Litoria naispela, Richards & Donnellan & Oliver, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5263.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:9EF23FE9-DDD8-46D4-A275-09A35243BF30 |
|
DOI |
https://doi.org/10.5281/zenodo.7814492 |
|
persistent identifier |
https://treatment.plazi.org/id/876BBBC1-3045-4239-A686-CD995DAFDDD4 |
|
taxon LSID |
lsid:zoobank.org:act:876BBBC1-3045-4239-A686-CD995DAFDDD4 |
|
treatment provided by |
Plazi |
|
scientific name |
Litoria naispela |
| status |
sp. nov. |
Litoria naispela , sp. nov.
Crater Mountain Treehole Frog
Figs 15–17 View FIGURE 15 View FIGURE 16 View FIGURE 17
https://zoobank.org/ urn:lsid:zoobank.org:act:
Holotype. SAMA R70216 ( FN JCUNQ [ SJR]3483). Adult male, forest adjacent Herowana Village, Eastern Highlands Province, Papua New Guinea ( 6.6220°S, 145.1962°E, 1400 m. a.s.l.), by local assistant for S. Richards on 5 December 2003. GoogleMaps
Paratypes. SAMA R70215 ( FN JCUNQ [ SJR]3482) , R70214 ( FN JCUNQ [ SJR]3475), adult males, same details as holotype except R70214 collected on 30 November 2003 .
Referred specimens. SAMA R71597 ( FN JCUNQ [ SJR]4858), recently metamorphosed juvenile, collected as tadpole on 20 November 1998, preserved on 12 December 1998, locality details as for holotype, collected by S.J. Richards ; SAMA R71594–71496 ( FN JCUNQ [ SJR]3132–3134), subadults, collected as tadpoles by S. Richards on 24–25 November 2001, raised in captivity by A. Mack and D. Wright, preserved on 25 January 2003 by S.J. Richards .
Diagnosis. Litoria naispela sp. nov. is distinguished from all other Litoria by the following unique combination of characters: size small (SVL of three males 25.5–25.8 mm); limbs long (TL/SVL 0.60–0.64); tympanum large (TYM/SVL 1.0–1.1, TYM/EYE 0.79–0.82), with predominantly transparent membrane; dorsum in life green with darker green spots, posterior of venter orange; vomerine teeth absent; finger webbing extensive; prominent dermal fold along outer edge of foot; pigmentation on nictitating membrane restricted to narrow band at dorsal margin; and is genetically diagnosable from L. singadanae at 48 sites in the 787 base pair alignment of mitochondrial ND4 gene and flanking tRNA ( Table 1 View TABLE 1 ).
