Crocuta crocuta ( Erxleben, 1777 )
|
publication ID |
https://doi.org/10.1093/mspecies/seab002 |
|
publication LSID |
lsid:zoobank.org:pub:7780CF83-D996-4E2E-A940-578019EF6A23 |
|
persistent identifier |
https://treatment.plazi.org/id/03C4ED51-9725-BE37-6BAB-92F2F7C6294F |
|
treatment provided by |
Felipe |
|
scientific name |
Crocuta crocuta ( Erxleben, 1777 ) |
| status |
|
Crocuta crocuta ( Erxleben, 1777) View in CoL
Spotted Hyena
Canis crocuta Erxleben, 1777:578 . Type locality “ Guinea, Aethiopia, ad caput bonae spei in terrae rupiumque caueis,” restricted to “Senegambia” by Cabrera (1911a:95).
Hyaena crocuta: Schreber, 1777 :Plate LXXXXVI B. Name combination.
Hyaena maculata Thunberg, 1811:302. Type locality “Africes australis.”
Hyaena capensis Desmarest, 1817:499 View in CoL . Type locality “aux environs du Cap de Bonne Esperance, … au midi de l’Afrique.”
Hyaena rufa Desmarest, 1817:499. Type locality not given; stated as Cape of Good Hope by Ellerman et al. (1953).
Hyana capensis Desmarest, 1820:216 . Type locality “Le midi de l’Afrique, aux environs du Cap de Bonne Esperance.”
Hyana rufa Desmarest, 1820:216. Type locality not given, “inconnues.”
Hyaena croacuta A. Smith, 1826:12. Incorrect subsequent spelling of crocuta Erxleben, 1777 .
Hyaena encrita A. Smith, 1827:461. Misprint ( Ellerman et al. 1953).
Hyaena cuvieri Boitard, 1842 (1845):233. Type locality “au Cap,” Cape of Good Hope.
H [ yaena]. crocuta capensis: de Blainville, 1844:82 . Name combination.
H [ yaena]. crocuta habessynica de Blainville, 1844:82. Type locality not given, Ethiopia implied.
Hyaena ( Crocotta) wissmanni Matschie, 1900a:22 . Type locality “Epikuro in Deutsch-Südwest-Afrika” Namibia ( Jenks and Werdelin 1998).
Hyaena ( Crocotta ) gariepensis Matschie, 1900a:25. Type locality “Bambusbergen im Oranje-Gebiet ” refined to “ Bamboos Mountains … 31 o 30’S., 26 o 20’E.” by Ellerman et al. (1953).
Hyaena ( Crocotta) germinans Matschie, 1900a:26 . Type locality “Rukwa-See” (Lake Rukwa), Tanzania.
Hyaena ( Crocotta ) thierryi Matschie, 1900a:30. Type locality “Sansanne Mangu, in Nord-Togo.”
Hyaena ( Crocotta ) togoensis Matschie, 1900a:31. Type locality “Volta, West-Togo,” restricted to “Kete Krachi, Togo,” by Jenks and Werdelin (1998).
Hyaena ( Crocotta ) noltei Matschie, 1900b:211, 215. Type locality “Yoko, Kamerun ” refined to “Yoko, upper Sanaga, south Cameroon,” by Jenks and Werdelin (1998).
Crocotta weissmanni: Trouessart, 1904:243 . Name combination and incorrect subsequent spelling of wissmanni Matschie 1900a .
Hyaena ( Crocuta) leontiewi Satunin, 1905:556 . Type locality “ Abyssinia ” = Ethiopia and Eritrea.
Crocotta kibonotensis Lönnberg, 1908 (1910) :16. Type locality “Kibonoto Steppe,” Kilimanjaro, Tanzania.
Crocotta germinans: Lönnberg, 1908 (1910) :16. Name combination.
Crocotta panganensis Lönnberg, 1908 (1910) :18. Type locality “Kibonoto,” Kilimanjaro, Tanzania.
Crocuta crocuta: Cabrera, 1911a:95 View in CoL . First use of current name combination.
Crocuta wissmanni: Cabrera, 1911a:95 . Name combination.
C [ rocuta]. gariepensis: Cabrera, 1911a:95. Name combination.
C [ rocuta]. germinans: Cabrera, 1911a:95 . Name combination.
C [ rocuta]. thierryi: Cabrera, 1911a:95. Name combination.
C [ rocuta]. togoensis: Cabrera, 1911a:95. Name combination.
C [ rocuta]. noltei: Cabrera, 1911a:95. Name combination.
C [ rocuta]. kibonotensis: Cabrera, 1911a:95 . Name combination.
C [ rocuta]. panganensis: Cabrera, 1911a:95 . Name combination.
Crocuta capensis: Cabrera, 1911a:96 . Name combination.
Crocuta leontiewi: Cabrera, 1911a:97 . Name combination.
Crocuta rufopicta Cabrera, 1911a:97 . Type locality “Odueina, Boran Country.”
Crocuta thomasi Cabrera, 1911a:98 . Type locality “Ankole, Uganda.”
Crocuta nyasae Cabrera, 1911a:99 . Type locality “Mount Milanji, South Nyasaland.”
Crocuta nzoyae Cabrera, 1911b:199 . Type locality “Meseta de Uasingishu, Africa Oriental Inglesa” = Guas Ngishu Plateau, Kenya.
Crocuta crocuta fisi Heller, 1914:5 . Type locality “Merelle Waterholes, Marsabit Road, British East Africa ” = Kenya.
Crocuta crocuta fortis Allen, 1924:214 . Type locality “Faradje, northeastern Belgian Congo.”
C [ rocuta]. c [ rocuta]. Germinans: Allen, 1924:214. Name combination.
Hyaena ( crocotta) wismanni Matthews, 1939a:256. Incorrect subsequent spelling of wissmanni Matschie 1900a .
Hyaena ( crocotta) leontiewi: Matthews, 1939a:256 . Name combination.
Crocotta kibotonensis Matthews, 1939a:256 . Incorrect subsequent spelling of kibonotensis Lönnberg 1908 .
Crocuta nyassae Matthews, 1939a:256 . Incorrect subsequent spelling of nyasae Cabrera 1911a .
Crocuta croucta Wilson and Reeder, 2005:1795 . Incorrect subsequent spelling of crocuta Erxleben, 1777 View in CoL .
CONTENT AND CONTEXT. Context as for genus. Crocuta crocuta View in CoL has no currently recognized subspecies.
NOMENCLATURAL NOTES. The genus name was used for an insect by Meigen (1800), but Meigen’s pamphlet was suppressed for the purposes of zoological nomenclature by the International Commission on Zoological Nomenclature (1963). Synonymy is modified from Matthews (1939a), Jenks and Werdelin (1998), and Wozencraft (2005).
Crocuta View in CoL is a Latinized version of the Greek “krokúttas” or “krokottas,” which itself comes from a Sanskrit word that references a female golden jackal ( Funk 2010). Ancient texts often referred to any of the three hyenas as jackals. The first recorded association of the spotted hyena with the designation crocuta View in CoL was in 1681 ( Funk 2010:55). An additional common name in English is tiger wolf, and a list of common names from over 95 languages is available online via Wikipedia (2019).
