Sphyrna gilberti, Quattro, Joseph M., Driggers Iii, William B., Grady, James M., Ulrich, Glenn F. & Roberts, Mark A., 2013
|
publication ID |
https://doi.org/10.11646/zootaxa.3702.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:E1157350-496E-4FD5-9301-8A67153E4530 |
|
DOI |
https://doi.org/10.5281/zenodo.6162983 |
|
persistent identifier |
https://treatment.plazi.org/id/03C5B858-FFEE-F214-B2AF-1B3C57A2AA2E |
|
treatment provided by |
Plazi |
|
scientific name |
Sphyrna gilberti |
| status |
sp. nov. |
Sphyrna gilberti View in CoL sp. nov.
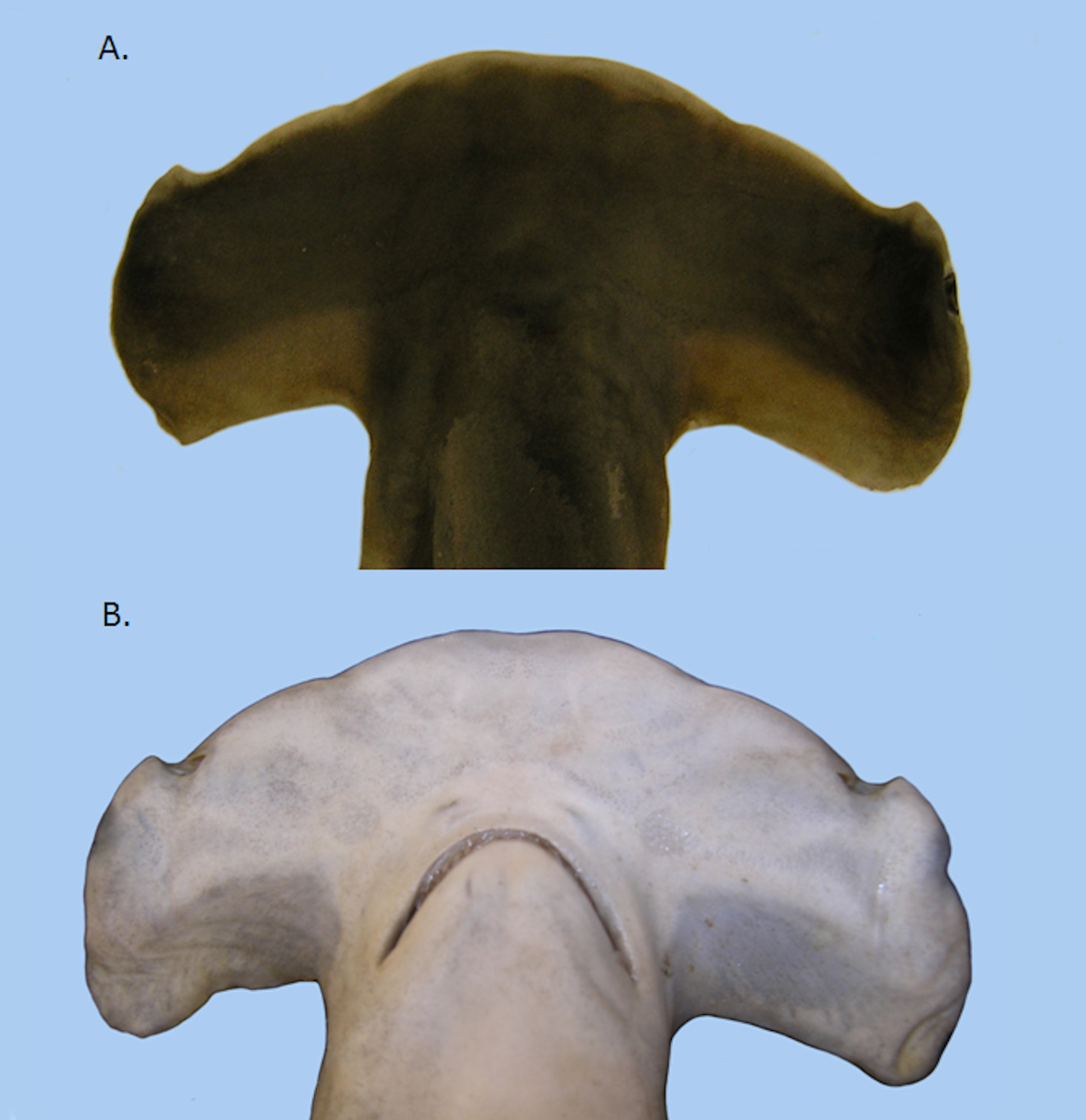
( Figures 5–6 View FIGURE 5 View FIGURE 6 , Table 1)
Proposed common name. Carolina hammerhead
This name was selected based on the type locality of S. gilberti sp. nov..