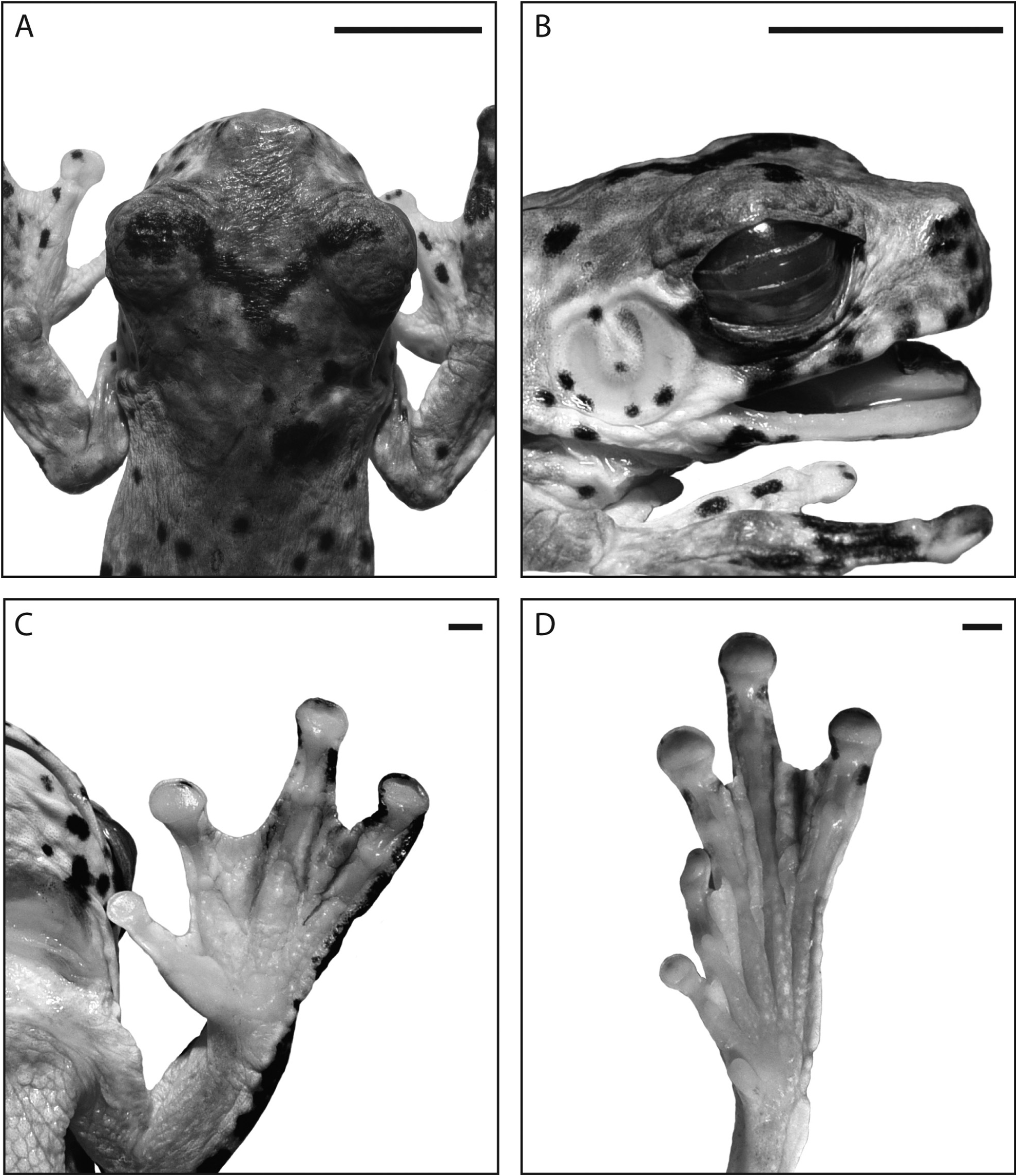
Description of holotype. An adult male ( Fig.15A View FIGURE 15 ) with vocal slits and nuptial pads. Measurements are presented in Table 5 View TABLE 5 . Body moderately slender. Limbs long (TL/SVL 0.60). Head slightly longer than wide (HW/HL 0.90). Vomerine teeth absent. Vocal slits short, located laterally in floor of mouth, extending anteriorly approximately 2.0– 2.5 mm from corner of jaws. Snout short, truncate, bluntly rounded in dorsal view ( Fig. 16A View FIGURE 16 ), nearly vertical in lateral view ( Fig. 16B View FIGURE 16 ); canthus rostralis poorly defined, broadly rounded; loreal region steeply sloping, slightly concave; eyes large (EYE/SV 0.14), protruding distinctly in dorsal and ventral views; pupil horizontal when contracted; pigmentation on nictitating membrane restricted to narrow band along dorsal edge; nares near tip of snout, directed laterally, not visible in dorsal view; tympanum large, conspicuous, (TYM/EYE 0.81), annulus prominent except dorsally where it merges with short, slightly curved supratympanic fold ( Fig. 16B View FIGURE 16 ). Tympanic membrane transparent except for central connective tissue at attachment of columella, scattered tiny white flecks, and several clumps of black pigment spots (< 0.5 mm diameter) predominantly around periphery of membrane ( Figs 15A View FIGURE 15 , 16B View FIGURE 16 ). Tongue broadly cordiform with shallow posterior notch.
Skin dorsally smooth with scarcely detectable fine, shallow grooves, more prominent laterally; throat very finely pitted anteriorly, a translucent fold of skin from vocal sac covers throat posteriorly; chest and abdomen coarsely granular. Outer edge of forearm with series of low, pale tubercles; outer edge of tarsus and foot with prominent white dermal fold; patch of low tubercles below vent.