DIAGNOSIS
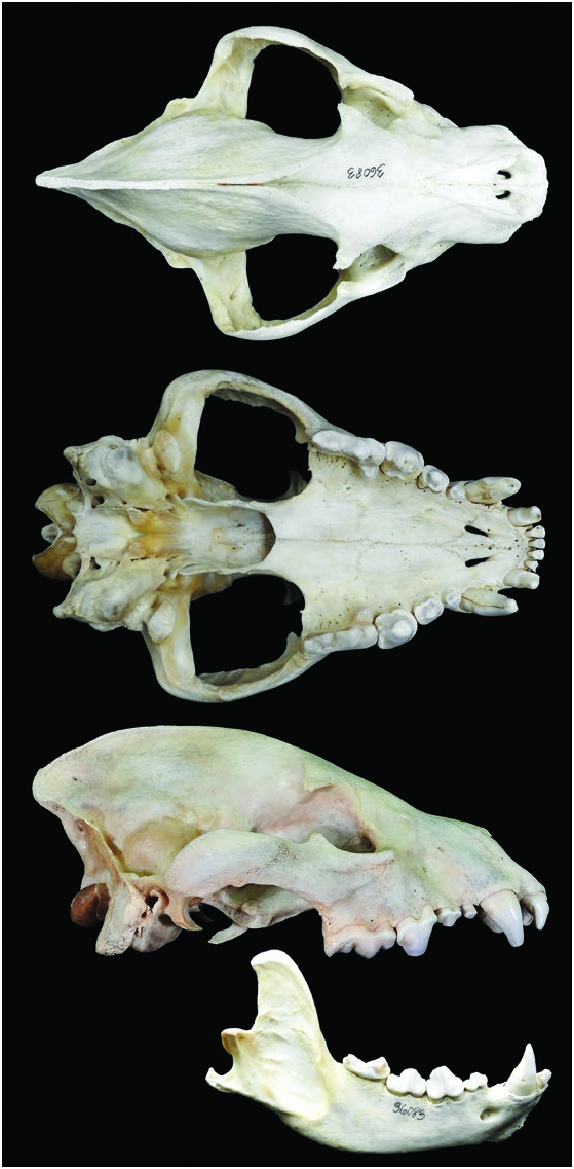
Crocuta crocuta ( Fig. 1 View Fig ) is sympatric with the brown hyena ( Hyaena brunnea) in the southern parts of its range and the striped hyena ( H. hyaena ) in the east ( Kruuk 1972). The genus Crocuta has rounded ears (pointed in Hyaena), lacks a dorsal mane (present in Hyaena), and has a spotted pelage (striped or uniform in Hyaena — Ellerman et al. 1953). The sagittal crest of the Crocuta skull ( Fig. 2 View Fig ) is largest in the middle region (more posterior in Hyaena), and the origin of the temporalis is more anterior than in Hyaena ( Buckland-Wright 1969:19, figure 1). Small differences in the occipital, squamosal, condylar, nuchal crest, otic, basis cranii, zygomatic arch, and orbital regions as well as the axis and atlas vertebrae have been detailed (BucklandWright 1969).
GENERAL CHARACTERS
Crocuta crocuta is a large ( 40–80 kg) carnivore with a female-dominant social system. Ground color of pelage varies widely from light gray through yellows to reds, with dark brown to black spots ( Allen 1924). Spots fade with age ( Kruuk 1972). The adult pelage is rough to the touch; younger individuals have soft and fluffy fur ( Matthews 1939b; K. E. Holekamp, pers. comm.). The round ears are erect with short, dirty-white and gray hair inside ( Deanne 1962). The nose is black and smooth ( Deanne 1962). Four toes, with nonretractable claws, are present on each foot ( Deanne 1962).
The well-developed neck and forequarters relative to the hindquarters give C. crocuta a sloping back most obvious when the animal runs away from a perceived danger ( Fig. 3 View Fig ). The body shape also allows the animal to carry off large clumps of meat well above the ground and drag heavy carcasses away from kill sites ( Kruuk 1972).
Mean external measurements (mm; parenthetical range if available, n) of northern C. crocuta from Ethiopia, Kenya, Tanzania, and Uganda for females, males, and specimens of unknown sex, respectively, were: total length, 1,515 (NA, 23), 1,477 (NA, 24), and 1,494.5 (1,475.0–1,501.0, 28); length of head and body, 1,333 (NA, 1), 1,295 (NA, 1), and 1,204.5 (1,191.0–1,209.0, 28); length of tail 233.8 (231.0–296.0, 23), 233.4 (232.0–267.0, 25), and 330.2 (NA, 2); length of hind foot, 235.9 (234.0–236.0, 24), 237.8 (234.0–238.0, 25), and 230.0 (NA, 28— Allen 1924; Matthews 1939a; Deanne 1962; Hamilton et al. 1986). Mean external measurements (mm; n) for females and males, respectively, from Namibia were: height at shoulder, 767 (21) and 775 (19); girth, 888 (17) and 845 (16); neck diameter, 533 (21) and 521 (20— Hamilton et al. 1986). Mean external measurements (mm) of two female C. crocuta from the Faradje, Democratic Republic of the Congo were: total length, 1,665; length of head and body, 1,340; length of hind foot, 257 ( Allen 1924). Measurements (mm) of a specimen of unknown sex from Chad were: total length, 1,676; length of tail, 317.5 ( Deanne 1962). Mean mass (kg; parenthetical range, n) for females, males, and specimens of unknown sex, respectively, were 55.0 (50.0–55.6, 22), 48.7 (NA, 25), and 54.8 (NA, 1— Deanne 1962; Neaves et al. 1980; Hamilton et al. 1986). In the Serengeti eight adult females averaged 55.3 kg (range 44.5–63.9) and 12 adult males averaged 48.7 kg (range 40.5–55.0— Kruuk 1972). To detect female-biased sexual-size dimorphism a sample needs 71 individuals of each sex ( McElhinny 2009).
Mean external measurements (mm; parenthetical range if available, n) of southern C. crocuta from Botswana, Malawi, Namibia, South Africa, Zimbabwe, and Zambia for females, males, and specimens of unknown sex, respectively, were: total length, 1,590.1 (1,440.0–1,794.0, 20), 1,569.8 (1,439.0–1,735.0, 19), and 1,625 (NA, 1) and length of tail, 254.6 (130.0–315.0, 20), 242.2 (208.0–290.0, 17), and 311 (NA, 1— Deanne 1962; Skinner and Chimimba 2005). Mean measurements (mm; parenthetical range, n) for females and males, respectively, were: height at shoulder 822 (735–885, 22) and 809.2 (700–870, 24); length of ear, 111.8 (80–127, 25) and 114.9 (80–138, 25); length of the hind foot with claw, 247.6 (200–270, 17) and 246.9 (230– 270, 15); and length of the hind foot without claw, 228 (215– 250, 5) and 230 (220–245, 9— Skinner and Chimimba 2005). Mean mass (kg; parenthetical range, n) for females, males, and specimens of unknown sex, respectively, were 68.4 (55.0–77.1, 46), 60.9 (49.0–79.0, 50), and 62.4 (47.0–78.2, 24— Deanne 1962; Wilson 1968; Smuts 1973; Racey and Skinner 1979; Mills 1984; van Jaarsveld et al. 1984; Skinner and Chimimba 2005). Mean measurements (mm or kg, parenthetical range) of 12– 13 females and 11– 13 males, respectively, from Natal were: length of head and body, 1,327 ( 1,220 –1,440) and 1,339 ( 1,250 –1,420); heart girth, 938 (850–1,040) and 924 (860–1,010); mass, 70 (56– 80) and 66.6 (55–79— Whateley 1980).
Cranial and dental measurements of C. crocuta are provided here roughly from north to south. Condylobasal length of a specimen of unknown sex from Sudan was 255 mm ( Matthews 1939a). Cranial and dental measurements (mm) for an individual of unknown sex from Ethiopia were: interorbital breadth, 55.5; lower carnassial length, 26.0; lower carnassial width, 10.0; length of lower tooth series, 103.0; distance from condyle to mandible, 172.0; postorbital constriction, 41.0; rostral breadth on canines, 58.0; upper carnassial length, 36.0; upper carnassial width, 19.0; length of upper tooth series, 98.0; width of palate across the carnassials, 99.0; zygomatic breadth, 153.0 ( Cabrera 1911a).
Cranial and dental measurements (mm) for a female from Odueina, Somalia, were: condylobasal length, 240.0; interorbital breadth, 53.5; lower carnassial length, 28.0; lower carnassial width, 11.0; length of lower tooth series, 106.5; distance from condyle to mandible, 178.0; postorbital constriction, 40.0; rostral breadth on canines, 59.0; upper carnassial length, 36.0; upper carnassial width, 20.0; length of upper tooth series, 103.0; width of palate across the carnassials, 97.0; zygomatic breadth, 159.0 ( Cabrera 1911a).
Cranial and dental measurements (mm) for a male from Ankole, Uganda, were: condylobasal length, 245.0; interorbital breadth, 54.0; lower carnassial length, 26.0; lower carnassial width, 10.5; length of lower tooth series, 107.0; distance from condyle to mandible, 175.0; postorbital constriction, 41.0; rostral breadth on canines, 58.0; upper carnassial length, 35.0; upper carnassial width, 18.0; length of upper tooth series, 100.0; width of palate across the carnassials, 101.0; zygomatic breadth, 165.0 ( Cabrera 1911a). Zygomatic breadth of a specimen of unknown sex from Uganda was 182 mm ( Matthews 1939a).
