Triandra pellabergensis Mart., 2021
|
publication ID |
https://doi.org/10.11646/phytotaxa.487.1.5 |
|
persistent identifier |
https://treatment.plazi.org/id/03D08785-FF9E-FF9C-5DF6-FF6AFB2EE9A4 |
|
treatment provided by |
Marcus |
|
scientific name |
Triandra pellabergensis Mart. |
| status |
N.R. |
Triandra pellabergensis Mart. View in CoL -Azorín, M.B.Crespo, M.Á.Alonso, N.R. Crouch & M.Pinter, sp. nov. ( Figs. 1f View FIGURE 1 , 2 View FIGURE 2 , 3a–b View FIGURE 3 )
Triandra pellabergensis resembles Urginea revoluta in flower and leaf morphology, but the former species differs in consistently producing flowers with three stamens that are associated with the inner perianth whorl (not six, three also with the outer perianth whorl), and elliptical, flattened, winged seeds with a prominent embryo (not subpyramidal and irregularly compressed). Triandra occurs in the desert regions of northwestern South Africa, unlike U. revoluta which occurs in the fynbos vegetation of the southwestern Cape; phylogenetically, they represent distinct lineages.
Type:— SOUTH AFRICA. Northern Cape. Pofadder (2919): Pella, Pella se Berge , ca. 25 km W of Pofadder, rocky slopes at base of large south facing cliffs (−AA), 919 m elevation, among quartz and mica rocks, fl. ex hort. at University of Alicante on 29 June 2018, M. Martínez-Azorín, M.B. Crespo, M.Á. Alonso et al. MMA1671b ( holotype: GRA; isotypes: ABH, K, PRE) .
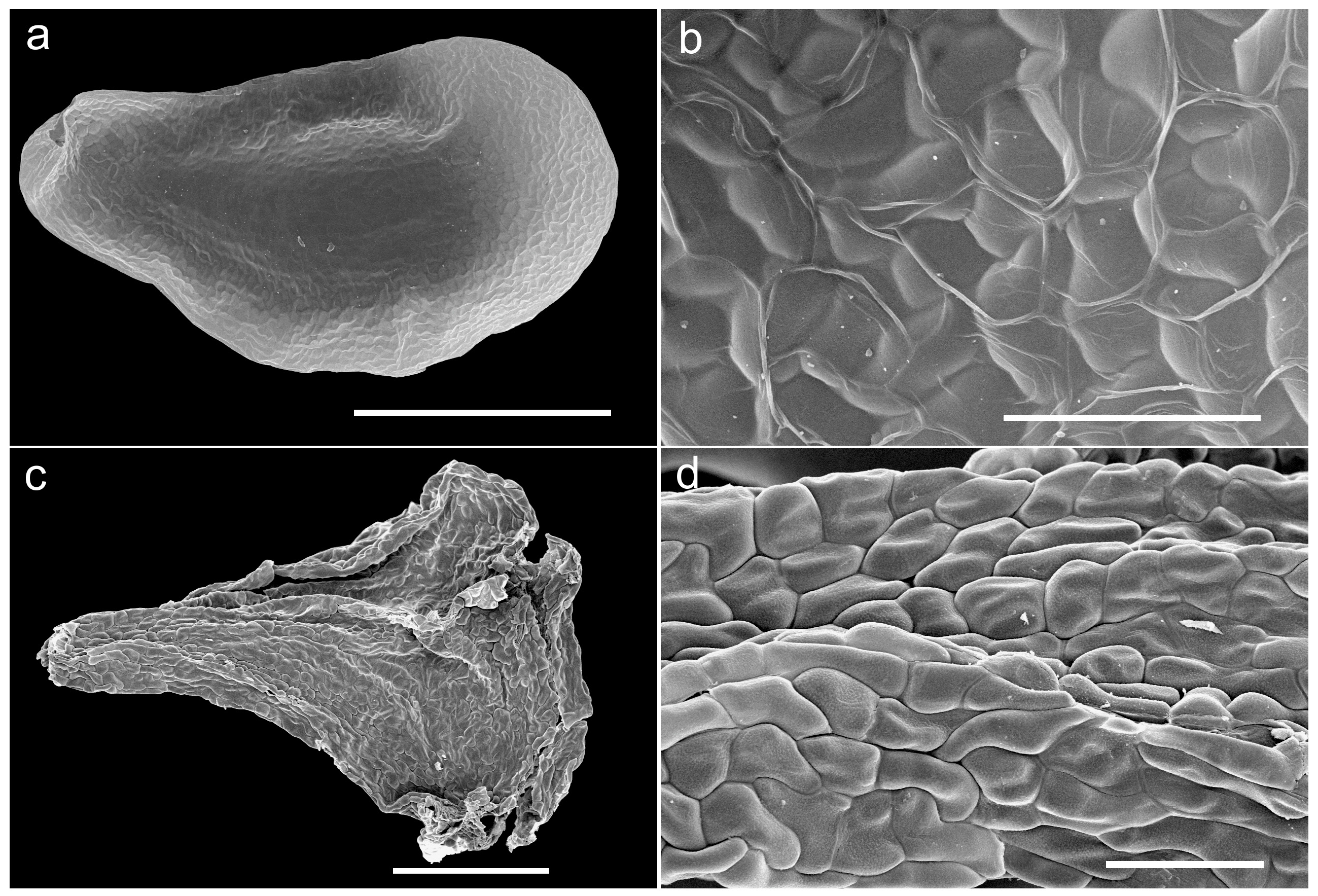
Description:—Small, herbaceous, deciduous geophyte. Bulb hypogeal, ovoid to subglobose, 12−22 × 8−18 mm, depressed in old plants, solitary, with compact, white, thickened scales, and pale brown, membranous to papery outer tunics. Roots fleshy, white, branched, 8−20 × 0.5−1 mm. Leaves 2−6, proteranthous and withered at flowering time, narrowly linear-filiform, hypogeal portion white, 2−10 mm long, aerial portion bright green, 20−70 × 0.8−1 mm, spreading, somewhat curved-sinuous, slightly fleshy-succulent, subterete to slightly flattened, glabrous, smooth. Inflorescence an erect, lax raceme, 30−60 mm long, with 4−7, long pedicellate flowers; peduncle at anthesis 30−50 mm long, greyish to purplish-brown, erect, glabrous, smooth, with minute paler maculae at base; pedicels 12−15 mm long at anthesis, spreading and curving downwards, smooth; bracts small, ovate-lanceolate, 0.6−0.9 mm long, clasping the pedicels, the lowermost with a spur ca. 0.4 mm long. Flowers pentacyclic, trimerous, stellate, nodding, opening by dusk and withering before sunrise, 1−3 flowers open at a time, flower buds subcylindrical and somewhat constricted at the middle; tepals 6, entire, whitish with a brownish-green longitudinal central band on the abaxial side, slightly glandulous at the apex, biseriate, outer overlapping inner at the base, free or very shortly connate for 0.2 mm at base, spreading and strongly reflexed at full anthesis; tepals monomorphic, 4.4−5.5 × 1−1.2 mm, narrowly lanceolate, slightly narrowed in the central