surface of carpus light green, green-brown, or olivaceous; region adjacent to and including furrow green-brown to green; carpus spine orange, orange brown or cream. Merus green-brown, grey, or olivaceous brown. Podomeres of pereopods light blue, cream, or grey-blue; joints of pereopod podomeres pink. Dorsal and dorsolateral surface of abdomen same colors as carapace; tergal margins brown to olivaceous brown; abdomen lacking dorsal stripe. Uropods same colors as abdomen. Ventral surface of abdomen and carapace cream. Dorsal ridge of form I gonopod central projection amber; body of central projection, gonopod, and mesial process tan. Form II gonopod and all associated processes cream. Cephalic portion of annulus ventralis pink to pink-cream; ridge of fossa pink; caudal region of annulus ventralis ranges from pink to cream colored.
Type locality. Pipestem Creek at intersection of Tom-Honaker Road (CR 20-3) and State Route 20, 3.3 km (2.04 mi) north-east of Pipestem , Summers County, West Virginia . The type series was collected on 11 October, 2016 by Z. J. Loughman. At this site ( Fig. 4 View FIGURE 4 ), Pipestem Creek is 4–10m wide, and consists of a series of high gradient riffles and glides, followed by moderate to low gradient runs with occasional pools. Substrates utilized by C. appalachiensis included large slab-rock, boulders, and large cobbles, as well as leaf packs. Riparian conditions ranged from residential yards to intact mesophytic forest bordered by SR 20. Water depth ranged from 0.2–1.0 m deep. Here, C. appalachiensis was both abundant and the only crayfish species observed. The type series was collected (n=3), along with several additional specimens (n = 15).
Disposition of types. The holotype, allotype, and morphotype are deposited in the North Carolina Museum of Science ( NCSM), Raleigh, NC. (catalogue numbers NCSM 1251657, 1251658 , 1251659, respectively) . Paratypes consisting of 1 M1, 1 MII, and 1 F are deposited in the West Liberty University Astacology Collection ( WLU 3003 View Materials ), the United States National Museum ( USNM 1422178 View Materials ) and the Ohio State Museum of Zoology ( OSUMC 9916 ) .
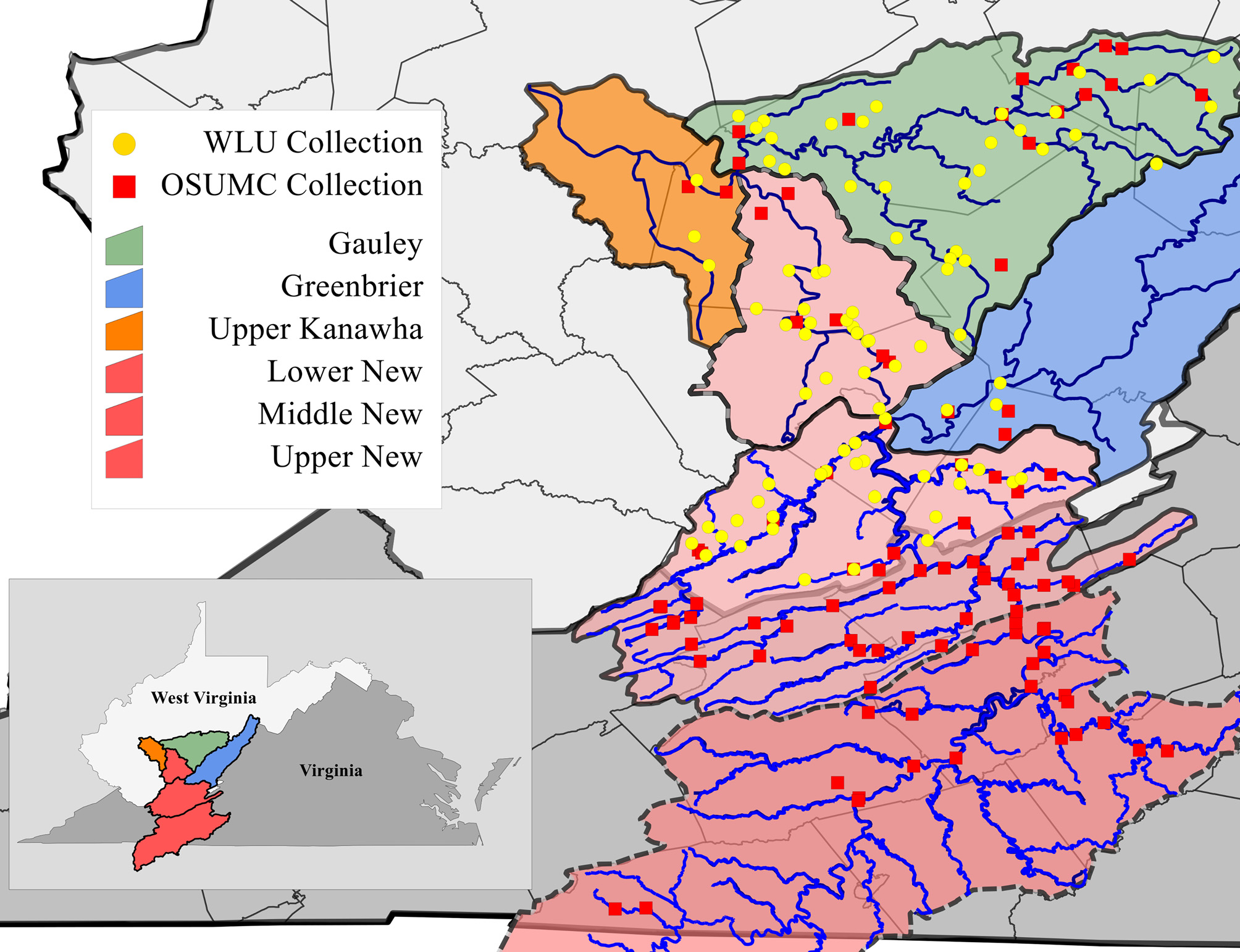
Range and specimens examined. Cambarus appalachiensis ( Fig. 5 View FIGURE 5 ) occurs in the Ridge and Valley, Appalachian Plateau, and Allegheny Mountain physiographic provinces in the upper New River watershed in the middle New River basin in Virginia and West Virginia, and the lower New, upper Kanawha, Gauley and Greenbrier river basins in West Virginia ( Fig. 5 View FIGURE 5 ). The southern/upstream portion of the range of C. appalachiensis occurs in the Middle New River mainstem. Robust populations currently persist in the New River, as well as Peak Creek and Little River and their associated tributaries.