Materials examined. Type specimens were collected in the coastal waters of South Carolina ( Figure 1 View FIGURE 1 ) and placed in the collection of the Florida Museum of Natural History. In parentheses following each specimen’s identification code is the CR haplotype and the LDHA6 genotype.
Holotype. UF 183577 (5, 6/6), 467 mm STL, female, Bulls Bay, South Carolina, U.S.A. July 2002 , collected by W.B. Driggers III, D. Oakley and G.F. Ulrich.
Paratypes. All from Bulls Bay, South Carolina, U.S.A. and collected by W.B. Driggers III, D. Oakley and G.F. Ulrich. UF 183579 (both 5, 6/6), two males, 468–471 mm STL, July 2001; UF 183578 (both 5, 6/6), two females, 471–506 mm STL, July 2002.
Comparative material. All specimens listed below housed at the University of South Carolina, Department of Biological Sciences, Conservation Genetics Laboratory and collected by W.B. Driggers III, J. M. Grady, D. Oakley and/or G.F. Ulrich in the nearshore waters of South Carolina, U.S.A.
S. gilberti : JQNSP2 (5, 6/6), 441 mm STL, male, Bulls Bay, July 2001; JQNSP3 (5, 6/6), 466 mm STL, female, Bulls Bay, July 2001; JQNSP4 (5, 6/6), 456 mm STL, female, Bulls Bay, July 2001; JQNSP9 (5, 6/6), 481 mm STL, male, Bulls Bay, July 2001; JQNSP10 (5, 6/6), 481 mm STL, Bulls Bay, July 2001; JQNSP11 (5, 6/6), 430 mm STL, male, Bulls Bay, July 2001; JQNSP16 (5, 6/6), 447 mm STL, female, Bulls Bay, June 2002; JQNSP17 (5, 6/6), 430 mm STL, male, Bulls Bay, June 2002; JQNSP19 (5, 6/6), 538 mm STL, male, Bulls Bay, August 2001; JQNSP20 (7, 6/6), 421 mm STL, female, Bulls Bay, August 2001; JQNSP24 (5, 6/6), 420 mm STL, female, Bulls Bay, May 2002; JQNSP25 (5, 6/6), 406 mm STL, female, Bulls Bay, May 2002; JQNSP26 (7, 6/6), 392 mm STL, female, Bulls Bay, May 2002; JQNSP27 (5, 6/6), 675 mm STL, male, Charleston Harbor, June 2002; JQNSP28 (5, 6/6), 462 mm STL, male, Bulls Bay, June 2002; JQNSP29 (5, 6/6), 438 mm STL, female, Bulls Bay, June 2002; JQNSP30 (5, 6/6), 495 mm STL, female, Bulls Bay, July 