Mean cranial and dental measurements (mm; parenthetical range) of 38 C. crocuta from Kenya with sexes combined were: breadth at base of canines, 59.0 (54–65); condylobasal length, 232.0 (221–254); greatest length of nasals, 56.7 (47– 67); interorbital breadth, 53.6 (48–60); length of lower tooth series, 104.7 (101–109); mastoid breadth, 94.9 (90–100); postorbital constriction, 43.6 (37–49); upper carnassial length, 35.0 (32.0–37.6); upper carnassial width, 19.8 (17.9–21.7); length of upper tooth series, 97.0 (94–106); zygomatic breadth, 162.3 (147–175— Allen 1924).
Mean cranial and dental measurements (mm; parenthetical n) of C. crocuta from the Balbal plains in Tanzania were: P2 length, 14.38 (97); P3 length, 21.11 (97); P4 length, 34.97 (97); c length, 14.47 (7); d2 length, 8.62 (4); d3 length, 13.48 (4); d4 length, 17.85 (4); m1 length, 26.67 (95); p2 length, 14.49 (96); p3 length, 19.86 (98); p4 length, 21.66 (91); prosthion to basion length, 228.3 (9—Kurtén 1956). Zygomatic breadth and condylobasal length (mm) of one female and one male, respectively, from the Balbal plains in Tanzania were 176, 247 and 177, 242. Condylobasal length of a specimen of unknown sex from Tanzania was 254 mm ( Matthews 1939a).
Mean cranial and dental measurements (mm; parenthetical range, n) for females, males, and specimens of unknown sex, respectively, from Faradje, Democratic Republic of the Congo were: breadth at base of canines, 62.2 (61.0–63.4, 2), 63.2 (60.0–65.7, 3), and 65.0 (63.5–67.0, 4); condylobasal length, 250.0 (242.0–258.0, 2), 248.7 (242.0–252.0, 3), and 251.0 (245.0–257.0, 4); greatest length of nasals, 63.5 (61.0–66.0, 2), 59.3 (56.6–63.4, 3), and 62.8 (54.2–67.7, 4); greatest length of skull, 281.0 (270.0–292.0, 2), 282.0 (275.0–291.0, 3), and 284 (278.0–288.0, 4); interorbital breadth, 56.5 (55.0–58.0, 2), 57.3 (54.8–58.7, 3), and 58.6 (58.0–59.0, 4); upper carnassial length, 35.7 (35.5–35.9, 2), 37.8 (37.4–38.1, 3), and 36.6 (35.0–38.2, 4); upper carnassial width, 19.2 (18.9–19.4, 2), 21.7 (21.0–22.5, 3), and 19.8 (19.1–21.0, 4); length of upper tooth series, 101.2 (99.5– 102.8, 2), 102.6 (100.6–105.4, 3), and 100.6 (95.0–103.0, 4); length of lower tooth series, 108.4 (105.4–111.4, 2), 110.7 (109.4–112.0, 3), and 110.3 (108.0–111.2, 4); mastoid breadth, 104.0 (102.0–106.0, 2), 103.3 (100.0–108.0, 3), and 105.0 (102.0–108.0, 4); postorbital constriction, 45.8 (44.0–47.5, 2), 47.8 (46.0–49.0, 3), and 47.0 (43.0–51.2, 4); zygomatic breadth, 178.5 (174.0–183.0, 2), 182.0 (177.0–191.0, 3), and 181.3 (179.0–184.0, 4— Allen 1924).
Cranial and dental measurements (mm) for a female from Mount Mulanji, Malawi, were: condylobasal length, 260.0; interorbital breadth, 61.0; upper carnassial length, 38.0; upper carnassial width, 20.0; length of upper tooth series, 111.0; lower carnassial length, 32.0; lower carnassial width, 12.0; length of lower tooth series, 119.0; distance from condyle to mandible, 190.0; postorbital constriction, 48.0; rostral breadth on canines, 69.0; width of palate across the carnassials, 110.0; zygomatic breadth, 179.0 ( Cabrera 1911a).
Mean greatest skull length (mm, parenthetical range) of five females and three males, respectively, from Zimbabwe were 226.0 (214.2–243.8) and 224.7 (210.0–254.5— Skinner and Chimimba 2005).
Cranial and dental measurements (mm) for an individual of unknown sex from Linyanti, Botswana, were: condylobasal length, 256.0; interorbital breadth, 64.0; upper carnassial length, 35.0; upper carnassial width, 20.0; length of upper tooth series, 113.0; lower carnassial length, 32.0; lower carnassial width, 11.0; length of lower tooth series, 117.0; distance from condyle to mandible, 190.0; postorbital constriction, 53.0; rostral breadth on canines, 66.0; width of palate across the carnassials, 112.0; zygomatic breadth, 185.0 ( Cabrera 1911a). Condylobasal length of one specimen of unknown sex from Linyanti, Botswana, was 256 mm; the zygomatic breadth of another specimen from the same location was 185 mm ( Matthews 1939a).
Mean cranial and dental measurements (mm; parenthetical range, n) of C. crocuta from South Africa were: breadth across zygomatic arches, 175.9 (146.0–188.0, 13); C breadth, 12.1 (10.7–12.9, 8); C length, 16.7 (15.5–17.5, 8); M1 breadth, 5.2 (4.7–5.5, 3); P1 breadth, 6.9 (6.1–8.2, 13); P2 breadth, 12.0 (10.5–13.0, 12); P2 length, 16.5 (14.7–19.2, 13); P3 breadth, 17.2 (15.8–18.0, 13); P3 length, 22.8 (21.0–24.5, 13); P4 length, 37.3 (35.0–39.7, 13); breadth of blade of P4, 11.3 (10.9–12.0, 13); length of metastyles of P4, 16.4 (15.2–17.8, 12); length of paracone of P4, 13.4 (12.0–14.5, 11); greatest anterior breadth across protocone of P4, 21.1 (19.9–22.5, 12); height of coronoid process, 85.6 (66.0–91.8, 14); c breadth, 12.4 (11.3–13.6, 10); c length, 16.1 (14.7–17.4, 10); m1 breadth, 12.0 (10.9–13.1, 14); m1 length, 27.6 (27.5–31.2, 14); m1 length of talonid, 2.9 (2.4–3.8, 14); p2 breadth, 11.0 (10.1–12.0, 12); p2 length, 16.1 (15.1–18.1, 14); p3 breadth, 15.2 (14.2–16.2, 14); p3 length, 20.8 (19.4–22.0, 14); p4 breadth, 13.3 (12.5–14.0, 14); p4 length, 22.6 (21.3–23.9, 14); p4 length of protoconid, 12.2 (11.0–13.6, 13); minimum dorsoventral depths of mandible anterior to p2, 37.3 (32.7–42.7, 14); minimum dorsoventral depths of mandible posterior to m1, 45.7 (35.0–49.7, 14); buccal-lingual breadth of mandibular corpus below the center of p3, 19.4 (17.9–21.4, 14); distance from anterior face of p2 to posterior margin of m1, 83.9 (79.0–88.7, 14); distance from anterior face of lower canine to the midpoint of posterior face of lower mandibular condyle, 177.5 (149.0–189.0, 14— Turner 1984). Condylobasal length of one specimen of unknown sex from South Africa was 258 mm; zygomatic breadth of another specimen from the same location was 179 mm ( Matthews 1939a).
Cranial and dental measurements (mm) for an individual of unknown sex from the Cape Peninsula of Africa were: condylobasal length, 236.0; interorbital breadth, 57.0; upper carnassial length, 35.0; upper carnassial width, 22.0; length of upper tooth series, 100.0; lower carnassial length, 30.0; lower carnassial width, 12.0; length of lower tooth series, 111.0; distance from condyle to mandible, 180.0; postorbital constriction, 42.0; rostral breadth on canines, 64.0; width of palate across the carnassials, 114.0; zygomatic breadth, 176.0. Cranial and dental measurements (mm) for an individual of unknown sex from West Africa were: length of lower tooth series, 109.3; length of upper tooth series, 99.8; width of palate across the carnassials, 107.1 ( Cabrera 1911a).