Cambarus appalachiensis View in CoL is the dominant tertiary burrower in the middle New River basin, with large populations occurring in the New River mainstem and the Bluestone River, as well as all major New River tributaries. Greenbrier watershed populations are limited to the lower reaches of the basin, with the farthest upstream populations reported in Wolf Creek, Monroe County. Within the lower New River, C. appalachiensis View in CoL occurs within the mainstem as well as all large tributaries. Gauley River drainage populations are stable and robust in the mainstem of the Gauley River, as well as the Williams, Cherry, Cranberry and Meadow Rivers and all associated tributaries, where C. appalachiensis View in CoL is the dominant Cambarus View in CoL species. Streams immediately downstream of Kanawha Falls in the upper Kanawha River are the only waterbodies within the basin that maintain C. appalachiensis View in CoL . Downstream of Kanawha falls C. appalachiensis View in CoL is replaced by its ecological equivalent, Cambarus robustus Girard, 1852 View in CoL .
Material examined included 1,089 specimens from 189 collections housed at either The Ohio State University Museum of Biological Diversity Collection or the West Liberty University Astacology Collection and are presented in Appendix 1.
Habitat and life history notes. The within-basin abundance and distribution of Cambarus appalachiensis differs among drainage basins. In both the upper and middle New River, C. appalachiensis is abundant in larger ordered streams, as well as the New River mainstem. As the New River increases in order, C. appalachiensis occurrence decreases in the mainstem, and is limited primarily to larger ordered tributaries. Throughout the Gauley River system C. appalachiensis is the dominant tertiary burrowing crayfish and occurs in all stream orders from small headwater systems through the Gauley River mainstem. In all situations, C. appalachiensis occurs in streams with substrates of mixed cobble and gravels with isolated large boulders and slabs, which are the species preferred habitat (RFT and ZJL personal observation). Cambarus appalachiensis also occurs in a myriad of gradients, from slack water eddies and slow runs, to rapids and high gradient step-pool streams.
