Crossodactylus franciscanus, Pimenta, Bruno V. S., Caramaschi, Ulisses & Cruz, Carlos Alberto Gonçalves, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3955.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:6E10D6BD-0574-4EA8-A160-A00F9CB9346E |
|
DOI |
https://doi.org/10.5281/zenodo.5616022 |
|
persistent identifier |
https://treatment.plazi.org/id/03D01A5F-5E7D-FF8E-09AE-56CAFAB32E62 |
|
treatment provided by |
Plazi |
|
scientific name |
Crossodactylus franciscanus |
| status |
sp. nov. |
Crossodactylus franciscanus View in CoL sp. nov.
Crossodactylus cf. trachystomus — Haddad et al. 1988.
Holotype. MNRJ 40137, adult male, collected at Baixo da Casca d’Anta (aprox. 20º13’S, 46º22’W, 870 m elevation), Parque Nacional da Serra da Canastra, municipality of São Roque de Minas, Minas Gerais state, Brazil, in 28 January 1981, by A.J. Cardoso, G.V. Andrade, and C.F.B. Haddad ( Figs. 4–5 View FIGURE 4 View FIGURE 5 ).
Paratypes. MNRJ 50784–9, four adult males and two adult females, collected at Mata do Batatal, Serra da Canastra, municipality of Passos (20º43’S, 46º37’W), state of Minas Gerais, Brazil, in 23 April 1943, by H. Veloso; MNRJ 50790–1, two adult males, collected at Mata da Colina, Serra da Canastra, municipality of Passos, state of Minas Gerais, Brazil, in April 1943, by H. Veloso.
Diagnosis. (1) body slender; (2) head longer than wide; (3) snout rounded in dorsal view, protruding in lateral view; (4) canthus rostralis sharp; (5) tympanum distinct; (6) vocal sac subgular, bilobate; (7) thumb spines developed; (11) toe and tarsal fringes broadly developed in males, reduced in females; (9) finger tips undilated; (10) toe tips truncate, undilated; (11) postrictal tubercle continuous; (13) a narrow glandular crest in the anterior surface of the arm, from arm insertion to the elbow; (14) presence of dorsal and dorsolateral folds; (15) a poorly delimited area marbled/dotted of brown over light-brown background from snout to shoulder; (16) presence of partial oblique lateral stripe; (17) belly immaculate.
Comparison with other species. Character states for the other species are shown in parenthesis. Crossodactylus franciscanus differs from C. dispar and C. grandis by the smaller size of males (males 20.4–22.1 SVL in C. franciscanus ; combined male SVL in C. dispar and C. grandis 23.6–42.0 mm), slender body (robust), head longer than wide (nearly as long as wide in C. dispar and wider than long in C. grandis ), sharp canthus rostralis (rounded), males with extensively fringed feet (moderate), truncate toe tips (rounded), and postrictal tubercle continuous (fragmented into small granules). Crossodactylus franciscanus also differs from C. grandis by the smaller size in females (females 20.3–23.6 mm SVL in C. franciscanus and 29.6–39.2 mm in C. grandis ).
Crossodactylus franciscanus could be distinguished from C. cyclospinus by its smaller size (males 20.4–22.1 mm and females 20.3–23.6 mm SVL in C. franciscanus ; males 22.8–24.5 mm and female 29.0 mm in C. cyclospinus ), from C. caramaschii and C. gaudichaudii by the smaller size of males (combined male SVL in these species 22.5–30.4 mm), and from C. aeneus , C. lutzorum , and C. schmidti by the smaller size of females (combined female SVL in these species 23.8–31.7 mm). It differs from C. aeneus , C. dantei , C. gaudichaudii , and C. werneri by its slender body (robust).
The rounded snout in dorsal view distinguishes Crossodactylus franciscanus from C. boulengeri , C. caramaschii , C. cylospinus , C. dantei , C. lutzorum , and C. timbuhy (nearly pentagon-shaped; variable in C. aeneus and C. gaudichaudii ). It differs from C. schmidti and C. werneri by the sharp canthus rostralis (rounded). Crossodactylus franciscanus differs from C. aeneus , C. cyclospinus , C. dantei , C. gaudichaudii , C. lutzorum , C. schmidti , and C. trachystomus by its bilobate subgular vocal sac (not expanded in C. dantei and C. lutzorum ; median subgular in the other species).