Mean cranial and dental measurements (mm; parenthetical range, n) of C. crocuta with sexes combined from unknown locations were: breadth across zygomatic arches, 166.3 (154.0–173.0, 4); C breadth, 11.8 (10.6–13.0, 4); C length, 16.6 (16.0–17.4, 3); P1 breadth, 6.5 (5.7–7.4, 3); P2 breadth, 11.3 (10.5–11.9, 4); P2 length, 15.6 (14.8–16.4, 4); P3 breadth, 16.9 (15.9–18.1, 4); P3 length, 21.8 (20.0–22.7, 4); P4 length, 37.4 (35.9–38.3, 4); breadth of blade of P4, 11.43 (10.4–11.9, 4); length of metastyles of P4, 16.4 (15.0–17.3, 4); length of paracone of P4, 13.4 (13.2–14.0, 4); greatest anterior breadth across protocone of P4, 20.5 (19.6–21.3, 4); height of coronoid process, 81.2 (71.2–86.0, 4); c breadth, 12.2 (11.5–12.7, 4); c length, 15.8 (15.1–16.4, 3); m1 breadth, 11.7 (11.2–12.6, 4); m1 length, 28.2 (26.4–29.6, 4); m1 length of talonid, 2.8 (2.4–3.2, 4); p2 breadth, 10.7 (10.0–11.4, 4); p2 length, 15.5 (14.7–16.5, 4); p3 breadth, 14.4 (14.2–14.6, 4); p3 length, 20.9 (19.6–22.2, 4); p4 breadth, 12.7 (12.1–13.0, 4); p4 length, 22.8 (21.9–23.2, 4); p4 length of protoconid, 11.9 (11.2–12.6, 3); minimum dorsoventral depths of mandible anterior to p2, 34.5 (33.2–36.4, 4); minimum dorsoventral depths of mandible posterior to m1, 44.2 (37.0–49.3, 4); buccal-lingual breadth of mandibular corpus below the center of p3, 17.8 (16.5–19.0, 4); distance from anterior face of p2 to posterior margin of m1, 83.5 (80.2–85.8, 4); distance from anterior face of lower canine to the midpoint of posterior face of lower mandibular condyle, 173.3 (159.0–185.0, 4); nasals length, 42.8 (39.0–47.0, 10— Turner 1984).
Mean cranial and dental measurements (mm; parenthetical n) of C. crocuta of unknown sex from an unknown location (probably Ethiopia) were: P2 length, 18.48 (4); P3 length, 24.22 (5); P4 length, 41.8 (5); d2 length, 10.20 (1); d3 length, 14.10 (1); d4 length, 20.00 (1); m1 length, 32.78 (6); p2 length, 16.00 (1); p3 length, 23.33 (6); p4 length, 24.60 (6); prosthion to basion length, 232.0 (2—Kurtén 1956); alveolar length of lower canine, 25.7 (24.5–26.5, 3); alveolar length of the upper canine, 33.5 (33.0–34.0, 3); basilar length, 192.8 (186.5–204.0, 3); greatest breadth of the “hindhead,” 87.8 (84.0–91.5, 3); width of palate at upper border of palate-pterygoid suture, 31.3 (31.0– 32.0, 3); midline length of basisphenoid, 25.7 (25.0–27.0, 3); infraorbital foramen distance at upper inner margin, 50.3 (46.5– 53.0, 3); nasal width, 23.8 (23.0–25.0, 3); smallest interorbital width, 46.9 (44.0–52.3, 3); narrowest zygomatic arch measured at upper border of zygomatico-temporalis suture, 16.9 (15.5– 19.0, 3); total midline length from front edge of premaxilla to rear edge of sagittal crest, 231.8 (218.0–245.5, 2); zygomatic breadth, 142.0 (133.0–156.0, 3— Satunin 1905).
Cranial and dental measurements are also available for fossil remains of C. crocuta . Mean lower carnassial lengths (mm; parenthetical range, SD, n) of late Pleistocene C. crocuta from various locations in Britain were: Kirkdale Cave, 31.505 (30.0–34.4, 1.013, 21); Barrington, 31.770 (30.3–33.7, 1.1148, 10); Torcourt Cave, 31.376 (29.5–33.6, 1.186, 17); Tornewton Cave, 31.596 (28.7–34.6, 1.304, 90); Joint Mitnor Cave, 31.350 (31.0–31.7, 0.495, 2); Pin Hole, 32.766 (30.0–40.7, 2.212, 44); Coygan Cave, 32.137 (29.5–34.8, 1.585, 16); Picken’s Hole, 32.288 (30.0–34.7, 1.419, 25); Badger Hole, 32.762 (30.7–34.0, 1.054, 8); Hyaena Den, 32.310 (31.1–34.5, 1.195, 8); Kent’s Cavern, 32.493 (26.9–36.3, 1.404, 118); Brixham Cave, 32.240 (31.4–34.0, 1.101, 5— Klein and Scott 1989).
Mean dental measurements (mm; parenthetical range, n) of late Pleistocene C. crocuta from Fouvent-Saint-Andoche were: C breadth, 13.2 (12.0–14.9, 15); C length, 17.5 (17.0–18.0, 13); P1 breadth, 7.6 (6.0–9.0, 20); P1 length, 7.7 (7.0–9.0, 21); P2 breadth, 13.5 (12.0–14.8, 26); P2 length, 17.7 (16.0–19.0, 16); P3 breadth, 17.5 (15.0–19.0, 22); P3 length, 24.5 (23.0–26.0, 20); P4 breadth, 21.6 (19.0–23.0, 19); P4 length, 39.7 (35.5–42.0, 16); D2 breadth, 7.6 (7.0–9.0, 8); D2 length, 12.5 (11.6–14.0, 8); D3 breadth, 13.5 (13.0–15.0, 6); D3 length, 22.6 (21.3–23.7, 13); D4 breadth, 12.6 (12.0–15.0, 7); D4 length, 10.2 (9.0–11.0, 6); c breadth, 13.7 (12.0–16.0, 41); c length, 16.3 (14.4–19.0, 40); p2 breadth, 12.2 (10.5–13.7, 20); p2 length, 16.5 (14.0–18.5, 21); p3 breadth, 16.4 (15.0–17.3, 41); p3 length, 22.5 (21.0–24.0, 44); p4 breadth, 14.9 (12.0–17.4, 62); p4 length, 24.0 (21.5– 26.0, 64); m1 breadth, 13.4 (12.0–14.5, 46); m1 length, 31.7 (27.5–34.0, 42); d2 breadth, 6.3 (5.0–7.2, 11); d2 length, 10.8 (9.5–12.0, 11); d3 breadth, 7.1 (5.4–8.0, 15); d3 length, 13.9 (12.7–15.0, 15); d4 breadth, 8.0 (7.0–9.0, 21); d4 length, 19.6 (19.0–21.0, 17— Fourvel et al. 2015).
Mean dental measurements (mm; parenthetical n) of late Pleistocene C. crocuta from Kent’s Cavern were: P2 length, 14.38 (97); P3 length, 21.11 (97); P4 length, 34.97 (97); c length, 14.47 (7); p2 length, 14.49 (96); p3 length, 19.86 (98); p4 length, 21.66 (91); m1 length, 26.67 (95); d2 length, 9.19 (10); d3 length, 13.74 (11); d4 length, 19.91 (34); prosthion to basion length, 249.6 (5—Kurtén 1956).