2002; JQNSP31 (5, 6/6), 488 mm STL, male, Bulls Bay, July 2002; JQNSP32 (5, 6/6), 484 mm STL, female, Bulls Bay, July 2002; JQNSP33 (5, 6/6), 495 mm STL, male, Bulls Bay, July 2002; JQNSP37 (5, 6/6), 541 mm STL, male, Bulls Bay, August 2002; JQNSP38 (5, 6/ 6), 458 mm STL, female. Bulls Bay, August 2002; JQNSP40 (5, 6/6), 603 mm STL, female, St. Helena Sound, September 2002; JQNSP41 (5, 6/6), 529 mm STL, male, St. Helena Sound, September 2002; JQNSP42 (5, 6/6), 572 mm STL, female, St. Helena Sound, September 2002; JQNSP43 (5, 6/6), 540 mm STL, female, St. Helena Sound, September 2002; JQNSP44 (5, 6/6), 538 mm STL, female, St. Helena Sound, September 2002; JQNSP46 (5, 6/6), 529 mm STL, male, St. Helena Sound, August 2002; JQNSP50 (5, 6/6), 587 mm STL, male, St. Helena Sound, August 2002; JQNSP60 (5, 6/6), 694 mm STL, female, Charleston Harbor, October 2002; JQNSP78 (5, 6/ 6), 391 mm STL, male, coastal South Carolina, 2003; JQNSP79 (5, 6/6), 370 mm STL, male, coastal South Carolina, 2003; JQNSP81 (5, 6/6), 406 mm STL, female, coastal South Carolina, 2003; JQNSP82 (5, 6/6), 404 mm STL, female, coastal South Carolina, 2003;JQNSP84 (5, 6/6), 387 mm STL, male, coastal South Carolina, 2003; JQNSP85 (5, 6/6), 375 mm STL, male, coastal South Carolina, 2003; JQNSP86 (5, 6/6), 326 mm STL, male, coastal South Carolina, 2003; JQNSP87 (5, 6/6), 370 mm STL, female, coastal South Carolina, 2003; JQNSP88 (5, 6/6), 420 mm STL, male, coastal South Carolina, 2003; JQNSP89 (5, 6/6), 443 mm STL, male, coastal South Carolina, 2003; JQNSP90 (5, 6/6), 415 mm STL, female, coastal South Carolina, 2003; JQNSP91 (5, 6/6), 409 mm STL, male, coastal South Carolina, 2003; JQNSP93 (5, 6/6), 389 mm STL, female, Bulls Bay, May 2003; JQNSP94 (5, 6/6), 377 mm STL, female, Bulls Bay, May 2003; JQNSP95 (5, 6/6), 400 mm STL, female, Bulls Bay, May 2003; JQNSP96 (5, 6/6), 406 mm STL, male, Bulls Bay, May 2003; JQNSP98 (5, 6/6), 390 mm STL, female, Bulls Bay, May 2003; JQNSP105 (5, 6/6), 412 mm STL, male, Bulls Bay, May 2003.