Crossodactylus franciscanus could be distinguished from C. aeneus , C. boulengeri , C. cyclospinus , C. dantei , C. gaudichaudii , C. lutzorum , C. trachystomus , C. schmidti , C. timbuhy , and C. werneri by its undilated finger tips (dilated). Crossodactylus franciscanus is separated from C. aeneus , C. caramaschii , C. cyclospinus , C. gaudichaudii , and C. timbuhy by the developed thumb spines (small). Crossodactylus franciscanus is distinguished from C. dantei , C. lutzorum , and C. schmidti by the truncate toe tips (rounded), and from these species and C.
aeneus , C. boulengeri , C. caramaschii , C. cyclospinus , C. gaudichaudii , C. timbuhy , C. trachystomus , and C. werneri by the undilated toe tips (dilated).
The developed postrictal gland distinguishes Crossodactylus franciscanus from C. caramaschii and C. lutzorum (a discrete ridge). Crossodactylus franciscanus is different from C. dantei and C. lutzorum due to the presence of dorsolateral folds (absent), and from these species and C. aeneus , C. caramaschii , C. cyclospinus , C. gaudichaudii , and C. schmidti by the presence of a narrow glandular crest on the anterior surface of the upper arm (absent).
Crossodactylus franciscanus differs from C. bokermanni , C. caramaschii , C. cyclospinus , C. schmidti , and C. trachystomus by having a poorly delimited area marbled/dotted of brown over light-brown background from snout to shoulder (a white or cream stripe from the snout to the shoulder; variable in C. werneri ). The presence of an oblique lateral stripe separates C. franciscanus from C. dantei , C. dispar , C. lutzorum , and C. timbuhy (absent; variable in C. boulengeri and C. caramaschii ). The immaculate belly distinguishes C. franciscanus from C. boulengeri , C. caramaschii , C. cyclospinus , and C. trachystomus (reticulated; with brown scattered blotches and short stripes in C. cyclospinus ).
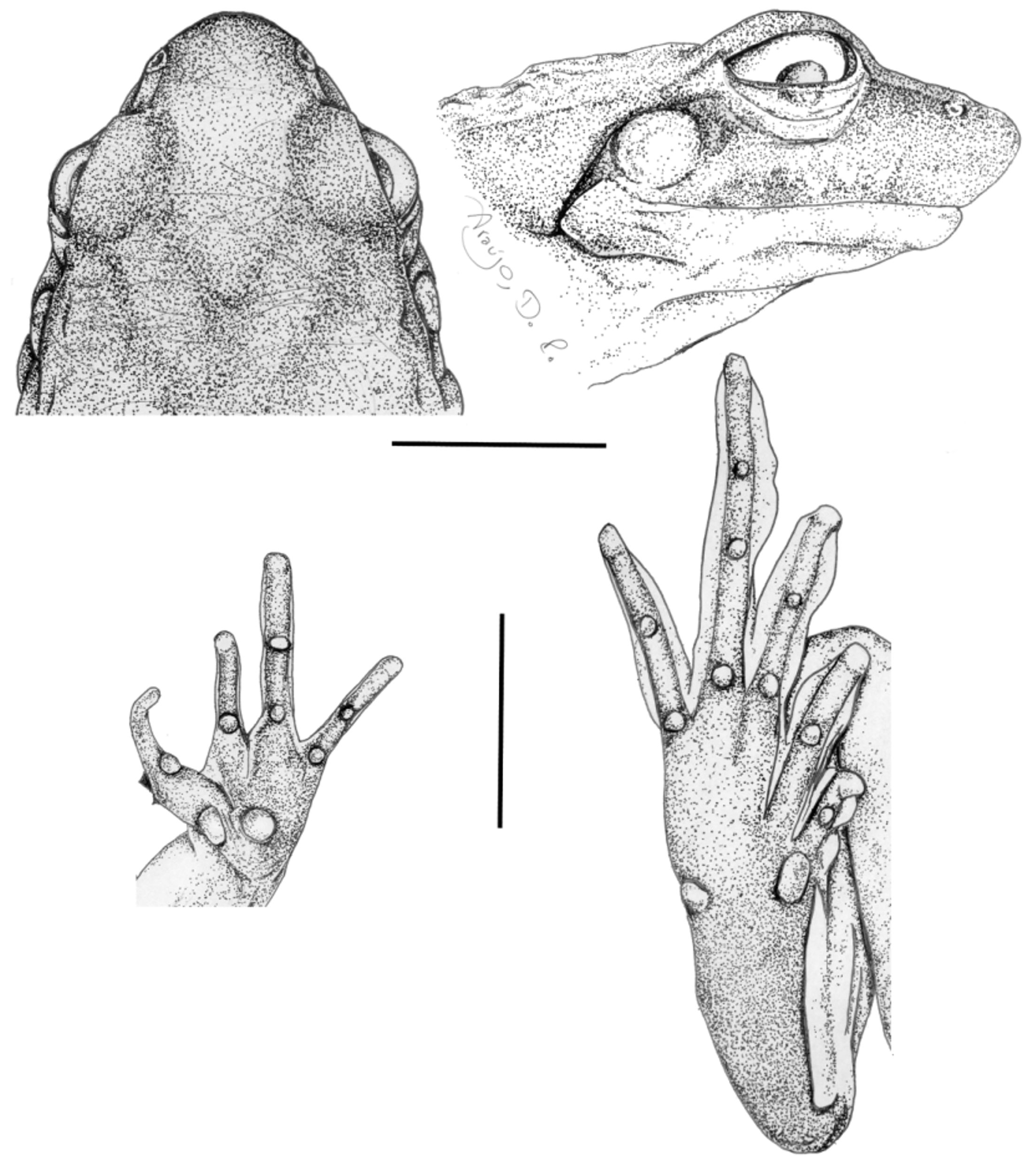
