Rossella antarctica Carter, 1872
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3692.1.6 |
|
publication LSID |
lsid:zoobank.org:pub:E86E41ED-D12B-4E3D-9FA3-25C8B2923183 |
|
DOI |
https://doi.org/10.5281/zenodo.5631272 |
|
persistent identifier |
https://treatment.plazi.org/id/03C387F0-AB15-FFD4-FF6A-FB130CB1FEC0 |
|
treatment provided by |
Plazi |
|
scientific name |
Rossella antarctica Carter, 1872 |
| status |
|
Rossella antarctica Carter, 1872 View in CoL
( Figs. 2 View FIGURE 2 A–B, 3, Tab. 2)
Synonymy:
Rossella antarctica Carter, 1872: 414 –417, pl. 21, figs. 1–10;1875: 117–118, pl. 10, fig. 4. Schulze 1887: 139–142, pl. 55, figs. 1–15. Schulze & Kirkpatrick 1910b: 15–17, pl. 1, figs. 2– 2v. Burton 1929: 405–407, fig. 1a. Koltun 1969: map 2; 1976: 165, pl. 1, fig. 1. Barthel & Tendal 1994: 90–93, fig. 32–34. Tabachnick 2002: 1444–1447, figs. 1–3.
Rossella antarctica solida Kirkpatrick, 1907: 5 –11, pl. 1, figs. 1–4, pl. 4, figs. 2–3.
Rossella antarctica gaussi Schulze & Kirkpatrick, 1910a: 296 ; 1910b: pl. 2, figs. 1– 1f.
Rossella antarctica intermedia Burton, 1932: 254 –255, fig. 3b.
Acanthascus grossularia Schulze, 1886: 49 ; 1887: 145–147, pl.56, figs. 1–12; 1897: 536–537.
? Acanthascus dubius Schulze, 1886: 49 ; 1887: 147–148, pl. 57, figs. 8–13.
? Rhabdocalyptus australis Topsent, 1901a: 6 ; 1901b: 37–38, pl. 2, figs. 5–6, pl. 4, figs. 14–24, pl. 5, fig. 1. Burton 1929: 407.
Material examined. 26 specimens from station 48-1 (SMF 11734, 11735, 11908–11915, 11916–11930). Other Material examined: BMNH 1887.10.20.49 from Kerguelen island, BMNH 1894.9.20.1 (lectotype) from Antarctica , BMNH 1910.10.28.5. ZMH S2925 ( Rossella antarctica gaussi , material from Schulze & Kirkpatrick 1910a, b).
Description. The habitus ( Fig. 2 View FIGURE 2 A–B) shows a well-developed velum of protruding pentactins, that usually covers the whole surface of the sponge. Single protruding diactins are also present. Big specimens sometimes show a fringe of diactins surrounding the osculum, but this is not regularly found. The body-form in general is barrel- or sack-shaped with a big inner cavity and usually one osculum at the top. A basis of long anchoring spicules at the bottom is usually present. We here chose two typical specimens from our material for detailed inspection. One is relatively small (SMF 11913), 7 cm high and 4 to 5 cm wide, has a very distinct velum of protruding pentactins, breached by long protruding diactins, regularly distributed over the whole surface, and shows a distinct spicular fringe around the osculum. Rooting spicules at the basis are present. The other specimen (SMF 11922) is one of the biggest specimens we obtained of this species, it is 12 cm high and 8 cm wide. It too shows a distinct velum of pentactins and many protruding diactins, but has no distinct fringe around the osculum. This specimen has a very thick body wall of up to 3 cm. It also has rooting spicules at the base. Both specimens show close associations with other invertebrates, especially bryozoans, which are very numerous especially within the spicular roots of the sponges, but also inside the velum, which serves as a kind of anchoring substrate for many small animals.