Mean cranial and dental measurements (mm; parenthetical range if available, n) of C. crocuta fossils from Sterkfontein Valley, South Africa, were: breadth across zygomatic arches, 156.0 (NA, 1); C breadth, 10.2 (NA, 1); C length, 15.9 (14.3–17.5, 2); M1 breadth, 7.4 (NA, 1); P1 breadth, 7.2 (6.0–8.3, 2); P2 breadth, 12.5 (11.5–13.9, 5); P2 length, 17.1 (16.0–18.4, 5); P3 breadth, 17.1 (15.0–19.5, 6); P3 length, 23.3 (21.1–25.7, 6); P4 length, 37.9 (35.0–41.4, 4); breadth of blade of P4, 12.4 (11.2–13.0, 3); length of metastyles of P4, 16.2 (14.6–19.2, 3); length of paracone of P4, 13.4 (12.6–14.0, 4); greatest anterior breadth across protocone of P4, 22.0 (19.1–25.0, 5); c breadth, 12.9 (11.1–14.6, 2); c length, 15.4 (14.5–16.2, 2); m1 breadth, 12.0 (11.1–12.4, 5); m1 length, 28.3 (27.8–29.5, 4); m1 length of talonid, 3.7 (3.4–3.8, 4); p2 breadth, 10.7 (9.7–12.1, 7); p2 length, 15.9 (13.9–17.6, 6); p3 breadth, 15.4 (13.0–24.7, 8); p3 length, 20.1 (18.8–21.7, 8); p4 breadth, 13.1 (12.0–14.0, 7); p4 length, 22.4 (19.5–24.2, 7); p4 length of protoconid, 12.3 (11.5– 12.8, 6); minimum dorsoventral depths of mandible anterior to p2, 35.4 (31.5–38.0, 4); minimum dorsoventral depths of mandible posterior to m1, 40.0 (38.0–42.0, 2); buccal-lingual breadth of mandibular corpus below the center of p3, 18.3 (15.6–20.1, 7); distance from anterior face of p2 to posterior margin of m1, 84.1 (83.0–85.2, 2— Turner 1984).
DISTRIBUTION
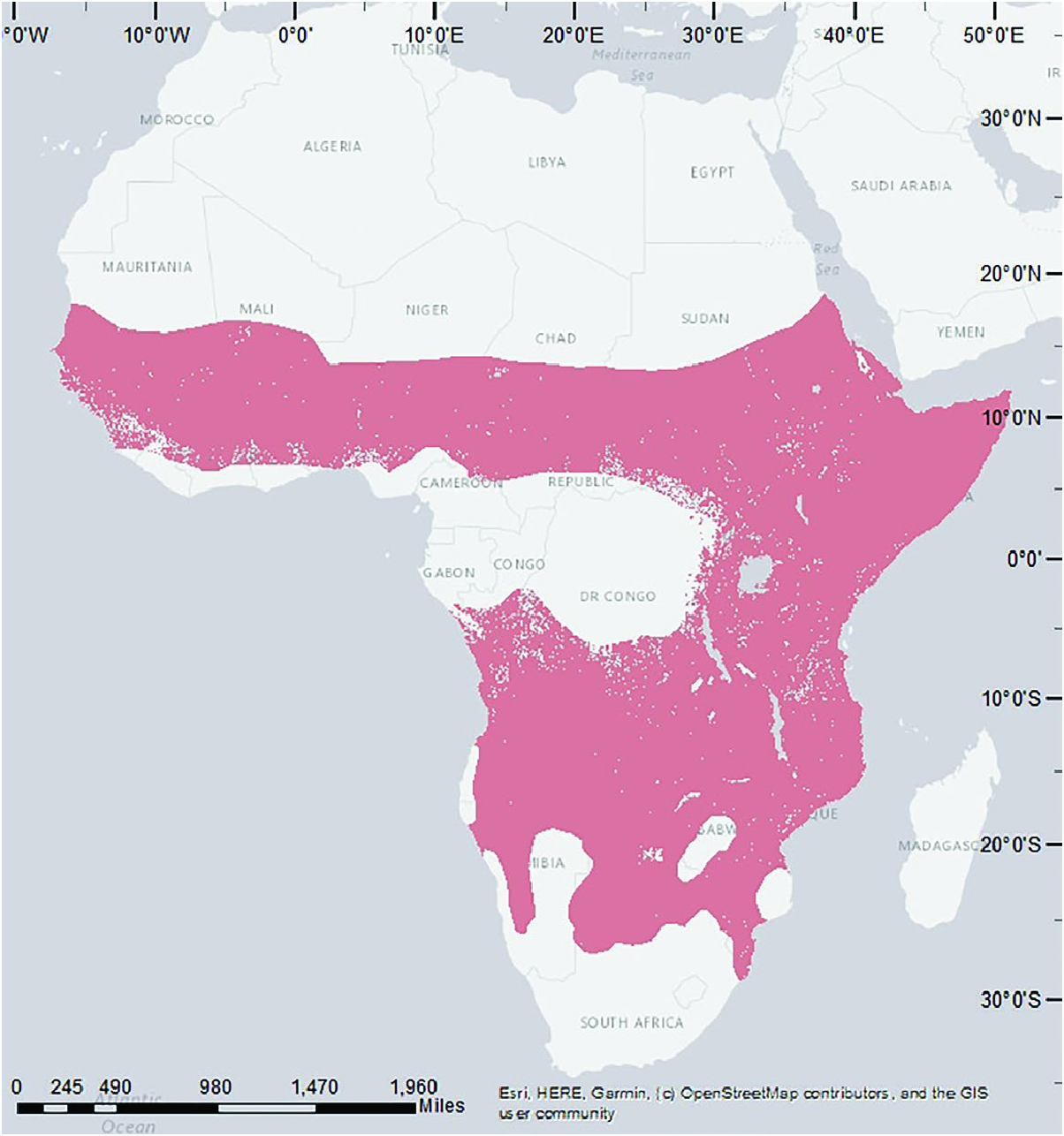
Geographically ( Fig. 4 View Fig ) Crocuta crocuta is widespread across sub-Saharan Africa except rain forests in the Congo basin and much of southern Africa, thus from about 17°N to 28°S ( Kruuk 1972; Holekamp and Dloniak 2010). It occurs from sea level to near the snow line of Kilimanjaro and up to 4,000 m ( Matthews 1939b; Kruuk 1972).
FOSSIL RECORD
The fossil record of Crocuta is extensive especially in Eurasia and Africa, but the interpretation of that record is in flux. The extant African Crocuta crocuta is closely related to extinct Eurasian, late Pliocene, and Pleistocene cave hyenas ( Rohland et al. 2005; Sheng et al. 2014). The origin and evolutionary diversity of Crocuta is complex and not resolved with evidence of bidirectional gene flow between Eurasian and African Crocuta ( Sheng et al. 2014; Westbury et al. 2020).
FORM AND FUNCTION
Form.— The adult dental formula is i 3/3, c 1/1, p 4/3, m 1/1, total 34 ( Fig. 2 View Fig ; Pournelle 1965). The neonatal dental formula is i 3/3, c 1/1, p 0/0, m 0/0 ( Pournelle 1965). Bonecrushing teeth include the third upper and lower premolars, whereas the carnassial shear (upper fourth premolar and lower first molar) can slice thick pieces of hide or tendon ( Kruuk 1972). Bite force increases up to 5 years of age; thus, subadults when weaned cannot crush the same bones as adults ( Binder and Van Valkenburgh 2000).
Crocuta crocuta has a thick hide ( Kruuk 1972). Descriptions and inventory of the muscles of the head, neck, back, thorax, abdomen, forelimb, and hind limb are available ( Watson and Young 1879:plates V, VI). Muscles of the thorax, forelimb, and associated synovial sheets have been detailed ( Gomerčić 1985). Masses of> 50 individual limb muscles from an adult female are available ( Spoor and Badoux 1989).
The dissection of an adult male provides the best description of the major anatomical features of C. crocuta (Watson andYoung 1879). The dorsally papillated tongue is elongate, flattened, and thin with a central oval surface of blunted papillae and a marginal surface of recurved papillae. The tonsil is oval with oblique glandular ridges; the short, soft palate lacks a uvula. The thickly muscular esophagus can be dilated and has a dense, tough mucous coat with longitudinal rugae. An empty stomach measured 23 by 18 cm, length by breadth, with thick walls and internal rugae of various robustness and orientations ( Watson and Young 1879:82, figure 1). The small pyloric opening was 0.3 cm in diameter. The small intestine ( 82 cm in length) has constrictions at irregular intervals, and the internal mucous surface is covered in villi. Eight Peyer’s patches increase in size from the stomach to the lower ilium. The 15-cm cecum marks the entrance to the 67-cm-long, well-muscled large intestine. The two segments of the liver are each divided into lobes ( Watson and Young 1879:85, figure 3). A pyriform gallbladder is present, as is a long, narrow pancreas (3 by 3 cm), and an elongate, tongue-shaped spleen ( 41 cm long, 3–8 cm in width— Watson and Young 1879).
The epiglottis guards the cartilaginous larynx, which merges into the 28-cm-long trachea that has 49 cartilaginous rings of varying breadth. The two unconnected halves of the thyroid extend from the cricoid cartilage of the larynx to the sixth tracheal ring. The right lung has six lobes, whereas the left lung has only three ( Watson and Young 1879:figure 4). The heart has a clearly defined fossa ovalis. The kidneys are globular with no lobes ( Watson and Young 1879). The pyriform bladder is 7 cm long when empty ( Matthews 1939c).
Weight of the left adrenal of five adult females (mean body mass 51.6 kg) was 6.9 g (± 1.2 SE) and of the left gonad 2.7 g (± 0.3 SE), whereas five adult males (mean body mass 43.6 kg) had smaller left adrenals, 4.8 g (± 0.4 SE) and larger left gonads, 6.3 (± 1.2 SE — Neaves et al. 1980). Subcutaneous fat is primarily in the axilla and groin. Abdominal fat is prominent on the omentum and mesenteries, whereas retroperitoneal fat is primarily around the kidneys ( Matthews 1939b).
The brain lacks a central sulcus between motor and somatosensory cortex ( Sakai et al. 2011). The hemispheres of the brain of one adult were each 8 cm in length and together 7 cm in breadth ( Watson and Young 1879:90, figure 5). Mean endocranial volume of 32 C. crocuta was 160.06 ml (± 9.353 SD) with mean regional volumes as follows: anterior cerebrum, 39.18 ml (± 8.671 SD), posterior cerebrum, 90.88 ml (± 8.735 SD), and cerebellum plus brain stem, 26.47 ml (± 1.831 SD); mean basal length of the skulls was 217.91 mm (± 6.81 SD — Sakai et al. 2011). Endocranial volumes for 88 C. crocuta were 155–220 ml ( Mann et al. 2018). In six adult females the hSDN nucleus in the medial preoptic area and anterior hypothalamus is one-half the size of that in four adult males ( Fenstemaker et al. 1999). Extensive brain images and atlases of C. crocuta (catalog # 64-352) are part of the online Comparative Mammalian Brain Collection ( Welker et al. 2019).
Crocuta crocuta has a zonary, hemochorial placenta that has received histological, ultrastructural, and genomic study ( Matthews 1954; Morton 1957; Oduor-Okelo and Neaves 1982; Enders et al. 2006; Funk et al. 2019). Females have paired ovaries, oviducts, and uterine horns ( Watson 1877). Ovarian capsules, whose walls are “heavily loaded with fat,” surround each of the ovoid ovaries and have a 2- to 3-cm slit that opens to the fimbria of the oviducts ( Matthews 1939c:22). Two fat pads encase the ovaries and the oviducts on each side ( Cunha et al. 2003:206, figure 5). The coiled oviduct has a complexly folded, vascularized mucosa with both ciliated and nonciliated epithelial cells as well as a musculature ( Cunha et al. 2003). Histological evidence indicates ovulation can occur from alternate ovaries and that follicular development may occur during lactation ( Matthews 1939c; van Jaarsveld et al. 1992a). Females with 1–2 embryos had 2–4 recent corpora lutea, and up to 13 corpora lutea may be present in the ovaries of old females ( Matthews 1939c).
Uterine horns are 7–8 cm in length in nonpregnant females with layers of longitudinal and circular muscle with associated vasculature and connective tissue around the endometrium ( Cunha et al. 2003). The lumen of the uterine horns is coiled, and uterine glands are embedded in the endometrium ( Cunha et al. 2003). The muscular portions of the uterine horns fuse to form the body of the uterus that connects to the vagina and urogenital sinus ( Cunha et al. 2003). The indistinct cervix is a histological transition between vagina and uterine horns, and paired bulbourethral glands are situated at the junction of the vagina and the urogenital sinus ( Cunha et al. 2003). A number of internal longitudinal folds line the 6- to 13-cm-long vagina as well as the bicornuate uterus ( Matthews 1939c).
The urogenital canal runs from the tip of the elongate clitoris (~ 17 cm) to the vagina after passing the junction with the urethra ( Matthews 1939c). The urethra runs along the ventral aspect of the reproductive tract ( Cunha et al. 2003). A large fold of tissue may prevent urine from entering the vagina ( Matthews 1939c).
In prepubertal females and adult males the base of the clitoris (2.5–3.0 cm in diameter) or penis ( 2.5–3.5 cm in diameter) is 20–28 cm anterior to the root of the tail and 15–20 cm anterior to the center of the anus. Both the clitoris and the penis are pendulous with an anterior and downward direction and contain erectile tissue ( Matthews 1939c; Cunha et al. 2003). The proximal portion of the elongate, ovoid, dark gray-black glans has backward-directed small protuberances but is smooth distally. The meatus is a longitudinal, slit opening on the dorsal tip. Covering most of the glans is a much wrinkled black prepuce. Posterior to the glans and anterior to the anus are two shallow pouches, each divided into hairy anterior and smooth posterior parts that appear as low eminences on the perineum. In females the pouches contain fibrous and adipose tissue and are formed from fused labia ( Matthews 1939c; Cunha et al. 2003). In males, the testes may occupy the scrotal sac or lie under the perineal skin. Minor sex differences exist in the abdominal hair pattern around the external genitalia. Both prepubertal females and adult males have a pair of 3–5 mm in diameter nipples on the abdomen centered in 1.3-cm-diameter areolae ( Matthews 1939c).
The prepuce of sexually mature, nulliparous females is enlarged and becomes slack and baggy after the first birth; its opening is also increased. The glans, itself, also hypertrophies as does the tip meatus. The distal shape of the glans becomes more truncated and rounded as the enlarged meatus ( 1.5 cm) becomes more ventral ( Matthews 1939c). Glans measurements (mean and parenthetical SE, in g or mm) for five adult females, five adult males, and three juvenile females, respectively, were: mass, 49 (2), 56 (4), 59 (5); meatus width, 58 (6), 5(1), 16 (7); total length, 171 (2), 193 (4), 163 (8); glans diameter, 17 (1), 17 (1), 18 (2); shaft diameter, 22 (2), 21 (1), 24 (3— Neaves et al. 1980). Photographs, drawings, and detailed text of the internal anatomy and external genitalia of adults are available ( Matthews 1939c; Davis and Story 1949; Cunha et al. 2003; Cunha et al. 2014).
For parous females the gray-black prepuce (diameter at base 3.75–5.25 cm) hangs in slack, baggy folds that reach maximum size near parturition and through lactation and then tighten up. Parturition often results in lacerations of the prepuce opening ( Fig. 5 View Fig ). Nipples in parous females are black, about 2.5 cm long by 1.0– 1.8 cm in diameter, and centered on conical areolae that are 5.0– 5.5 cm in diameter ( Matthews 1939c). One of 116 females had four nipples; the remainder had two ( Kruuk 1972). If supernumerary nipples are present, they enlarge during lactation. At peak lactation, each mammary gland is 26 cm long, 10 cm wide, and 5 cm thick. Mammary tissues and associated structures regress after lactation but seldom completely ( Matthews 1939c).
The neuroanatomy of the clitoris and penis is similar with a single opening at the tip. The glans is wide and blunt in females and tapered in males. In both sexes, the dorsal nerves track along both sides of the corporeal body at the 5 and 7 o’clock positions. In females the dorsal nerves fan out laterally on the clitoral body ( Baskin et al. 2006). Females have fewer spinal motoneurons innervating the bulbocavernosus and ischiocavernosus perineal muscles ( Forger et al. 1996).
Males have pyriform Cowper’s glands and a highly variable prostate but no seminal vesicles. Testes of adults are 4 cm long and 2 cm wide ( Matthews 1939c). Mean testes volume (mm 2) varies; 1,685.58 (± 55.27 SE) for 16 immigrant males and 1,200.3 (± 127.89 SE) for four adult natal males, but the volume for 30 immigrant males (940, ± 57 SE) did not differ from that for 12 adult natal males (1,030, ± 86 SE — Holekamp and Smale 1998; Curren et al. 2013). Ejaculate and sperm characteristics (mean and parenthetical SE) of 16 immigrant and four natal males, respectively, are concentration (sperm/ml × 106), 18.52 (5.96), 2.23 (1.24); percent motile, 56.47 (7.78), 25.75 (15.10); sperm length (μm), 45.97 (0.33), 40.25 (2.63); sperm midpiece length (μm), 5.81 (0.09), 5.50 (0.20); sperm head length (μm), 3.75 (0.10), 3.50 (0.29); ejaculate volume (ml), 6.40 (0.76), 2.38 (0.92— Curren et al. 2013). No baculum is present ( Estes 1991).
Both sexes have extensive anal glands including a pair of large glands, one on either side of the rectum with numerous smaller median glands. The ducts open to the dorsal part of the anal pouch ( Matthews 1939b, 1939c). The pasty secretion is semisolid ( Matthews 1939b).
Function. —Vision during the day appears similar to humans but at night is far superior ( Kruuk 1972). Hearing is acute; Crocuta crocuta can hear conspecific vocalizations at a carcass up to 10 km distant (mean 4.2 km — Mills 1990). They can also use contact calls to distinguish individuals (BensonAmram et al. 2011).
Stomachs rarely contain soft tissues but primarily hold bones, hair, and feathers ( Matthews 1939b). C. crocuta can completely digest bones, hair, and teeth, but may also regurgitate slimy masses of hair and bone slivers ( Kruuk 1972). Regurgitation of these remains attracts other individuals, who, along with the regurgitator will roll in the expelled material ( Kruuk 1972). Feces are primarily minerals from bones with some hair or feathers and are often deposited in latrine areas ( Matthews 1939b). Fresh scat is green and turns white as it dries ( Matthews 1939b).
Mass-specific total body water was 66.3% (± 4.3 SD) for six free-living C. crocuta . Water flux was 64.4 ( ± 30.0 SD) ml/ kg-day in and 63.9 ( ± 29.1 SD) ml/kg-day out ( Green et al. 1984). Mean serum sodium was 139 (± 4.0 SD) mmol/l with an influx of 3.47 ( ± 1.66 SD) mmol/kg-day and an efflux of 3.43 (± 1.59 SD) mmol/kg-day ( Green et al. 1984).
Mean plasma cortisol levels were about 100 ng /ml ( van Jaarsveld and Skinner 1992) and did not differ between immigrant and natal males ( Holekamp and Smale 1998). Mean fecal glucocorticoid (fGC) levels for four clans were 103–185 ng /g ( Van Meter et al. 2009). Intense anthropogenic disturbance (not tourism) and social instability, but not ecological factors, elevate fGC levels ( Van Meter et al. 2009). Young juveniles (<6 months) have higher mean fGC levels ( 37 females, 35.4 ng /g; 50 males, 45.2 ng /g) than older (6–24 months) juveniles ( 28 females, 19.2 ng /g; 31 males 17.7 ng /g— Benhaiem et al. 2012). Lactating females ( n = 76, 112.6 ng /g, ± 35.5 SE) had higher levels of fGC than 34 nonlactating females (40.0 ng/g, ± 9.3 SE) with 20 pregnant females intermediate ( 59.5 ng /g, ± 17.3 SE — Goymann et al. 2001b).
ONTOGENY AND REPRODUCTION
Ontogeny. —The genital tubercle of Crocuta crocuta enlarges by day 31 of gestation, but the reproductive tract has not differentiated ( Cunha et al. 2005). Fetal ovaries begin to differentiate by day 45 of gestation, and testicular differentiation is apparent by day 30 ( Browne et al. 2006). At day 50 of gestation the Müllerian ducts are well-developed in females and absent in males, and the Wolffian ducts are beginning to degenerate in females ( Lindeque and Skinner 1982a). From at least 60 days, fetal gonads and adrenal glands synthesize androgens ( Lindeque and Skinner 1982a; Browne et al. 2006). Although steroid hormones have complicated roles in the development of the external genitalia, growth of both the clitoris and the penis is largely independent of androgens ( Drea et al. 1998; Cunha et al. 2014).
Gestational age can be estimated by femur length using transabdominal ultrasound ( Place et al. 2002). C. crocuta can be roughly aged by the closure of the frontoparietal and basilar sutures in the skull and the amount of tooth wear ( Matthews 1939c; Lindeque and Skinner 1984; van Jaarsveld et al. 1987). Ages of subadults may be estimated by tooth-eruption models ( Van Horn et al. 2003).
Cubs are born in isolated underground dens where they spend their first weeks of life before being carried to a communal den ( White 2007; Holekamp and Dloniak 2010). At birth, the dark brown neonates can walk, react to sound and movements, and have open eyes and erect ears, as well as erupted incisor ( 2–4 mm long) and canine ( 6–7 mm long) teeth ( Matthews 1939c; Pournelle 1965; Golding 1969; Frank et al. 1991). Cheek teeth begin to erupt at 31 days, and adult dentition is present by 15–18 months ( Pournelle 1965; Kruuk 1972; Mills 1990; Van Horn et al. 2003). Cubs can move rapidly at 10 days ( Kruuk 1972). At 6–11 weeks molting to the adult pelage starts at the head and forequarters and is nearly complete by 9–18 weeks except for a middorsal stripe ( Pournelle 1965; Golding 1969), although the legs may remain dark for a year ( Kruuk 1972). Average mass of six neonates was 1.49 kg ( Pournelle 1965; Golding 1969). A 1.6 kg captive neonate was 3.2 kg at 25 days, 4.8 kg at 37 days, and 14.5 kg at 100 days ( Pournelle 1965), but two other 1.6 kg captive neonates grew at a slower rate, being only 8.6–10.9 kg at 3 months ( Golding 1969). Fetal growth curves are available from 30 days to near term and postnatal curves from birth to 25 years ( van Jaarsveld et al. 1988).
An ethogram of behavior during the first month of life includes aggressive, dominance and submissive, play, and exploration behaviors ( Drea et al. 1996). Littermates establish relative dominance rank as early as the first day of life ( Smale et al. 1995). During the first week mother–cub interactions predominate ( Drea et al. 1996), and vocalizations are apparent ( Kruuk 1972). The “giggle” (rarely called “chuckle”) vocalization is “sharper and higher pitched” than that of adults ( Pournelle 1965:503). Social and interactive play between cubs begins the second week of life, with locomotor and object play emerging in weeks 3 and 4 ( Drea et al. 1996). Adult behavioral displays, such as leg lifting with genital erections, begin at 1 month ( Kruuk 1972).
First solid food is at 3 months, but only rarely do juveniles under 6 months eat from a kill ( Kruuk 1972). Juveniles begin following their mothers at a few months of age, gain independence from the communal den at 8.9 months ( n = 22 juveniles from 11 litters), and begin to hunt at 8–10 months of age ( Kruuk 1972). Typically weaning is 12–18 months but can last up to 2 years and, rarely, may be as short as 7 months ( Hofer and East 1995; Holekamp et al. 1996). In Kenya, females are larger than males for many traits because females grow faster after weaning ( Swanson et al. 2013). Development of the massive skulls needed for bone cracking is not complete until 35 months, well after weaning and sexual maturity ( Watts et al. 2009; Tanner et al. 2010). For the first 2 years of life, females and males do not differ in their use of space ( Boydston et al. 2005).
Females are sexually mature at 2–3 years, and the first estrus may result in pregnancy ( Matthews 1939c; Kruuk 1972). Age at first birth is 30–45 months ( Mills 1990), but varies with dominance rank, 2–3 years for high-ranking females versus 5–6 years for low-ranking females ( Holekamp et al. 2012). Males are sexually active at 2 years ( Kruuk 1972). Females rarely disperse, but in their third year (30–45 months) nearly all males leave their natal clan and move into another clan (~ 8–10 km distant) where they initially assume a low rank as immigrants ( Mills 1990; Van Horn et al. 2003; Boydston et al. 2005). Consequently, for females rank has stronger effects on social networks than does rank for males ( Turner et al. 2018). Rarely, males may be nomadic for short periods ( Mills 1990).
Reproduction. —The female urogenital sinus traverses the clitoris and functions in urination, copulation, and birth ( Cunha et al. 2003). Crocuta crocuta anatomy gives females complete control over mate choice ( Holekamp and Dloniak 2010). Copulation lasts 8–12 min ( Matthews 1939c) and requires the coordinated efforts of females and males to align the flaccid forward-projecting clitoris and the erect penis ( Cunha et al. 2003). A “male must squat behind the female and hop around while thrusting blindly upward and backward” until intromission occurs ( Holekamp and Dloniak 2010:196). Swelling of both clitoris and penis may result in a partial lock during coitus ( Cunha et al. 2003). Reproductive females mate with multiple males during estrus, and males can mate with multiple females ( Engh et al. 2002; East et al. 2003; Holekamp and Dloniak 2010). At least 20–35% of cubs from twin litters have different sires ( Engh et al. 2002; East et al. 2003). In 24 observed copulations, females copulated only with immigrant males ( Holekamp and Smale 1998); in addition, paternity tests indicated that only one of 71 cubs conceived over 13 years was sired by a natal male ( Engh et al. 2002; Holekamp et al. 2012).