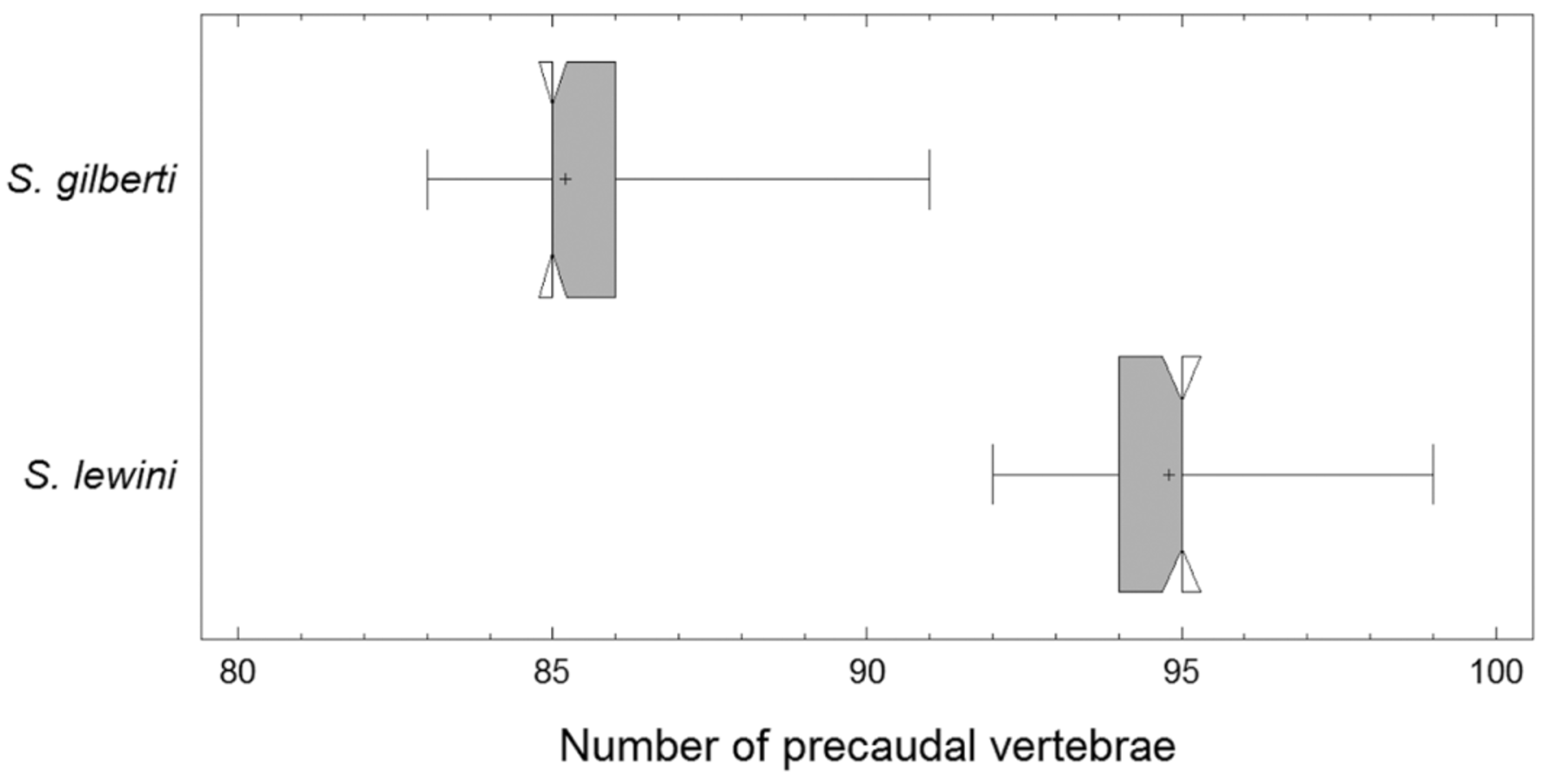
S. lewini : JQNSP12 (1, 2/2), 445 mm STL, male, Bulls Bay, June 2002; JQNSP18 (1, 1/2), 525 mm STL, male, Bulls Bay, June 2002; JQNSP21 (1, 1/1), 436 mm STL, female, Bulls Bay, May 2002; JQNSP22 (1, 1/2), 437 mm STL, female, Bulls Bay, May 2002; JQNSP23 (1, 1/2), 476 mm STL, female, Bulls Bay, May 2002; JQNSP34 (1, 2/2), 906 mm STL, female, Charleston Harbor, July 2002; JQNSP35 (1, 2/2), 883 mm STL, female, Charleston Harbor, July 2002; JQNSP36 (1, 1/2), 918 mm STL, female, Charleston Harbor, July 2002; JQNSP39 (1, 2/2), 469 mm STL, female, Bulls Bay, August 2002; JQNSP45 (1, 1/2), 690 mm STL, male, St. Helena Sound, August 2002; JQNSP48 (1, 1/2), 676 mm STL, male, St. Helena Sound, August 2002; JQNSP49 (1, 1/1), 648 mm STL, female, St. Helena Sound, August 2002; JQNSP51 (1, 1/1), 632 mm STL, female, St. Helena Sound, August 2002; JQNSP52 (8, 2/2), 653 mm STL, male, St. Helena Sound, August 2002; JQNSP53 (8, 2/2), 690 mm STL, male, St. Helena Sound, August 2002; JQNSP54 (1, 2/2), 733 mm STL, female, Charleston Harbor, September 2002; JQNSP58 (1, 2/2), 775 mm STL, female, Charleston Harbor, September 2002; JQNSP59 (1, 2/2), 739 mm STL, female, Charleston Harbor, September 2002; JQNSP61 (1, 1/1), 729 mm STL, male, Charleston Harbor, September 2002; JQNSP100 (1, 1/2), 527 mm STL, female, Bulls Bay, May 2003; JQNSP101 (1, 2/2), 485 mm STL, male, Bulls Bay, May 2003; JQNSP103 (1, 2/2), 452 mm STL, male, Bulls Bay, May 2003; JQNSP104 (1, 1/1), 444 mm STL, male, Bulls Bay, May 2003; JQNSP-C (1, 1/2), 877 mm STL, male, North Edisto, June 2003; JQNSP-D, 526 mm STL, female, North Edisto, June 2003; JQNSP-E (1, 1/2), 801 mm STL, female, Charleston, November 2002. Diagnosis. Sphyrna gilberti sp. nov. can be distinguished from congeners by having a head length greater than 20% of STL, cephalofoil with median indentation, inner narial groove present, pelvic fins with straight rear margins, and 91 or fewer precaudal vertebrae.