Based on collections examined to complete the description, C. appalachiensis appears to have a life history similar to that of other larger tertiary burrowing Cambarus species in Appalachia ( Jezerinac et al. 1995; Foltz et al. 2016). Form I males dominate over Form II males during autumn, winter and early spring months. Population wide molts occur in West Virginia beginning in mid-May, and are complete by mid-June (Z.J.L. personal observation). The majority of adult males are form II from the latter part of June through mid-September, when another population wide molt occurs, at which time the majority of males are Form I. This was evident during the collection of the type series in Pipestem Creek , Summer County West Virginia. Not all animals captured were retained, though basic biological data was ascertained. Fifty-one males were collected on 11 October 2016; only three were Form II.
Mating likely occurs in the fall, winter and spring months. During the collection of the type series on three separate instances Form I males were collected alongside two to three females under large slabrock boulders. While mating was not observed, it was possible cohabitation in this scenario was associated with possible mating efforts. Active glare glands were not observed on any females collected in the type series (n = 89). Females collected in early and mid-summer in both June and July in West Virginia frequently display active glare glands (ZJL personal observation).
Ovigerous females as well as females carrying instars have been collected on several occasions in both Virginia and West Virginia ( Table 2 View TABLE 2 ). The earliest recorded date for females carrying eggs in this study was 24 July, 1989 with the latest occurrence of eggs on 19 August ( Table 2 View TABLE 2 ). The earliest recording of juveniles was 2 August when a female was collected with stage I juveniles. Females likely maintain juveniles throughout the fall, and possibly into the spring. During the collection of the type series two females were collected maintaining 56 and 78 stage IV juveniles capable of living freely from their mothers (11 October, 2016). Mean egg counts for nine females was 105.9 eggs; mean number of juveniles for 6 females was 43.9 juveniles. The highest fecundity observed was 337 juveniles from a female collected in the Gauley River, Webster County, West Virginia ( Table 2 View TABLE 2 ).
Conservation status. Cambarus appalachiensis should be listed as currently stable (CS) using the American Fisheries Society criteria ( Taylor et al. 2007), and assigned a G4/G5 ranking using the Master (1991) global conservation criteria for conservation listing because of its broad range and stable status. Cambarus appalachiensis should be listed as least concern (LC) using the International Union for the Conservation of Nature ( IUCN 2001) criteria because of its broad distribution (ZJL and RFT personal observation).
Crayfish associates. Cambarus appalachiensis has been collected with Cambarus (Cambarus) carinirostris Hay, 1914 , Cambarus (Hiaticambarus) chasmodactylus James 1966 , Orconectes (Crockerinus) sanbornii ( Faxon,1884) , Orconectes (Gremicambarus) virilis ( Hagen, 1840) , and Orconectes (Procericambarus) cristavarius Taylor, 2000 .
Variation. As in many Cambarus species, C. appalachiensis undergoes an ontogenic change in morphology, with juveniles and sub-adults being noticeably more spinose than adults. Specifically, juvenile postorbital ridges as well as acumen terminate in well pronounced spines, compared to adults whose postorbital ridges and acumens often terminate in either tubercles or spinose tubercles, rarely in defined spines. Juvenile/sub-adult rostra also are less broad and more lanceolate than adult rostra, which normally consist of parallel margins that are thickened, ending in strongly angled acumen, which are often absent in juveniles/sub-adults. Finally, chelae architecture is reduced in juvenile/subadult C. appalachiensis and defined in adult animals as explained below.