Ovarian hormones facilitate the “development of a large clitoral canal and a highly elastic urogenital meatus” ( Cunha et al. 2003:207). For one nulliparous female, 3–4, 10-s contractions occurred in bouts about every 78 s, 1–5 h before birth, whereas in the hour before birth contractions occurred singly about every 44 s ( Frank and Glickman 1994).
For birth to occur the fetus “moves along an exceptionally tortuous route, first following a caudal-ventral path from the uterus through the bony pelvic outlet, and then making a sharp turn in an anterior direction to traverse the clitoral canal to emerge through the meatus of the glans clitoris” ( Cunha et al. 2003:207). The meatus tears during delivery of primiparous mothers; thus, subsequent deliveries are easier. However, firstterm births have higher neonatal mortality (~60% in one captive colony), especially if the placenta detaches early in labor and the cub becomes anoxic ( Frank and Glickman 1994; Cunha et al. 2003). Also, both mother and offspring may die if a cub becomes wedged during labor ( Morton 1957). Births from a single litter may occur within an hour ( Henschel and Skinner 1990; Frank et al. 1991) or up to days apart ( Matthews 1939c). Placentophagia occurs ( Henschel and Skinner 1990; Frank and Glickman 1994).
Birth typically occurs at the entrance to the natal den, with only the posterior portion of the female plugged into the narrow opening (K. E. Holekamp, pers. comm. to VH). A photo essay of the birth of a cub at Kruger National Park is available ( Adams and Cox 2019).
Overall cub mortality is inversely related to rank as low-ranking mothers lose more cubs from birth to 1 month of age ( Holekamp et al. 1996; White 2002; Watts et al. 2009; Swanson et al. 2011). Causes of cub mortality are infanticide (including facultative siblicide), starvation, disease, weather, injury, humans, and predation ( Hofer and East 1997; Smale et al. 1999; White 2005; Wahaj et al. 2007).
Crocuta crocuta does not have seasonal births in equatorial regions ( Matthews 1939b), but births may be restricted to winter in southern latitudes ( Pournelle 1965; Lindeque and Skinner 1982b), although conceptions are positively correlated with food abundance ( Holekamp et al. 1999b). Gestation is 110 days. Stillbirths and in utero resorption of embryos are common in captivity and in the wild. Approximately 27–33% of litters have higher litter size in utero than at birth ( Wahaj et al. 2007). Mean litter size declines from pregnancy to den emergence: 78 in utero litters, mean 1.90; 70 litters at birth, mean 1.63; 1,117 litters at den emergence, mean 1.55 ( Zuckerman 1952; Reitz 1972; van Jaarsveld et al. 1988; Holekamp and Dloniak 2010). Singletons are common for first litters (~80% captive— Frank et al. 1991; ~50% wild— White 2005), and triplets are rare ( Smale et al. 1999; Wahaj et al. 2007). Litter composition is biased toward mixed-sex litters ( van Jaarsveld et al. 1988), in part due to sibling aggression in same-sex litters ( Frank et al. 1991; Golla et al. 1999). However, at 1–2 months, fighting rates did not differ between 26 mixed- and 20 same-sex litters ( Wahaj and Holekamp 2006), and “mothers endeavor to keep all offspring alive for as long as possible” ( White 2008:353). Males provide no parental care ( Holekamp and Dloniak 2010).
The minimum inter-litter interval is 9–10 months ( Matthews 1939a, 1939b) if offspring survive, but in the wild the average inter-litter interval is 14–19 months ( Mills 1990; Holekamp et al. 1996). Estrus may occur as early as 2 weeks after the loss of a litter ( Matthews 1939c), but in the wild litters are produced 3–5 months after the loss of cubs ( Mills 1990).
Sex steroids as well as reproductive protein hormones vary by sex, age, reproductive condition, and social status. Plasma estrogens and progesterone (nmol/l) are higher in females (estradiol: four females, mean 1.38, no SE, five males, mean 0.41 ± 0.04 SE; estrone: four females, mean 1.00, no SE, five males mean 0.11 ± 0.008 SE; progesterone: four females, mean 96.9, no SE, five males, mean 6.42 ± 3.21 SE — Racey and Skinner 1979). In females, progesterone levels vary from 4 to 92 ng / ml with some correlation to reproductive state in some females ( Racey and Skinner 1979; Gombe 1985; van Jaarsveld et al. 1992a). Estrogen patterns are even less clear ( van Jaarsveld et al. 1992a). Lactating females have 25–33% lower prolactin levels than other age, sex, or reproductive classes ( van Jaarsveld et al. 1992b). Mean plasma gonadotropins (ng/ml) are similar for females and males (luteinizing hormone: five females, 8.40 ± 5.12 SE, five males, 3.22 ± 0.29 SE; follicle-stimulating hormone: five females, 29.04 ± 1.36 SE, five males, 25.08 ± 1.36 SE — Racey and Skinner 1979).
Plasma androgen levels vary across studies ( Goymann et al. 2001a). Reported levels of androgens use different units that are not directly comparable (ng/100 ml plasma, ng/g gonadal or adrenal tissue— Racey and Skinner 1979; nmol/l plasma— Lindeque et al. 1986; ng/ml plasma— Frank et al. 1985; ng/g feces— Dloniak et al. 2004). Androgen levels vary with age ( Glickman et al. 1992) and, in adults, variation occurs both with reproductive state in females (highest during pregnancy) and with residency in males (highest in immigrant males— Dloniak et al. 2004). Androgen levels are also positively correlated with social rank in multiparous females ( Jones 2019). In males, androgen levels vary with dispersal status and may increase with age or tenure within a clan ( van Jaarsveld and Skinner 1991; Holekamp and Smale 1998; Goymann et al. 2001a). In adrenal glands, amounts of testosterone and androstenedione are similar in females and males ( Racey and Skinner 1979). Gonadectomy reduces plasma androgen levels to near zero in both sexes ( Frank et al. 1985). Androgen binding in plasma is greater for females than males ( van Jaarsveld et al. 1992c). Fecal androgen levels follow the same patterns (sex and reproductive differences) as plasma levels ( Dloniak et al. 2004).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Crocuta crocuta ( Erxleben, 1777 )
| Hayssen, Virginia & Noonan, Paula 2021 |
Crocuta croucta
| WILSON, D. E. & D. M. REEDER 2005: 1795 |
Crocotta kibotonensis
| MATTHEWS, L. H. 1939: 256 |
Crocuta nyassae
| MATTHEWS, L. H. 1939: 256 |
Crocuta crocuta fortis
| ALLEN, J. A. 1924: 214 |
Crocuta crocuta fisi
| HELLER, E. 1914: 5 |
Crocuta crocuta :
| CABRERA, A. 1911: 95 |
Crocuta wissmanni :
| CABRERA, A. 1911: 95 |
Crocuta capensis :
| CABRERA, A. 1911: 96 |
Crocuta leontiewi :
| CABRERA, A. 1911: 97 |
Crocuta rufopicta
| CABRERA, A. 1911: 97 |
Crocuta thomasi
| CABRERA, A. 1911: 98 |
Crocuta nyasae
| CABRERA, A. 1911: 99 |
Crocuta nzoyae
| CABRERA, A. 1911: 199 |
( Crocuta ) leontiewi
| SATUNIN, K. A. 1905: 556 |
Crocotta weissmanni :
| TROUESSART, E. - L. 1904: 243 |
( Crocotta ) wissmanni
| MATSCHIE, P. 1900: 22 |
( Crocotta ) germinans
| MATSCHIE, P. 1900: 26 |
Hyana capensis
| DESMAREST, A. G. 1820: 216 |
capensis
| DESMAREST, A. G. 1817: 499 |
Canis crocuta
| CABRERA, A. 1911: 95 |
| ERXLEBEN, J. C. P. 1777: 578 |