Description. Direct measures and counts for the holotype of S. gilberti sp. nov. (UF 183577) are listed below and reported in mm. Values in parentheses represent proportion of each measure as a percentage of STL. Proportions, expressed as percent of STL, of all collected specimens of S. gilberti sp. nov. are presented in Table 1.
Female; precaudal length 309 (66); fork length 358 (77); natural total length 454 (97); STL 467; head length 113 (24); pre-first dorsal length 130 (28); pre-second dorsal length 281 (60); prepectoral length 106 (23); prepelvic length 217 (46); preanal length 270 (58); snout-vent length 225 (48); interdorsal space 102 (22); second dorsalcaudal space 26 (6); pectoral-pelvic space 89 (19); pelvic-anal space 33 (7); anal-caudal space 34 (7); pelvic-caudal space 86 (18); vent-caudal length 97 (21); head width 138 (30); median-lateral indentation space 34 (7); lateral indentation-nacelle space 33 (7); head posterior margin 35 (7); snout length 36 (8); nacelle length 47 (10); nacelle height 13 (3); eye length 12 (2); eye height 10 (2); internarial space 65 (14); inner narial groove length 30 (6); nostril length 12 (3); mouth width 34 (7); mouth length 19 (4); dental formula U 13–1–13, L 13-1–13; intergill length 28 (6); first gill slit height 15 (3); second gill slit height 16 (3); third gill slit height 18 (4); fourth gill slit height 18 (4); fifth gill slit height 14 (3); pectoral anterior margin 53 (11); pectoral base 25 (5); pectoral inner margin 21 (4)pectoral posterior margin 44 (9); pectoral height 48 (10); first dorsal anterior margin 75 (16); first dorsal base 43 (9); first dorsal inner margin 15 (3); first dorsal posterior margin 60 (13); first dorsal height 60 (13); second dorsal anterior margin 19 (4); second dorsal base 15 (3); second dorsal inner margin 21 (4); second dorsal posterior margin 25 (5); second dorsal height 9 (2); pelvic anterior margin 21 (4); pelvic base 21 (4); pelvic inner margin 16 (3); pelvic posterior margin 27 (6); pelvic height 22 (5); anal anterior margin 20 (4); anal base 21 (4); anal inner margin 18 (4); anal posterior margin 27 (6); anal height 15 (3); dorsal caudal margin 144 (31); preventral caudal margin 49 (10); upper postventral margin 188 (25); lower postventral caudal margin 29 (6); caudal fork length 32 (7); caudal fork width 35 (7); subterminal caudal margin 11 (2); terminal caudal margin 29 (6); 87 precaudal vertebrae.