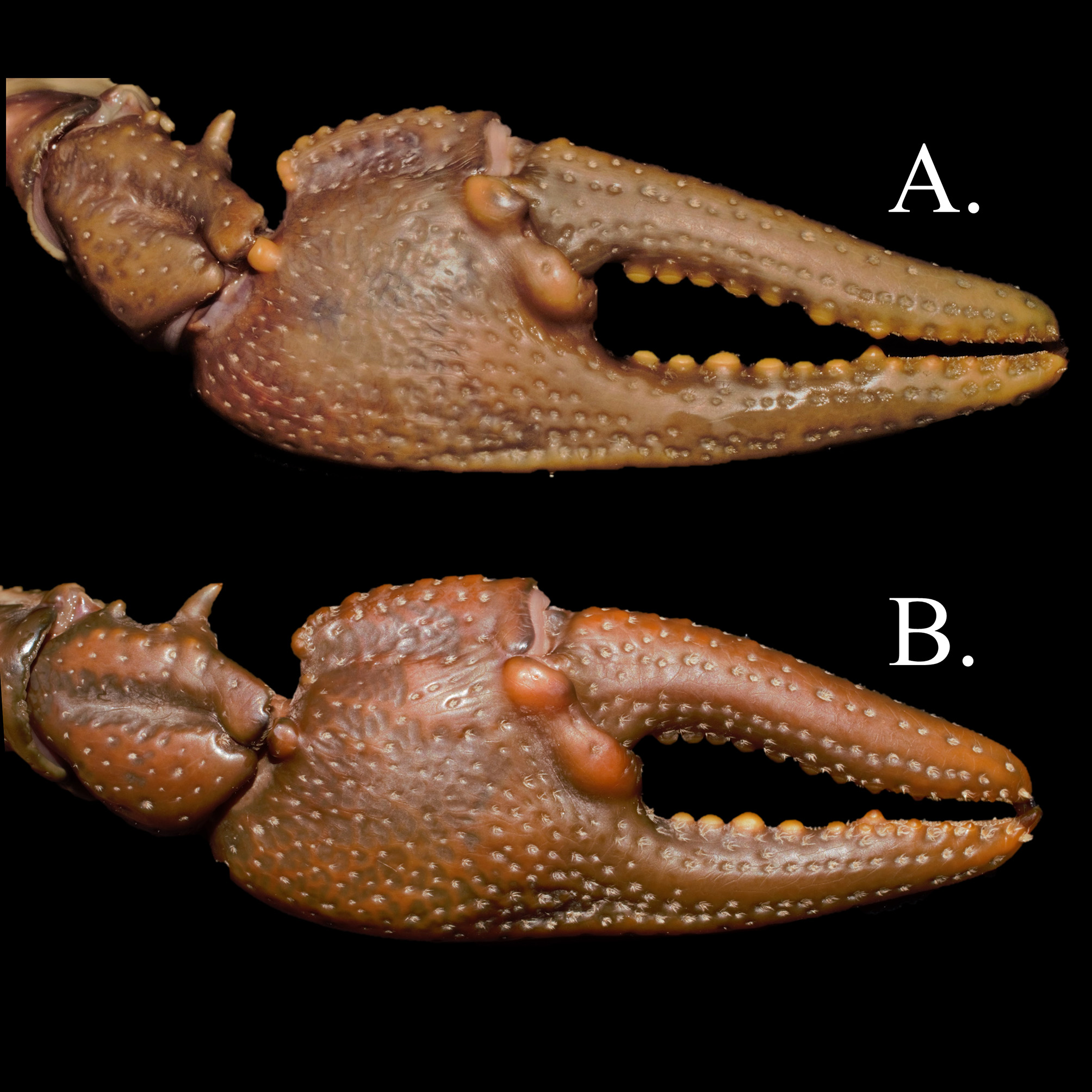
Two distinct chelae types exist in C. appalachiensis , with both forms present in Form I and Form II individuals ( Fig. 6 View FIGURE 6 A–B). Elongated chelae ( Fig. 6 View FIGURE 6 A) are far more frequently encountered than truncated chelae ( Fig. 6 View FIGURE 6 B). Among adults, there is variation between animals in the Greenbrier, the lower, middle, and upper New River Basins, and the Gauley River basin. Chelae of adult C. appalachiensis from the Greenbrier and New river basins lack well defined lateral impressions, lack extensive tuberculation on the dorsal surface of the dactyl, and often possess an extensive gape between the dactyl and fixed finger of the propodus when the dactyl is closed. Gauley River C. appalachiensis have a more defined lateral impression, moderate tuberculation on the dorsal surface of the dactyl, and moderate to weak gape between the dactyl and propodus when the dactyl is closed. In addition to differences in chelae architecture, Greenbrier and New river animal’s rostra are normally broad, with steeply angled acumen. Gauley River C. appalachiensis rostra can be less broad, occasionally approaching a sublanceolate condition, and can have less steeply angled acumen, though most animals observed possess rostra similar to those from other basins. The latter is an exception in the Gauley, but does occur with enough frequency to be mentioned (ZJL personal observation).
Relationships and comparisons. Cambarus appalachiensis is placed in the subgenus Cambarus based on (1) the presence of a subapical notch in the form I gonopod and (2) the lack of a well-developed mesial second tubercle row on the palm ( Hobbs 1969). Among described members of the subgenus, C. appalachiensis is most similar to C. sciotensis , C. hatfieldi , and C. angularis in overall body size and shape and thickening of the rostral margins.