portion, canaliculate. Stamens 3, free, opposite to the inner tepals whorl, erect and connivent to the style at full anthesis (initially spreading after bud opening – see Fig. 2b,c,d View FIGURE 2 , left sides– but soon becoming erect and connivent to the style); filaments white, filiform, subterete, ca. 4 × 0.1 mm, smooth; anthers yellow, ovate-oblong, ca. 0.3 mm long, dehiscing by longitudinal slits, with yellow pollen. Ovary pale yellow-green, ovate, truncate to the style, ca. 2 × 1.2 mm; style white, narrowly subclavate, erect, ca. 2.2 × 0.1 mm, subterete; stigma small, glandulose and minutely papillate. Capsule ovoid-globose, loculicidal, 4–4.5 × 3.5–4 mm, valves splitting to the base, with the withered perigone segments circumscissile below and forming an apical cap. Seeds black, shining, 2–2.7 × 1–1.2 mm, ellipsoid, flattened with prominent embryo and distinctly winged, testa surface reticulate, loose and easily detachable from the endosperm.
Etymology:—Named after the type locality of the species, Pella se Berge in the northwestern Northern Cape Province of South Africa close to the Orange River and the border with Namibia ( Fig. 1 View FIGURE 1 ). This mountain site is a type locality shared by two other remarkable Hyacinthaceae , Bowiea gariepensis van Jaarsveld (1983: 343) and Eliokarmos craibii Martínez-Azorín et al. (2015: 69) .
Phenology:— Triandra pellabergensis flowers from March to July in cultivation in the northern hemisphere and fruits appear from April to September. Leaves are proteranthous and completely withered at flowering time. Flowers are short-lived, opening by dusk and lasting few hours of a single night. In habitat, plants were in leaf in August (southern hemisphere Spring) at time of collection. Further studies are needed to evaluate its phenology in the wild.
Habitat:—This species is restricted to the Desert Biome and Eastern Gariep Rocky Desert (Dg 10) vegetation, growing in gravelly quartzitic soil among boulders, below south-facing large cliffs ( Fig. 1 View FIGURE 1 ) in positions that experience deep shade for most of the winter. The new species shares habitat with, among others, Bowiea gariepensis , Tylecodon sulphureus ( Toelken 1977: 191) Toelken (1978: 381) subsp. armianus van Jaarsveld (1989 : t. 1984) and Whiteheadia bifolia ( Jacquin 1791: 215) Baker (1873b: 226) . The region is characterized by annual precipitation ranging from 45 to 80 mm, peaking in late summer and early autumn, becoming more pronounced eastwards. Summer maximum temperatures often exceed 40ºC, occasionally reaching 50ºC at low elevations. Frost is very rare, but occurs at high elevations in the region ( Mucina & Rutherford 2006) where the type locality is located.
Distribution:— Triandra pellabergensis is only known from the type locality, in Pella se Berge, Northern Cape Province of South Africa ( Fig. 1 View FIGURE 1 ). Further research is needed to evaluate its full extent of occurrence, whilst cognisant of its cryptic character.