Body round to oval in cross section becoming rectangular at the caudal peduncle. Head laterally expanded with median indentation. Head width 25–32% of STL. Lateral indentation present on each side of head approximately equidistant between median indentation and anterior margin of nacelle. Inner narial groove extends from nostril to lateral indentation. Nacelle well defined and eye height usually greater than 50% nacelle height. Nictitating eyelid present. Posterior orbit on perpendicular with symphysis of upper jaw. Snout length approximately equal to mouth width. Corners of mouth even with posterior margin of head. Labial furrows small and inconspicuous. Head length approximately equal to interdorsal space. Third gill slit height approximately equal to anal fin height. Fourth gill slit near pectoral fin origin. Fifth gill slit height shorter than other gill slits and posterior to pectoral fin origin.
Pectoral fin relatively small and its height approximately equal to the pectoral fin posterior margin. Pectoral fin anterior margin. Pectoral fin anterior margin less than 50% of head width. Apex of pectoral fin rounded and posterior margin relatively straight with rounded free rear tip. First dorsal fin origin ranges from above pectoral fin insertion to above pectoral fin inner margin. First dorsal fin anterior margin straight with apex slightly rounded. First dorsal fin posterior margin straight to slightly concave and its height approximately equal to pectoral anterior margin. Length of first dorsal fin inner margin and pelvic fin inner margin approximately equal. Interdorsal ridge absent in life but can appear present in preserved specimens. Second dorsal fin origin posterior to anal fin origin. Second dorsal fin small and its height equal to or less than 20% of first dorsal fin height. Second dorsal fin inner margin length at least twice as long as second dorsal fin height. Origin of pelvic fin posterior to first dorsal fin free rear tip. Pelvic fin posterior margin straight. Anal fin base broad and equal to or less than anal fin height. Upper and lower precaudal pits present. Caudal fin deeply forked and greater than 25% of STL. Subterminal notch present.
Dental formula U 11 to 15–0 to 2–11 to 15, L 11 to 14–0 to 2–11 to 14 (dental formula should be considered preliminary as jaws were not removed from specimens and small posterior teeth could have gone unnoticed resulting in lower counts than actual number of teeth present). All teeth unicuspidate. Symphysial teeth smaller than adjacent anterior teeth, crown well differentiated from base, smooth edged to weakly serrated, and symmetrical. Upper anterior, lateral and posterior teeth with cusp at an oblique angle that becomes progressively greater moving from the symphysis toward the rear of the palatoquadrate. Mesial edge of upper anterior, lateral and posterior teeth smooth to weakly serrated. Distal edge of upper anterior, lateral and posterior teeth with a central notch and ranging from smooth to serrated from the tip of the cusp toward the base. Lower anterior teeth adjacent to the symphysis erect, symmetrical, smooth to weakly serrated and differentiated from base. Lower lateral and posterior teeth similar in shape to upper anterior, lateral and posterior teeth.
Number of precaudal vertebrae range from 83 to 91. One individual had greater than 87 precaudal vertebrae.
Color. Live specimens with grey to brown dorsal coloration fading to white ventrally. Ventral pectoral fin apex variably white to dusky (60% of specimens with dusky-tipped pectoral fins). Lower lobe of caudal fin with dusky to black tip. Frozen specimens retain similar, yet not as distinct, colorations to live individuals.
Size. Maximum size is unknown; however, the mean size of S. gilberti sp. nov. and S. lewini neonates with an open umbilicus collected during this study was 397 mm and 451 mm STL, respectively. Therefore, assuming equal brood size, lower size at birth for S. gilberti sp. nov. could indicate that it reaches a smaller maximum size than S. lewini .
Etymology. Named in honor of ichthyologist Carter R. Gilbert, who first reported (Gilbert 1967) an anomalous specimen of S. lewini collected off Charleston, SC (USNM 25180). Based on vertebral count and collection location, the anomalous specimen is likely the first recorded individual of S. gilberti sp. nov..
Distribution. All specimens reported herein and those examined by Quattro et al. (2006) were captured in the western North Atlantic Ocean. However, data presented by Pinhal et al. (2012) indicate a presence in the western South Atlantic Ocean. Based on the rarity of samples of S. gilberti sp. nov. relative to S. lewini and the absence of morphological attributes for field identification, no definitive information is available on geographic distribution and more data will be required to determine its precise range.