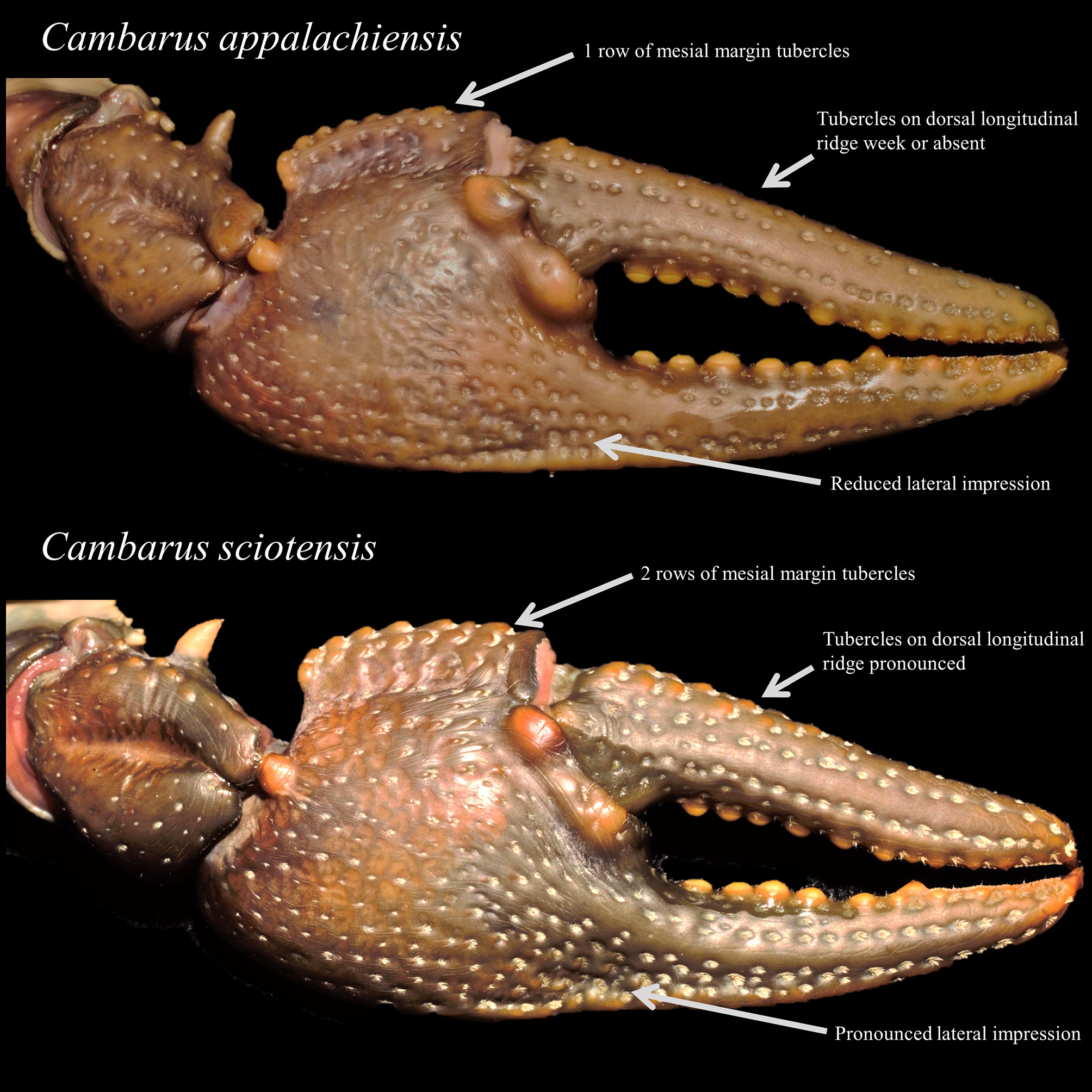
Cambarus appalachiensis and C. sciotensis were distinguished by morphometric ratios of palm length/width, and palm depth/length, as well as chelae architecture. Mean ± SE of palm length/width ratios of form I males, form II males, and females were 70.7% ± 0.49, 70.0% ± 0.40, and 70.6% ± 0.54 for C. appalachiensis and 62.6% ± 0.65, 64.2% ± 0.90, and 63.8% ± 0.80 for C. sciotensis , respectively. Also, mean ± SE of palm depth/length ratios of form I males, form II males, and females were 83.7% ± 0.82, 85.6% ± 0.52, and 85.1% ± 0.61 for C. appalachiensis and 93.1% ± 1.1, 90.8% ± 1.2, and 92.7% ± 1.1 for C. sciotensis , respectively. The result of the previously mentioned ratios is C. appalachiensis has a much more elongated chelae in profile than C. sciotensis , which possess a subrectangular chela.