Description of the holotype. Body slender. Head longer than wide; nostrils prominent, situated and directed dorsolaterally, closer to the tip of snout than to eye. Snout with approx. 30% of HL, rounded in dorsal view, protruding in lateral view ( Fig. 5 View FIGURE 5 ). Canthus rostralis well marked, sharp; loreal region oblique, slightly concave. Eyes approx. 30% of HL. Tympanum distinct, approx. 70% of ED, rounded; supratympanic fold well marked, extending from the posterior corner of the eye to the shoulder. Vocal sac median, subgular. Upper lip spines developed, brown, concentrated on the anterior region. Tongue almost covering the entire mouth floor surface, with a discrete posterior notch. Choanae ovoid, very distant from each other. No vomerine teeth.
Arms slender; forearms thicker than upper arms; fingers narrow, long, with undilated tips and slightly enlarged bases; finger lengths II~IV<I<III ( Fig. 5 View FIGURE 5 ); three developed spines on each thumb, arranged triangularly; the spine on the inner margin of thumb is smaller than the others; the region around their bases also cornified. Scutes poorly developed on upper surfaces of finger tips, more evident on fingers III e IV; small dermal folds with rounded margins on the joints of distal phalanges; fringes on fingers weakly developed. Carpal tubercle rounded, large; thenar tubercle elongated, as long as the diameter of carpal tubercle; subarticular tubercles rounded, protruding, more developed on finger I; supernumerary tubercles small, scarce ( Fig. 5 View FIGURE 5 ).
Legs slender; the sum of tibia, thigh, and foot lengths 1.8 times the SVL; toes slender, extensively fringed, with truncate, undilated tips; toe lengths I<II<V<III<IV. Scutes on upper surfaces of toe tips more developed than on fingers, less evident on toe I; small dermal folds with rounded margins on the joints of distal phalanges. Inner metatarsal tubercle elongated, protruding; outer metatarsal tubercle small, rounded, protruding; subarticular tubercles rounded, protruding; no supernumerary tubercles. Fringes joined at base; tarsal fringe very developed, continuous distally with outer fringe of toe I, almost reaching the joint with the tibia; outer fringe of toe V reaches the posterior margin of the basal tubercle of toe ( Fig. 5 View FIGURE 5 ).