Remarks. Concordant partitions among independent character sets are reliable indicators of both evolutionary divergence and speciation (Avise & Ball 1990; Grady & Quattro 1999). Based on extensive geographic sampling, mitochondrial gene trees recovered a deep divergence within S. lewini that was repeated in independent nuclear genes (Abercrombie et al. 2005; Quattro et al. 2006; Zemlak et al. 2009; Naylor et al. 2012; Pinhal et al. 2012). Although morphometric attributes did not delineate groups among nominal S. lewini , meristic characters defined two morphological groups that are consistent with the genetic lineages.
Analyses of the 67 measurements taken on 54 S. gilberti sp. nov. and 26 S. lewini specimens failed to reveal any external character useful in differentiating the two species. While significant differences were found in mean or median values for specific characters between species, the range of each measure overlapped. Gilbert (1967) suggested that chondrocranium structure and the distribution of ampullae on the ventral side of the head are taxonomically valuable for sphyrnids. Comparisons of radiographs of the heads of S. gilberti sp. nov. and S. lewini failed to reveal any structural differences (Figure 7). Visual comparisons of the distribution of ampullae also failed to reveal differences between the two species ( Figure 6 View FIGURE 6 ).
Consistency across characters would otherwise support Quattro et al. ’s (2006) suggestion of cryptic speciation in S. lewini but for the reliance on meristic attributes for morphological separation of the genetic groups. Among meristic attributes, only the number of precaudal vertebrae separates S. gilberti sp. nov. from S. lewini ( Figure 8 View FIGURE 8 ). While meristic characteristics, in particular vertebral counts, are subject to environmental influence in fishes, a phenomenon discussed as early as Jordan (1891), the taxonomic efficacy of vertebral counts in differentiating shark species was noted by Springer & Garrick (1964), who stated “of the genera containing two or more species, almost half include at least one species distinguishable on vertebral counts.” For example, Rhizoprionodon terraenovae (Richardson 1836) and R. porosus (Poey 1861) are two species of small sharks in the western North Atlantic Ocean recognized as distinct based on overlapping number of precaudal vertebrae (Springer 1964). Other examples of subspecies or species whose proper identification rely on vertebral counts include Mustelus canis canis (Mitchell 1815) vs. M. canis insularis Heemstra 1997 (Heemstra 1997) , Carcharhinus galapagensis (Snodgrass & Heller 1905) vs. C. obscurus (Lesueur 1818) (Garrick 1982) and C. limbatus (Valenciennes 1839) vs. C. tilstoni (Whitley 1950) (Last & Stevens 1994) .
The paucity of diagnostic features for S. gilberti sp. nov. could be related to the use of neonates and young juveniles in morphological comparisons. Adult S. gilberti sp. nov. and S. lewini specimens might reveal additional diagnostic morphological characters, assuming that the effect of allometry on characters is removed. Gilbert (1967) reported significant ontogenetic changes in head morphology, fin shape and fin coloration, but noted that other characters, such as chondrocranium structure and ampullae pore patterns, are reliably stable. Among the 67 morphometric attributes examined in this study, 22 were correlated with body length when standardizing each measure by STL. Therefore, morphological characters intended as taxonomic keys for S. gilberti sp. nov. should be carefully evaluated for developmental effects.