In addition to the previous ratios, C. appalachiensis is readily differentiated from C. sciotensis by its lack of a second mesial row of tubercles, lack of a pronounced lateral impression, and lack of pronounced tubercles on the longitudinal ridge of the dactyl ( Fig. 7 View FIGURE 7 ). Cambarus sciotensis has two mesial rows of tubercles, moderate to strong lateral impression, and pronounced tubercles on the dorsal longitudinal ridge of the dactyl ( Fig. 7 View FIGURE 7 ). Finally, C. sciotensis postorbital ridge terminate in a straight tubercle or spine; C. appalachiensis Post orbital ridges terminate in dorsally deflected spines or tubercles.
Cambarus appalachiensis View in CoL can be differentiated from both Cambarus angularis Hobbs and Bouchard, 1994 View in CoL and Cambarus hatfieldi Loughman et al. 2014 View in CoL by its more elongate chelae, compared to both the latter species subrectangular chelae. In addition to chelae shape, C. appalachiensis View in CoL has less thickened rostral margins than C. angularis View in CoL , and a more elongate rostrum than C. hatfieldi View in CoL . Cambarus appalachiensis View in CoL central projection does not extend past the gonopod shaft; both C. angularis View in CoL and C. hatfieldi View in CoL central projections typically extend past the gonopod shaft, and normally past the distal margin of the mesial process. Cambarus appalachiensis View in CoL is also on average longer in total body length ( = 74.4 mm; n = 98; SE ± 9.8%) than C. hatfieldi View in CoL ( = 67.6 mm; n = 52; SE ± 7.3 mm; Loughman et al. 2014).
Four additional Cambarus View in CoL species occur in the greater New River system that can easily be differentiated from C. appalachiensis View in CoL . Both Cambarus smilax Loughman et al. 2011 View in CoL and Cambarus cf. robustus Girard, 1852 View in CoL have two mesial rows of tubercles as well as lanceolate rostra, compared to C. appalachiensis’ single row of mesial tubercles and broad rostra. Cambarus smilax View in CoL does not appear to be sympatric with C. appalachiensis View in CoL , and is endemic to mid and headwater reaches of the Greenbrier River ( Loughman et al. 2011). Cambarus cf. robustus View in CoL , like C. smilax View in CoL , also is a headwater species and endemic to the upper New River system of Virginia and North Carolina, and does not occur in larger ordered streams typically utilized by C. appalachiensis View in CoL . Only two sites have been found to harbor both species.
Cambarus chasmodactylus is the third Cambarus species in the New River system, and does on occasion occur in sympatry with C. appalachiensis . Cambarus appalachiensis is readily differentiated from C. chasmodactylus by its elongate chelae, broad rostrum, and brown coloration compared to C. chasmodactylus’ broad chelae, lanceolate rostrum, and blue, green, turquoise coloration. Cambarus chasmodactylus also has an extremely large gape in the chelae, which is substantially larger than the chelae gape observed in C. appalachiensis . Cambarus carinirostris occurs in the northern New River system and functions as the basins secondary burrowing species. As such, it occurs primarily in headwater streams, and normally does not occur in sympatry with C. appalachiensis . Cambarus carinirostris has a broad rostrum like C. appalachiensis , but can be differentiated from C. appalachiensis by its subrectangular chelae which maintain a single row of appressed tubercles, compared to C. appalachiensis elongate chelae which possess a defined single mesial row of tubercles.
The upper Guyandotte River system is geographically proximate to the upper and middle New River system and is occupied by C. theepiensis . Cambarus appalachiensis and C. theepiensis have similar broad rostra, but can be differentiated from each other by the number of tubercles rows on the mesial margin of the palm as well as subpalmar tubercles. Cambarus theepiensis has two rows of tubercles on the mesial margin of the chelae, as well as 2–4 subpalmar tubercles ( Loughman et al. 2013). Cambarus appalachiensis has a single row of tubercles, and normally lacks or possesses only a single subpalmar tubercle.
Etymology. Cambarus appalachiensis is named after the Appalachian Mountains where C. appalachiensis entire range occurs. The common name Conhaway Crayfish is in reference to Conhaway River, which was the name of both New and Bluestone Rivers in the 1700’s, and was the name Christopher Gist referred to the New River by in his field journal. It is theorized that Conhaway was the Shawnee name for the New River. Gist’s expeditions throughout the New River basin in 1750 and 1751 were among the first expeditions throughout the New River valley by Europeans.
Common name. Conhaway Crayfish.
TABLE 1. Morphological measurements (mm) of Cambarus appalachiensis, new species.
| Holotype | Allotype | Morphotype | |
|---|---|---|---|
| Carapace | |||
| Total carapace length | 38.1 | 39.6 | 44.0 |
| Postorbital length | 32.1 | 33.5 | 37.8 |
| Length cephalic section | 24.8 | 25.7 | 27.8 |
| Width | 20.2 | 21.1 | 4.3 |
| Depth | 16.2 | 16.9 | 19.0 |
| Length rostrum | 8.7 | 9.1 | 9.1 |
| Length acumen | 2.6 | 2.9 | 2.8 |
| Length areola | 13.3 | 13.9 | 16.2 |
| Width areola | 4.4 | 4.4 | 4.5 |
| Abdomen | |||
| Width | 16.4 | 20.0 | 19.7 |
| Length | 38.8 | 41.1 | 43.0 |
| Cheliped | |||
| Length mesial margin palm | 11.8 | 10.0 | 13.3 |
| Width palm | 17.3 | 16.0 | 20.3 |
| Depth palm | 16.2 | 9.2 | 11.2 |
| Length dactyl | 24.5 | 20.0 | 19.7 |
| Length carpus | 11.7 | 12.2 | 15.6 |
| Width carpus | 9.6 | 8.7 | 11.5 |
| Length dorsal margin merus | 10.5 | 9.5 | 9.9 |
| Depth merus | 9.9 | 9.8 | 12.2 |
| Gonopod length | 8.1 | 9.9 |
TABLE 2. Cambarus appalachiensis pleopodal egg and juvenile counts.
| OSUMC # | # Eggs | # Instars | Date | County | Stream | County | State |
|---|---|---|---|---|---|---|---|
| 7647 | 159 | 0 | 7/24/1989 | Monroe | Turkey Cr. | Monroe | WV |
| 7647 | 117 | 0 | 7/24/1989 | Monroe | Turkey Cr. | Monroe | WV |
| 7647 | 98 | 0 | 7/24/1989 | Monroe | Turkey Cr. | Monroe | WV |
| 6869 | 82 | 0 | 8/2/2007 | Tazewell | Mud Fork | Tazewell | VA |
| 6874 | 0 | 58 | 8/2/2007 | Tazewell | Bluestone R. | Tazewell | VA |
| 7123 | 161 | 0 | 8/18/1988 | Monroe | Wolf Cr. | Monroe | WV |
| 7189 | 118 | 0 | 8/18/1988 | Monroe | Indian Cr. | Monroe | WV |
| 7189 | 88 | 0 | 8/18/1988 | Monroe | Indian Cr. | Monroe | WV |
| 7189 | 15 | 0 | 8/18/1988 | Monroe | Indian Cr. | Monroe | WV |
| 7124 | 115 | 0 | 8/19/1988 | Summers | Hungard Cr. | Summers | WV |
| 7276 | 0 | 10 | 8/20/1988 | Greenbrier | Little Clear Cr. | Greenbrier | WV |
| 7191 | 0 | 337 | 8/22/1988 | Webster | Gauley R. | Webster | WV |
| 7118 | 0 | 18 | 9/1/1989 | McDowell | Elkhorn Cr. | McDowell | WV |
| 7266 | 0 | 54 | 9/10/1988 | Nicholas | Cranberry R. | Nicholas | WV |
| 7543 | 0 | 62 | 9/10/1988 | Nicholas | Cherry R. | Nicholas | WV |
| X egg/instar count = | 105.9 | 150.8 | |||||
| SD egg/instar count = | 43.9 | 123.0 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cambarus (Cambarus) appalachiensis
| Loughman, Zachary J., Welsh, Stuart A. & Thoma, Roger F. 2017 |
Cambarus hatfieldi
| Loughman et al. 2014 |
Cambarus smilax
| Loughman et al. 2011 |
Cambarus angularis
| Hobbs and Bouchard 1994 |
Cambarus robustus
| Girard 1852 |
Cambarus cf. robustus
| Girard 1852 |