Dorsal skin posteriorly granular; discrete vertebral glandular ridge from interorbital region to the vent, interrupted on sacral region; dorsolateral folds from the posterior corner of eyes to the inguinal region, fragmented on a row of granules from midbody to groin; a pair of developed oblique glandular ridges between the dorsolateral folds and the vertebral ridge, from upper eyelids toward midbody. Ventral surfaces smooth. Upper eyelids and legs with small granules; flanks with sparse large granules; cloacal region with a few large granules. Postrictal tubercle elongated, continuous, touching the posterior margin of tympanum. A thin glandular crest on the anterior surface of upper arm, from arm insertion to elbow.
Color in preservative (70% ethanol) uniformly light brown. Poorly delimited area marbled/dotted of brown over light brown background from snout to shoulder; maxillary region brown; tympanum, postrictal tubercle, and oblique lateral stripe cream. Two poorly evident brown transversal bars on forearm. Three brown transversal bars on thigh, three on tibia, and four on tarsus-foot. Ventral surfaces cream; small brown blotches on gular region, chest and anterior region of belly; narrow longitudinal dark brown line on chest. Ventral surfaces of hands and feet brown with cream tubercles; fringes cream but transparent, with small brown dots. Several scratches on dorsal surfaces.
Measurements of holotype: SVL = 22.1; HL = 8.6; HW = 8.0; TL = 10.6; THL = 11.6; FL = 17.9; TD = 1.8; ED = 2.6; END = 1.7; NSD = 0.9; IND = 2.9; IOD = 2.4.
Variation. Toe and tarsal fringes are broadly developed in males and reduced in females, and forearms are thicker in males than in females. Upper lip spines are absent in females. Some specimens present a cream vertebral line from snout to vent. The number of transversal bars on legs varies from three to five. Paratypes present reticulated gular region and chest, but immaculate belly. Morphometric variation is shown on Table 2 View TABLE 2 .
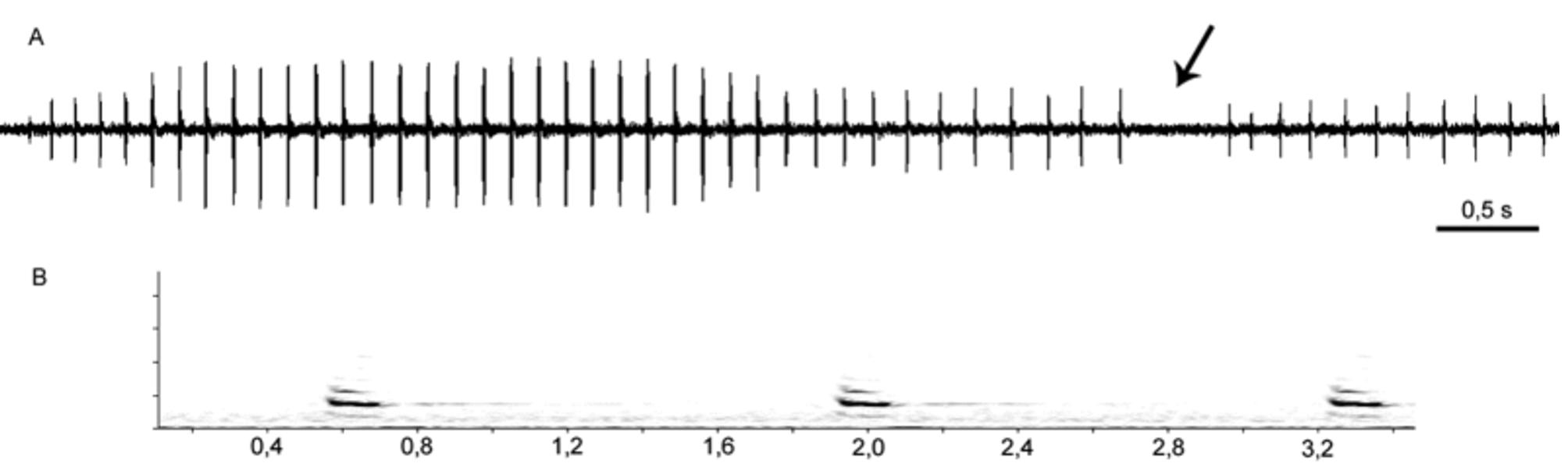
Males (N = 7) Females (N = 2) Vocalization. Some parameters of the advertisement call of Crossodactylus franciscanus were described in Haddad et al. (1988; referred as C. cf. trachystomus ) based on the recording of one male, but a better refinement is necessary in order to allow comparisons with calls known for the genus. The re-analysis of the recording AJC 010/ 1, the same used by Haddad et al. (1988), shows an advertisement call composed of harmonic notes and less intense initial notes followed by high intensity notes, subsequently decreasing on last notes ( Fig. 6 View FIGURE 6 ). Final portion of the call (i.e. the portion after peak intensity) contains most of the notes in some of the calls analyzed. Some calls seem to have been altered for unknown reasons (maybe the presence of the observer). We used only regular calls for description of the advertisement call and comparisons with other species whereas altered calls were analyzed separately.
Mean advertisement call duration (N = 6 calls) is 6.42 s and mean number of notes per call is 48. Mean note duration (N = 284 notes) is 12 ms and mean internote interval is 123 ms. Mean dominant frequency is 3460.1 Hz and is always situated on the second harmonic ( Fig. 6 View FIGURE 6 ). Table 3 View TABLE 3 presents standard deviations and ranges of each parameter analyzed and comparative data on acoustic parameters of the advertisement calls known for Crossodactylus : C. caramaschii , C. cyclospinus , C. franciscanus , C. gaudichaudii , C. schmidti , and C. trachystomus ( Weygoldt & Carvalho-e-Silva 1992; Bastos & Pombal 1995; Nascimento et al. 2005a; Pimenta et al. 2008; Caldart et al. 2011; present study). The advertisement call of C. timbuhy ( Weygoldt, 1986; under Crossodactylus cf. dispar ) still requires a detailed description and was not used for comparisons.
Dominant frequency (Hz) 2000–5500 3306 ± 649 3894 ± 525
2017–4280 (11 calls) 1802–4826 (1535) As observed for Crossodactylus trachystomus ( Pimenta et al. 2008; under C. bokermanni ), the first note of each call (N = 6 first notes) is less intense, with a slightly smaller duration (mean = 10 ms; SD = 4.8; range = 5–18 ms) and lower dominant frequency (mean = 3125 Hz; SD = 363.87; range = 2531–3656 Hz). First notes with less intense and lower mean dominant frequencies are attributes probably found in others Crossodactylus but have not yet been described. Lower dominant frequencies were also observed in Hylodes amnicola Pombal, Feio & Haddad, 2002 (Pombal et al. 2002) and in other anurans (e.g., Bastos & Haddad 2002; Castellano et al. 2002) and is considered a common effect associated with the beginning of the call ( Giacoma et al. 1997).
We considered signs of alteration on calls analyzed the interruptions followed by less intense calls after long intervals, the addition of very low intensity notes at the end of call, and also the emission of isolated notes. These alterations were also observed in Crossodactylus aeneus , C. gaudichaudii , and C. trachystomus during field activities during this study (B.V.S. Pimenta, pers. obs.). They appear to be associated with the calling male noticing the presence of an observer, the moment when a male resumed call activity after escaping and returning to call site, or the presence of a second male emitting calls with short intervals. When call was interrupted and followed by low intensity calls, the notes of these altered calls presented mean duration of 11 ms (SD = 2.6; range = 5-16 ms; N = 93 notes), mean internote interval of 124 ms (SD = 13.6; 99–175 ms; N = 93 intervals), and mean dominant frequency of 3228.6 Hz (SD = 231.41; range = 2531–3844 Hz; N = 89 notes). Low intensity notes added to the end of a call present mean duration of 11 ms (DP = 2.5; range = 7–17 ms; N = 28 notes), mean internote interval of 141 ms (SD = 45.8; range = 98–282 ms), and mean dominant frequency of 3170.6 Hz (SD = 262.95; range = 2531–3844 Hz; N = 22 notes). The only notes showing considerable differences from unaltered calls were the isolated notes, which present mean duration of 12 ms (N = 3 notes) and mean dominant frequency of 2031.3 Hz (SD = 107.96; range = 1969–2156 Hz; N = 3 notes).
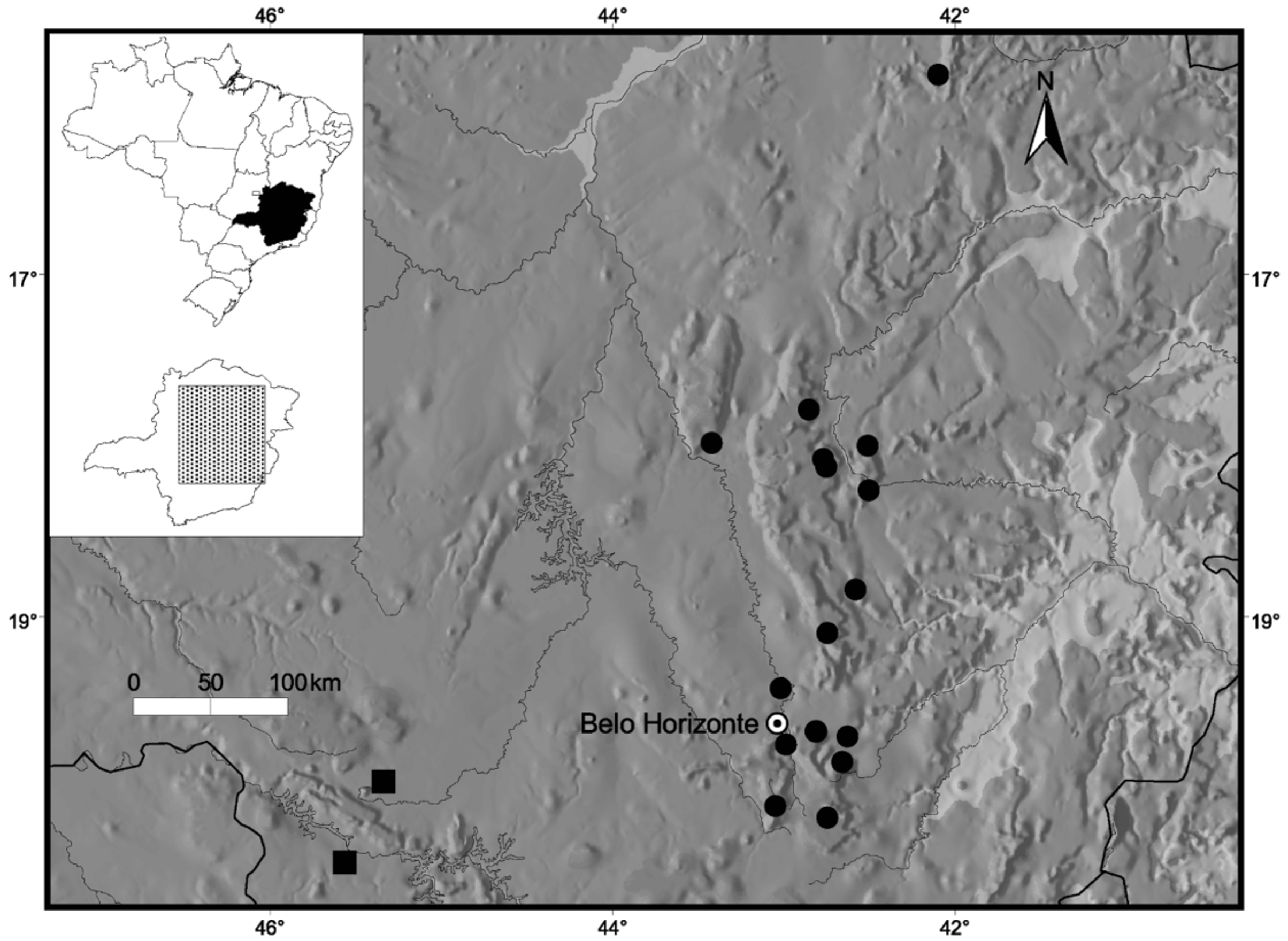
Geographic distribution, natural history, and conservation status. Crossodactylus franciscanus is currently known from two municipalities in the western region of the state of Minas Gerais, both included in the Cerrado biome ( Fig. 3 View FIGURE 3 ). The Parque Nacional da Serra da Canastra, on the municipality of São Roque de Minas, and the municipality of Passos are on opposite margins of the Rio Grande, which is dammed in this region, and distant approx. 60 km from each other. Passos is not included on the complex of elevations and plateaus collectively known as Serra da Canastra. The localities Mata do Batatal and Mata da Colina, situated at Passos according to the original jar label, were not located.