Diagnostic characters and taxonomic relationships:— Triandra pellabergensis is identified by the solitary bulb; the 2−6 proteranthous, filiform, glabrous, leaves; the lax raceme with 4−7, pedicellate, nodding, nocturnal flowers; the reflexed tepals at anthesis; the 3, erect stamens associated with the inner tepal whorl; the erect and narrowly subclavate style; the erect capsules with the withered tepals atop; and the flattened, winged seeds with prominent embryo ( Fig. 2 View FIGURE 2 , 3a–b View FIGURE 3 ). The new species is unique in Hyacinthaceae in having only 3 stamens per flower, with those associated with the outer tepal whorl being absent. Some genera with very specialized flowers in Hyacinthaceae , like those of Albuca Linnaeus (1762: 438) (subfam. Ornithogaloideae ), sometimes lack anthers on the 3 outer stamens, due to the specialized pollination mechanism ( Johnson et al. 2012), although their filaments, which are sometimes reduced, are always present. We have observed various plants of Triandra pellabergensis in cultivation during the past three years and all individuals and flowers consistently produced 3 stamens, and lacked the outer staminal whorl.
The nodding nocturnal flowers with reflexed tepals of Triandra superficially resemble those of other genera in Urgineoideae , such as Thuranthos , Vera-duthiea or some species of Indurgia Speta (2001: 169) . However, the latter genera clearly differ in androecial, gynoecial and vegetative structures (cf. Crouch et al. 2018, Martínez-Azorín et al. 2018b, 2019 b, Yadav et al. 2019). Our phylogenetic studies (Martínez-Azorín et al. submitted) have revealed that the latter three genera represent independent evolutionary lineages which present considerable convergence in flower morphology, adapted as they are to night pollinators. The above-mentioned phylogenetic study place a sample of Triandra as sister to the Madagascan endemic genus Rhodocodon , from which it can be readily separated on morphological grounds (cf. Knirsch et al. 2015, 2016, 2019); these molecular analyses further reveal Triandra to be distantly related to Thuranthos , Vera-duthiea and Indurgia .
In overall morphology, Triandra pellabergensis approaches Urginea revoluta , a similar-looking species that occurs in the coastal mountains of the southern Western Cape Province. However, this latter species produces larger bulbs (to 4 cm in diam.); fewer (1−2) leaves per bulb, each leaf to 25 cm long; a longer and flexuous raceme with more numerous (5−20) flowers; larger tepals ( 6−9 mm long), pedicels ( 10−15 mm long) which are reflexed in flower and articulated in their middle; six stamens per flower with longer filaments (ca. 5 mm long); subclavate style with slightly capitate stigma; and notably angular, wrinkled seeds 2−2.5 mm long, with rugulose testa ( Fig. 3 View FIGURE 3 c−d) ( Duthie 1928, Manning & Goldblatt 2018). Manning & Goldblatt (2018) considered that Urginea revoluta is unique in the genus ( Drimia , in their very broad sense covering nearly the whole subfamily Urgineoideae ) in producing pedicels that abscise near the middle if the flowers are not pollinated, leaving a short, recurved basal portion 4−5 mm long attached to the scape, a character not observed in Triandra . There are also notable seed morphological differences between Triandra and U. revoluta , with those of the former being ellipsoidal, flattened and winged with a prominent embryo and reticulate testa ( Fig. 3 View FIGURE 3 a−b), whilst in the latter they are subpyramidal, angular or irregularly compressed with a rugulose testa ( Fig. 3 View FIGURE 3 c−d). We consider that the value of seed morphological characters has largely been understimated in the taxonomy and systematics of Urgineoideae . The current report well examples how two species that represent independent evolutionary lineages can show similar flower morphology (with covergence probably driven by nocturnal pollinators), and yet clearly differ in seed features (unconnected to pollination syndromes); both characters are widely used in other plant families to segregate genera. This is also evident when comparing angular or irregularly compressed seeds in genera such as Litanthus or Geschollia Speta (2001: 169) , with those of genera (e.g. Urginavia , Thuranthos, Striatula , Vera-duthiea, etc.) that are better adapted to wind dispersal, being flattened and winged. Our unpublished phylogenetic analyses include a sample of U. revoluta from near Betty’s Bay, Western Cape Province of South Africa, which resolves sister to a clade that includes Boosia macrocentra ( Baker 1887: 702) Speta (2001: 169) and related taxa. The distinct genetic divergence between Urginea revoluta and Triandra (Martínez-Azorín et al. submitted) again indicates how the remarkable convergence of flower morphology may have been driven by nocturnal pollinators. To date, little work on pollinators of night-flowering urgineoids has been undertaken, but an extension of the work of Stirton (1976) should provide insights into the character of evolutionary processes that have led to the observed floral form convergence .
In summary, Triandra pellabergensis shows a unique syndrome of morphological characters within Urgineoideae , representing an independent evolutionary lineage for which a new genus is accordingly described.
| ABH |
Universidad de Alicante |
| K |
Royal Botanic Gardens |
| PRE |
South African National Biodiversity Institute (SANBI) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.