Fingers short, extensively webbed, relative lengths 3>4>2>1. Finger webbing thick, fleshy, on inside of Finger 4 and outside of Finger 2 reaching to base of discs, on both sides of Finger 3 to mid-way between subarticular tubercle at base of penultimate phalanx and disc with fleshy fringe continuing to disc, on outside of Finger 1 reaching to subarticular tubercle at base of penultimate phalanx ( Fig. 16C View FIGURE 16 ). Subarticular tubercles prominent, those on fingers 3 and 4 distinctly bi-lobed ( Fig. 16C View FIGURE 16 ), those on Finger 1 and 2 unilobed. Terminal discs moderately large (3FD/SVL 0.06, 3FD/3FP 1.6) with circum-marginal grooves. Nuptial pads narrow, comprising fine pale brown granules, extending ~ 1.8 mm along proximal inner edge of Finger 1. Toes fully webbed except Toe 4, webbed to proximal edge of subarticular tubercle at base of penultimate phalanx; relative lengths 4>5>3>2>1 on left foot, disc of Toe 5 on right foot damaged, so shorter than Toe 3. Subarticular tubercles prominent, distal tubercle on Toe 5 bilobed, on remainder of toes unilobed. Discs moderately large (4TD/SVL 0.06, 4TD/4TP 1.36) with circum-marginal grooves, discs on toes slightly smaller than those on fingers (3FD/4TD 1.07) ( Fig. 16C–D View FIGURE 16 ).
Colour in life ( Fig. 15A View FIGURE 15 ). Dorsum pale lime green with dark-green spots and small areas of cyan white. Darkgreen pigment patches on dorsum form large ‘Y’-shaped patch between eyes, round patch on one eyelid, and short irregular bars on hindlimbs; very dark-green pigmentation present along outer edge of hand and forearm and forming discrete spots on otherwise transparent tympanic membrane. Outer edge of tarsi white with dark-green blotches. Iris off-white with large brown patch anteriorly and posteriorly. Hidden surfaces of limbs orange, except small yellow patch inside knee; Ventral surfaces of legs orange, orange colour extending anteriorly onto posterior of abdomen, becoming egg-yolk yellow anteriorly; a dark-brown pigment patch laterally on throat just anterior of angle of jaws, remainder of throat with scattered brown pigment spots. Tubercles below vent white.
Colour in preservative. Dorsum blue grey with dark-brown and greenish spots; Fingers 1 and 2 white with dark spots; Fingers 3–4 blue grey dorsally with very dark (nearly black) pigment patches; ventrally hands and feet translucent cream, dermal ridges on all limbs and enlarged tubercles around vent white.
Variation. In life the two male paratypes were also pale green and both exhibited the prominent broad V shaped inter-ocular marking, which remains evident in preservative; however, they exhibited fewer dark-green markings, and in preservative they are pale-purple grey rather than blue grey, with scattered dark-brown and greenish spots. A recently metamorphosed specimen ( SAMA R71597 ; Fig. 17D View FIGURE 17 ) is predominantly brown and white with some patches of brown on the limbs. A subadult specimen ( SAMA R71596 ) raised from a tadpole collected from the treehole illustrated in Fig. 17A View FIGURE 17 is white with medium lime green mottling across head, and light turquoise green mottling and scattered dark-green spots across dorsum; ‘Y’-shaped green marking between eyes is evident. Iris offwhite with narrow brown pigment veins and large brown anterior and posterior pigment patches ( Fig. 17E View FIGURE 17 ) .
Comparisons. In its moderate size (male SVL 25.5–25.8 mm), slender body, green and brown dorsal colour, extensively webbed fingers, and males lacking a rostral spike, Litoria naispela sp. nov. most closely resembles the following 12 species: L. aplini , L. daraiensis sp. nov., L. gracilis sp. nov., L. haematogaster sp. nov., L. iris , L. lisae sp. nov., L. majikthise , L. nigropunctata , L. richardsi , L. singadanae , L. umarensis and L. verae . It can be immediately distinguished from all of these species except L. richardsi and L. singadanae by its very large tympanum (TYM/SVL 0.10–0.11, TYM/EYE 0.78–0.80 vs. TYM/SVL <0.06, TYM/EYE <0.50) and transparent (vs. pigmented) tympanic membrane. It can be distinguished from L. richardsi by lacking (vs. having) both irregular black lines on dorsum and extensive black markings ventrolaterally; and from L. singadanae by its smaller size ( 3 adult males SVL 25.5–25.8 vs. 2 adult males 28.7–28.8), longer legs (TL/SVL 0.60–0.64 vs. 0.57 & 0.57), though this difference is less evident when juvenile L. naispela are considered, larger eyes (EYE/SVL 0.13–0.14 vs. 0.11 & 0.11), near absence of pigmentation on throat except patch at angle of jaws (vs. extensive spotting across throat e.g., Fig. 15C View FIGURE 15 vs. 15E), and dense brown pigmentation ventrolaterally absent (vs. present; see Fig. 15C View FIGURE 15 vs. 15E). Litoria singadanae is the closest known relative of L. naispela sp. nov. It is known only from the mountains of the Huon Peninsula, approximately 190 km to the northeast of the type locality ( Fig. 4 View FIGURE 4 ) and these species are separated geographically by the lowlands of the Markham Valley and the high peaks (many to ~ 3,000 m a.s.l.) of the Finisterre/Saruwaged Mountains.
Distribution and ecology. Litoria naispela sp. nov. is known only from the vicinity of Herowana Village in Eastern Highland Province, on the southern slopes of Papua New Guinea’s Central Cordillera ( Fig. 4 View FIGURE 4 ). The habitat where the species was encountered is moderately disturbed lower montane rainforest, although large areas around the village had been converted to gardens and coffee plantations.
Observations on this species during three separate field expeditions suggest that L. naispela sp. nov. is an obligate treehole breeder. Treeholes utilised by L. naispela sp. nov. were between 1.0 and about 15.0 m above the ground and had openings of at least 10–15cm in diameter ( Fig. 17A View FIGURE 17 ). Clutch size was between 10– 15 eggs, which were glued to the vertical surfaces of tree trunks about 10–20 cm above the treehole openings ( Fig. 17B–C View FIGURE 17 ). Embryos developed into fully formed tadpoles within the egg capsule attached to the tree trunk ( Fig. 17C View FIGURE 17 ) before dropping into the treehole below, usually during heavy rain (S. Richards pers. obs.). This reproductive strategy is an example of Mode 26 of Haddad and Prado (2005) and is shared with its close relative L. richardsi , which has been observed vocalising adjacent to a treehole with egg clutches glued above the opening and free-swimming tadpoles inside (S. Richards, pers. obs.). The reproductive mode of L. singadanae , this species’ closest known relative, remains unknown, but Richards (2005) noted the absence of lentic waterbodies in the area where it was discovered, so it seems highly likely that it has a reproductive strategy like its two relatives.
In their contrasting dark brown and white dorsal patterns, recently metamorphosed froglets raised from tadpoles at Herowana bear a striking resemblance in colour and patterning to bird droppings ( Fig. 17D View FIGURE 17 ). All the treeholes containing L. naispela sp. nov. tadpoles around Herowana were known by local informants to be sources of drinking water frequented by birds. We hypothesis that this species has evolved a colour pattern resembling bird droppings to reduce their detectability to predators when they emerge from treeholes. This mimicry or masquerade behaviour may be convergent on distantly related treefrogs from the Neotropics ( Dendropsohus marmoratus Laurenti; Hylidae ) and Indochina ( Theloderma asperum Boulenger ; Rhacophoridae ) that have similar colouration and ecology ( Toledo & Haddad 2008).
IUCN Red-List Status. Litoria naispela sp. nov. is known only from the vicinity of Herowana village, where forest has been extensively cleared for gardens and coffee plantations. However, Herowana is within a protected area, the Crater Mountains Wildlife Management Area, and extensive tracts of primary forest remain in the region. Until the species’ distribution, habitat requirements and any potential threats are better documented we recommend that it be listed as Data Deficient by the IUCN.
Etymology. The word naispela is from the Melanesian pidgin meaning ‘pretty’, ‘beautiful’.
Molecular divergences. Based on analyses of a 787 base pair alignment from the mitochondrial ND4 gene and flanking tRNA L. naispela sp. nov. is closely related to and strongly supported as the sister taxon to L. singadanae from the Huon Peninsula ( dA between the taxa of 0.07, Table 2 View TABLE 2 ). dA between sister species pairs in other groups of Litoria ranges from 0.04 to 0.25 ( Donnellan et al. 2021, Rowley et al. 2021). The clade comprising these two taxa is in turn allied to L. richardsi , but this taxon is much more divergent from L. naispela sp. nov. ( dA of 0.17).
| SAMA |
South Australia Museum |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.