According to Haddad et al. (1988) males of Crossodactylus franciscanus (referred as Crossodactylus cf. trachystomus ) call during the day and shortly after sunset. Specimens were found in small ponds with shrubs and grass on margins or sitting on debris at the bottom of shallow rivulets, where tadpoles were also recorded (no specimens of tadpoles were found in collections). As far as we are aware, the last specimen of C. franciscanus which entered collection was the holotype, found in 1981.
Etymology. The specific epithet “ franciscanus ” aludes to the Rio São Francisco, which forms one of the most important Brazilian river basins. Its headwaters are located in the Parque Nacional da Serra da Canastra, type locality of the new species.
Remarks. Specimen ZUEC 4343, which was not found in the corresponding collection during our study, was collected at Serra da Canastra and recorded by A.J. Cardoso. The holotype’s original jar label says it is also a recording voucher. There is only one recording of Crossodactylus from Serra da Canastra deposited at the Arquivo Sonoro Neotropical da Universidade de Campinas (Neotropical Sound Archive, University of Campinas; AJC 010/ 1), so we could not determine which of these two specimens, ZUEC 4343 or MNRJ 40137, was recorded.
TABLE 2. Means, standard deviations (SD), and ranges of some measurements (in mm) of Crossodactylus franciscanus sp. nov. (N = number of specimens).
| Mean | SD | Range | Range | |
|---|---|---|---|---|
| SVL | 21.3 | 0.61 | 20.4–22.1 | 20.3–23.6 |
| HL | 7.9 | 0.36 | 7.6–8.6 | 6.9–8.4 |
| HW | 7.3 | 0.35 | 6.9–8.0 | 6.4–7.3 |
| TL | 10.2 | 0.53 | 9.4–10.8 | 9.6–11.1 |
| THL | 10.3 | 1.10 | 8.4–11.6 | 10.3–11.3 |
| FL | 16.4 | 0.98 | 15.3–17.9 | 14.2–17.9 |
| TD | 1.5 | 0.28 | 1.2–1.8 | 1.3–1.6 |
| ED | 2.8 | 0.24 | 2.5–3.1 | 2.9–2.9 |
| END | 1.6 | 0.18 | 1.4–2.0 | 1.6–2.1 |
| NSD | 0.9 | 0.10 | 0.8–1.0 | 1.0–1.2 |
| IND | 2.5 | 0.22 | 2.2–2.9 | 2.3–2.5 |
| IOD | 2.1 | 0.27 | 1.7–2.4 | 1.7–2.1 |
TABLE 3. Comparison of acoustic parameters of known advertisement calls of Crossodactylus. Data presented as mean ± SD; range (N, number analyzed) when available. * = data not available.
| C. caramaschii | C. cyclospinus | C. franciscanus | |
|---|---|---|---|
| Notes per call | 56.83 ± 5.41 49–69 (6) | 63.3 ± 11.9 35–98 (25) | 48 ± 18.2 36–84 (48) |
| Call duration (s) | 5.50 ± 0.54 4.71–6.09 (6) | 4.33 ± 0.74 3.57–6.25 (25) | 6.42 ± 2.5 4.8–11.6 (6) |
| Note duration (ms) | * | 28 ± 4 3–40 (1601) | 12 ± 2.6 6–20 (284) |
| Internote interval (ms) | * | 40 ± 3 29–65 (1576) | 123 ± 13.4 90–184 (284) |
| Dominant frequency (Hz) | ~5000 | 4981 ± 626.6 3488–5447 (25) | 3460.1 ± 282.5 2344–4031 (284) |
| continued. |
| MNRJ |
Museu Nacional/Universidade Federal de Rio de Janeiro |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.