Recently, Castro (2011) suggested that the cryptic species proposed by Quattro et al. (2006) could be S. couardi Cadenat 1950 , a species described from within S. diplana Springer 1941 (= S. lewini ). Cadenat (1950) briefly described what he considered a shark closely resembling S. diplana , except the unknown species had a “longer and narrower” head and the ventral sides of the pectoral fins were entirely white. Subsequent addition of diagnostic characters separating S. couardi from S. lewini , including ampullae pore patterns (Cadenat 1960), chondrocranium structure (Gilbert 1967) and position of first dorsal fin origin relative to the pectoral fin insertion (Compagno 1984), were thoroughly discussed by McEachran & Seret (1987). Based on examination of two heads placed in the Museum National d’Histoire Naturelle and Cadenat’s personal collections of jaws, a single skin sample, notes and photographs, McEachran & Seret (1987) concluded that none of the proposed diagnostic characters were valid and could be attributed to intraspecific variability with S. lewini . For example, after examining Cadenat’s personal photographs, McEachran & Seret (1987) discovered that several S. couardi specimens had pectoral apices with dusky coloration. The above factors, in addition to the lack of type specimens upon which to base comparisons, led McEachran & Seret (1987) to designate S. couardi as a junior synonym for S. lewini ; a status it tentatively retains in Compagno (2005). Our data indicate that the head shape and variability in ventral pectoral fin coloration are highly variable among S. gilberti sp. nov. and S. lewini . For example, head width ranged from 25.78–31.66 % STL for S. gilberti sp. nov. and 25.09–30.06 for S. lewini and coloration of the pectoral fins was variable with some individuals of both species having entirely white ventral pectoral apices and others having dusky apices. Based on our data and the conclusions of McEachran & Seret (1987), there is no reason to consider S. gilberti sp. nov. and S. couardi synonymous.
In his description of a new species of hammerhead shark, S. diplana, Springer (1941) noted specimens of this taxon collected off the coast of the Carolinas, Florida, Mississippi, Louisiana and Texas, thus a comparison between S. diplana and the species described herein is warranted. In the first major work examining chondrichthyan fauna in the western North Atlantic Ocean, Bigelow & Schroeder (1948) documented four species of sphyrnids, including S. diplana , that occurred in the region. However, Bigelow & Schroeder (1948) noted that S. diplana is “represented in the tropical-subtropical waters of the eastern and western Indo-Pacific by a form ( S. lewini Griffith, 1834 ) closely resembling diplana . ”. Nonetheless, the authors regarded S. lewini and S. diplana as distinct taxa due to possible differences in dentition. The validity of S. diplana was subsequently questioned by Tortonese (1950) and Fraser-Brunner (1950), with the latter considering S. diplana a junior synonym of S. lewini .
Fraser-Brunner (1950) reasoned that Springer (1941) had not considered a circumtropical distribution for S. lewini and based comparisons of S. diplana to S. lewini on a single specimen that was most likely incorrectly identified. Subsequently, others (e.g. Gilbert 1967, Compagno 1984, Castro 2011, Ebert & Stehmann 2013) continue to relegate S. diplana to a junior synonym for S. lewini . Despite the current taxonomic status of S. diplana , it could be suggested that S. gilberti sp. nov. and S. diplana are, in fact, synonymous. Unfortunately, extensive morphological comparisons between S. diplana and other members of the genus are hampered by a lack of comparative material as the type series of S. diplana consists of a male holotype (USNM 108451) and paratypes comprising a single head (USMN 108452) and two dry jaws (USNM 110296 and USNM 110297) (Howe & Springer 1993). Fortunately, Springer (1941) reports vertebral counts for seven S. diplana (without comment on specific location); however, it is not clear if counts were obtained from any of the type specimens. The number of total vertebrae for S. diplana reported by Springer (1941) range from 196–204 and he stated that roughly half of the total vertebrae occur after the precaudal pit. Given this, we suggest that precaudual vertebrae counts of 98–102 are consistent with Springer’s data. Clearly, Springer’s precaudal vertebrae counts for S. diplana (98–102) are most similar to S. lewini (92–99) and greater than those we report for S. gilberti sp. nov. (83–91). Furthermore, we obtained radiographs of the holotype from the Smithsonian Museum of Natural History, and counted 94 precadual vertebral for S. diplana . The precaudal vertebrae count for S. diplana (94) is within the range of S. lewini (92–99) and greater than those we report for S. gilberti sp. nov. (83–91), suggesting a clear distinction of S. gilberti sp. nov. from S. diplana but consistent with no distinction between S. diplana and S. lewini . Of note, Gilbert (1967) reported total vertebrae counts for eight S. lewini to range from 192–204, consistent with Springer’s counts for S. diplana . One animal studied by Gilbert (1967) had an ‘unusually low’ total vertebrae count of 174; we suggest that this aberrant animal represents the first report of S. gilberti sp. nov..
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |