Rhynchozoon Hincks, 1895
|
publication ID |
https://doi.org/10.1080/00222930500415195 |
|
persistent identifier |
https://treatment.plazi.org/id/03CE7B54-FF8F-FF8F-DEB2-1AFA8BF0BDF1 |
|
treatment provided by |
Felipe |
|
scientific name |
Rhynchozoon Hincks, 1895 |
| status |
|
Genus Rhynchozoon Hincks, 1895 View in CoL
Rhynchozoon tumulosum ( Hincks, 1882) View in CoL
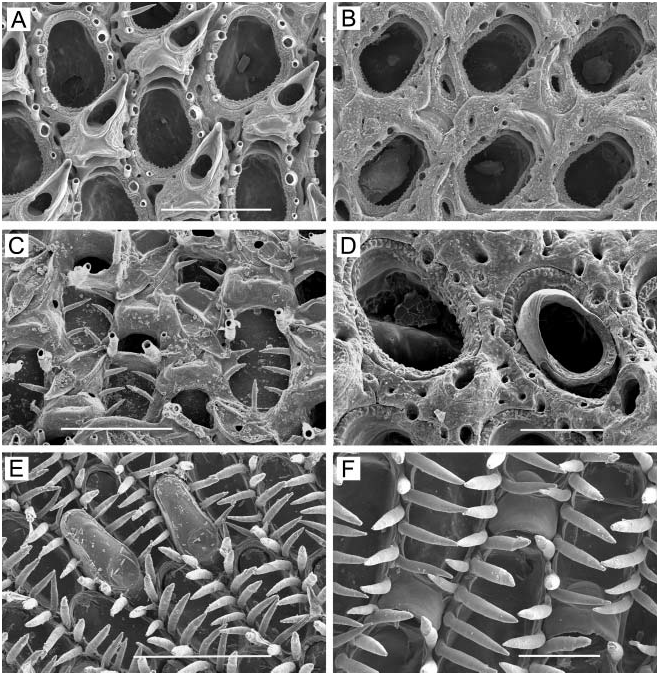
( Figure 25A–H)
Schizoporella tumulosa Hincks 1882, p 252 ; 1883, p 447, Plate 18, Figure 2.
Schizoporella tumulosa: Robertson 1908, p 293 , Plate 20, Figure 53; O’Donoghue and
O’Donoghue 1923, p 37, 1926, p 56 (in part?).
Rhynchozoon tumulosum: Osburn 1952, p 458 View in CoL , Plate 54, Figures 4–5 View Figure 4 View Figure 5 , 12.
Rhynchozoon rostratum: Soule and Soule 1964, p 33 View in CoL (in part); Dick and Ross 1988, p 84,
Plate 6I.
Description
Colony. Tan to light brown in colour; encrusting, unilaminar, occasionally multilaminar through frontal budding or overgrowth of older layers; circular or irregular; largest observed 2.5 cm across.
Zooids. Irregularly hexagonal; marginal zooids 0.40–0.58 mm long (average 50.460 mm, n 515, 3) by 0.28–43 mm wide (average 50.335 mm, n 515, 3); young zooids delineated by a deep groove with a calcified line formed by appressed marginal walls between adjacent rows of areolae; zooidal boundaries later become indistinct. Zooids interconnect by unusual raised, disk-like dietellae scattered irregularly around the distolateral and distal walls, each with a single tiny pore in the centre. Basal wall completely calcified, often with several irregularly distributed white punctae up to 0.05 mm in diameter.
Frontal wall. Shiny, vitreous; initially markedly convex, inflated, occasionally smooth but usually weakly to strongly costate ( Figure 25A, B) between the 11–19 (average514.4, n 524, 3) large areolar pores in total around the margin; the costal ridges of the primary layer soon become thickened by finger-like projections of secondary calcification ( Figure 25D) growing centripetally over them. Frontal wall at intermediate stage of calcification convex, heavily costate; proximal to orifice is a variably developed umbo that can be tall or short, cylindrical or conical, sharp or blunt. Frontal wall at late stage heavily calcified, flatter and irregular, often with only traces of costal ridges remaining around margin, but generally somewhat rugose, irregular.
Orifice. Primary orifice ( Figure 25A, C) slightly broader than long, 0.10–0.14 mm long (average 50.118 mm, n 515, 3) by 0.11–0.14 mm wide (average 50.125 mm, n 515, 3), with a shallow, curved proximal sinus between a pair of triangular projections, flanked on each side by a conspicuous, rounded condyle; orificial margin beaded with 14–19 (average516.5, n 522, 3) regularly spaced, rounded denticles. With increased frontal calcification, primary orifice lies deep in peristome; secondary orifice ( Figure 25E–G) irregular in shape, with a process at margin on each side, sharp and cylindrical or stout and blunt, angled toward orifice to a variable extent; with a broad pseudosinus between projection on one side and base of avicularian rostrum within the peristome.
Avicularia. Three types occur. One is the asymmetrically positioned suboral avicularium, which arises initially as a bulbous chamber ( Figure 25B) from an areolar pore lateral to proximal margin of orifice, on one side or the other; rostrum directed laterally toward side of origin and tilted in frontal direction, with a hooked end; mandible long-triangular with a small hook at end. As frontal wall thickens, avicularian chamber becomes completely immersed, covered by the umbo, and avicularium comes to lie completely in peristome. In addition to suboral avicularium, zooids can have a single frontal avicularium along proximal zooidal margin ( Figure 25E), equal to or larger than suboral avicularium in size, with a non-hooked rostrum angled upward from the frontal surface, the mandible longtriangular, pointing proximally or sometimes laterally. Zooids can have a third type of avicularium; this is a frontal avicularium occurring anywhere along the lateral margins ( Figure 25F), one or two per zooid. These lateral avicularia are less than or equal in size to the proximal avicularia and have an equilateral or long-triangular mandible usually directed perpendicular to zooidal margin. In heavily calcified zooids, chamber of frontal avicularia (proximal and lateral) can become completely immersed, the rostrum scarcely raised above the frontal surface. The complement of avicularia is variable; zooids within the same colony can have (in addition to the suboral avicularium) no frontal avicularia, only the proximal avicularium, the proximal and one or more lateral avicularia, or one or more lateral avicularia and no proximal avicularium.
Spines. Marginal zooids have two to five stout distal spines sometimes longer than zooid itself; in some colonies, most zooids have only two spines ( Figure 25B), whereas in others, zooids with three to five spines are common ( Figure 25A, C). Spines are ephemeral and restricted to marginal zooids; colony fragments without marginal zooids appear to lack them altogether.
Ovicell ( Figure 25F, G). Broader than long; 0.13–0.20 mm long (average 50.164 mm, n 522, 3) by 0.14–0.25 mm wide (average 50.197 mm, n 522, 3), immersed to the level of the colony surface, the globose top exposed at first but later weakly covered by frontal calcification from surrounding zooids; proximal face of ovicell lies in peristome and has a lumpy panel of exposed endooecium that is semicircular, blunt-triangular, transversely elliptical, or circular in shape, completely or incompletely bordered by ectooecium along the proximal margin. In fertile colonies, many zooids leave space for an ovicell before the ovicell develops; the result is a large secondary orifice that will be reduced in size when the ovicell forms.
Ancestrula . Roughly 0.40 mm in length and width; flattened; orifice semicircular with a straight proximal margin and about 15 rounded denticles around the curvature; possibly with several pairs of spines surrounding orifice, though if present these are obscured by periancestrular zooids in our specimens. Ancestrula surrounded by three zooids distally and distolaterally and a pair of larger zooids proximally ( Figure 25H). Form of periancestrular zooids is similar to that of later zooids; they have three or more thick distal spines; however, many zooids within four generations from the ancestrula lack a suboral (or any other) avicularium. Ancestrula is rapidly overgrown by periancestrular zooids and difficult to observe.
Remarks
Hincks (1882) described this species from Cumshewa Harbour, Queen Charlotte Islands; the type locality is thus only about 250 km south of Ketchikan. Hincks’s (1882, 1883) descriptions and illustration accord well with our specimens, indicating an orifice that is broader than long; occurrence of more than two distal spines (shown in Hincks 1883, Figure 2b but not mentioned in his descriptions), conspicuous frontal costation, and the occurrence of the proximal frontal avicularium (though Hincks 1883, Figure 2 shows this avicularium positioned more distally). Robertson (1908) provided an additional description and illustration of R. tumulosum ; she indicated young zooids having ‘‘tubelike ridges’’ radiating from the margin toward the centre, and her illustration shows an orifice similar to that of Hincks’s illustration. Unlike Hincks, she noted ‘‘avicularia scattered profusely over the whole surface of old colonies’’.
We have examined ( Dick and Mawatari 2005) a NHM specimen labelled ‘‘1886.3.6.49. Schizoporella tumulosa Hincks , Queen Charlotte Islands, Canada, Pt. of type’’, with the colony of interest indicated by an arrow in India ink, presumably done by Hincks. This colony, which lacks ovicells, shows the same suite of other characters as our specimens, and we are thus confident that our material is R. tumulosum (Hincks) .
Earlier, Busk (1856) had described a similar species, R. rostratum , from Mazatlán, Mexico. This species was not noted again until Hastings (1930) wrote a few descriptive remarks and provided illustrations for specimens she identified as R. rostratum from Panama, Colombia, and the Galapagos. She indicated an orifice with a shallow proximal sinus and small condyles, and mentioned only one frontal avicularium per zooid. Osburn (1952) distinguished between R. tumulosum and R. rostratum on the basis of the former having a distinctly ‘‘schizoporellidan’’ sinus and strong frontal costae, as compared to the latter having a shallower sinus and only weak marginal costation. Osburn (1952) considered R. tumulosum to be distributed from British Columbia southward to the San Benito Islands, Baja California, and R. rostratum to be distributed from Point Conception, California southward to the Galapagos Islands. He thus viewed the two species as overlapping from Point Conception to middle Baja California.
After re-examining Osburn’s specimens and additional material from the eastern Pacific, Soule and Soule (1964) declared R. tumulosum a junior synonym of R. rostratum , although they then curiously indicated the range for R. rostratum to be from southern California to the Galapagos, ignoring the records of R. tumulosum extending as far north as British Columbia. The Soules reported two to four oral spines for R. rostratum and noted that Osburn had overlooked the spines present in some of the specimens in the Hancock collections he had identified as R. tumulosum . Indeed, no previous authors had described spines for either R. rostratum or R. tumulosum , perhaps due to the ephemeral nature of the spines.
We consider it unlikely that R. tumulosum and R. rostratum are synonymous, partly because of the high-boreal to tropical distribution required by synonymy, and also because Hastings (1930) indicated for R. rostratum a characteristic faintly greenish colour strongly contrasting with the opaque white of the endooecial panel of the ovicell and the frontal processes. Hastings’s illustrations (1930, Plate 14, Figures 93–96) also indicate an uncinate process within the peristome at the base of the avicularian rostrum, which Osburn (1952) mentioned as a character diagnostic for R. rostratum ; our specimens lack this process. We have not examined tropical specimens of R. rostratum .
Distribution
Dick and Ross (1988) found a single Rhynchozoon specimen at Kodiak that was similar to R. tumulosum in Osburn’s Hancock collections, but that they identified as R. rostratum because of the synonymy ( Soule and Soule 1964). We have examined an additional specimen from Kodiak in which zooids are heavily costate, with three stout spines on many of the marginal zooids, characters indicative of R. tumulosum . In specimens from California, Robertson (1908) noted ‘‘tube-like ridges radiating toward the middle’’ in younger zooids, similarly suggestive of R. tumulosum . She noted this species as abundant in dredgings from San Pedro to San Diego. We consider the range of R. tumulosum to extend from the western Gulf of Alaska to California.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Rhynchozoon Hincks, 1895
| Dick, Matthew H., Grischenko, Andrei V. & Mawatari, Shunsuke F. 2005 |
Rhynchozoon rostratum : Soule and Soule 1964 , p 33
| Dick MH & Ross JRP 1988: 84 |
| Soule DF & Soule JD 1964: 33 |
Rhynchozoon tumulosum :
| Osburn RC 1952: 458 |
Schizoporella tumulosa :
| Robertson A 1908: 293 |
Schizoporella tumulosa
| Hincks T 1882: 252 |