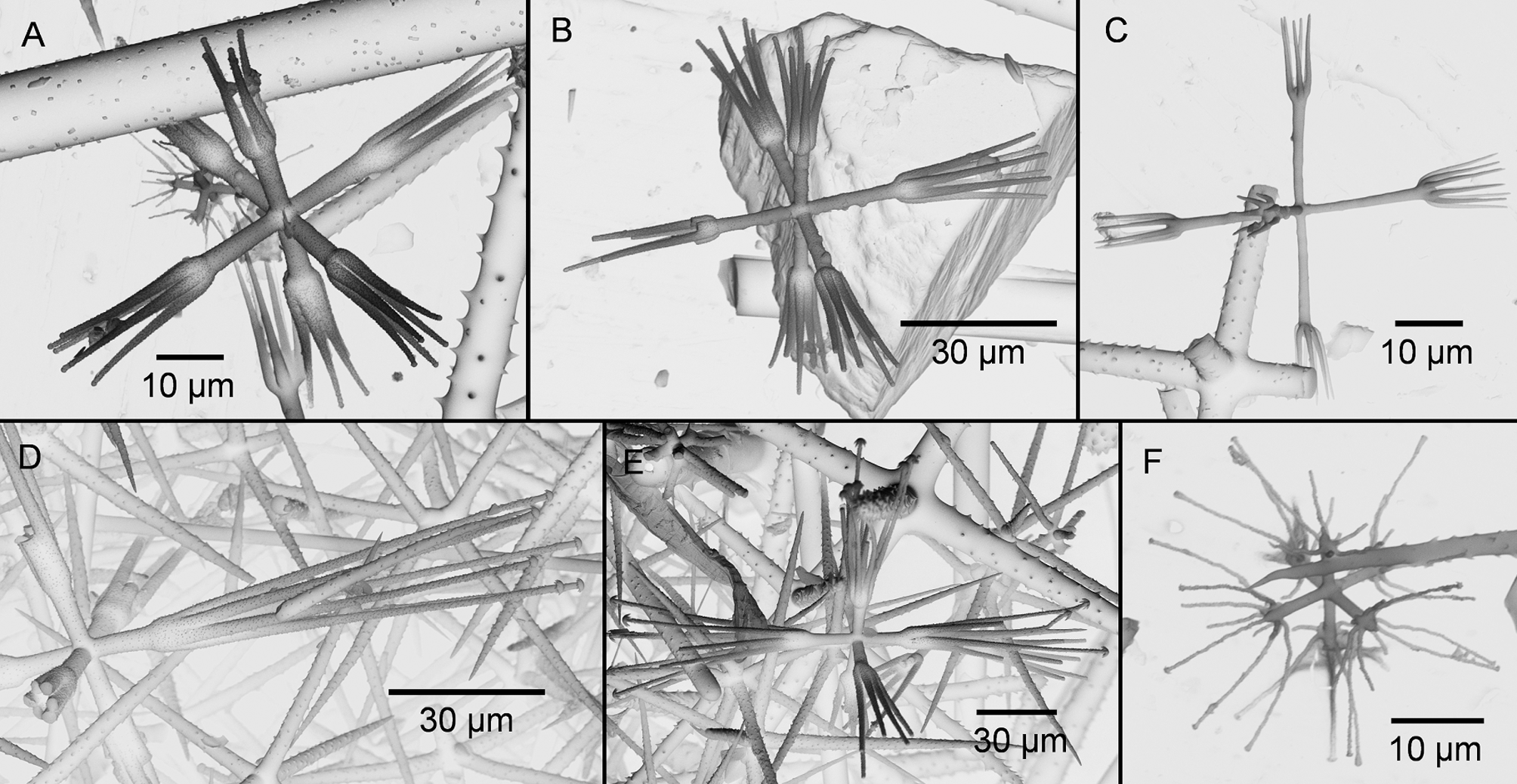
The species is best defined by two very characteristic microscleres: calycocomes and microdiscohexasters ( Fig. 3 View FIGURE 3 ). The calycocomes are usually rather small, 90 µm in diameter. They have relatively long primary rays of 17 µm length, which are commonly similar in length to the secondary rays of 26 µm. The middle pieces of the calycocomes are rather short, the 3 to 8 secondary rays are mostly straight or slightly curved. Two variations in shape of the common “ antarctica -calycocome” ( Fig. 3 View FIGURE 3 A) were present rather regularly: specimens with several knobs on the lateral surfaces of the primary rays ( Fig. 3 View FIGURE 3 B), and another ( Fig. 3 View FIGURE 3 C) which lacks the discoidal endings but instead has pointed secondary rays. These latter calycocomes seem to be growth stages of the common types. The microdiscohexasters ( Fig. 3 View FIGURE 3 F) are characterised by the lack of a capitulum at the end of their primary rays, instead, the secondary rays originate directly from the endings of the primary rays. The secondary rays are all of the same length and lead to a very characteristic, star-like appearance of the spicule, which is 35 µm in diameter. Characteristic are also the large dermal pentactins, which are rough and often have distinct lateral hooks (thorns), bowed towards the pointed end of the rays. Other microscleres are small oxyhexactins 160 µm, oxyhexasters 145 µm, rather rare mesodiscohexasters 52 µm.
We could also document one remarkable peculiarity: in some large specimens, we found a second kind of calycocome ( Fig. 3 View FIGURE 3 D–E), significantly larger than the usual ones of the species. These were 240 µm in diameter with primary rays of a similar length as those of the common small calycocomes. It seems very likely, that these represent normal calycocomes, which due to ecological or individual genetic factors have grown this large. We found these large calycocomes always in large specimens, so that it seems possible, that they are characteristic of adult, mature sponges. The specific, very characteristic small calycocomes of R. antarctica were found in all specimens possessing large calycocomes as well, and always in a high density. The large calycocomes are too numerous and appear too regularly, to be just contaminations, also the above mentioned similarities with the usual antarctica -calycocomes point to their identity as spicules really originating from R. antarctica specimens.
Remarks. The habitus changes, as in most Rossella spp., with age. In juvenile specimens, many characters are not very distinguished yet, as they develop during growth. Therefore, no general statement can be given, that Rossella antarctica is generally characterized by the velum and the oscular fringe, as stated e.g. by Kunzmann (1996). For an exact identification, one always has to study the sponges’ spicules.
As in many other species (see below) we found some extraordinary size ranges in several spicule types of the sponges from the SYSTCO I-expedition. A number of microscleres reached sizes much larger than previously reported (e.g., Schulze & Kirkpatrick 1910; Barthel & Tendal 1994). In R. antarctica , this is especially the case for the oxyhexactins, oxyhexasters and microdiscohexasters. Calycocomes on the other hand were within previously documented size ranges, with the exception of the additional large calycocomes found in most big specimens. These large calycocomes might on first sight be considered a reason for the erection of a new species, but the fact that they occur only in some large specimens of this highly polymorphic species makes it seem unjustified to describe a species just on the basis of this character. Schulze (1887a, b) reports similar large calycocomes in Acanthascus grossularia (considered here a synonym of R. antarctica ) but does not give measurements. The large growth of many spicule types is probably caused by environmental factors, most likely a high silicon concentration within the local water, but might also reflect regional variations in the sponges population genetics. This has to be further examined in the future.
Many taxa have been synonymized with R. antarctica (see above). One example present in our material is the subspecies Rossella antarctica gaussi designated by Schulze and Kirkpatrick (1910), which is characterized by its irregular, somewhat “deformed”, calycocomes bearing lateral knobs at their primary rays. We found such calycocomes in most of our specimens ( Fig. 2 View FIGURE 2 B), but usually in very low quantities. Consequently we propose that this is within the range for the species and therefore this variant is a valid synonym of R. antarctica .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Rossellinae |
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Rossellinae |
|
Genus |