Siren sphagnicola, Fedler & Enge & Moler, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5258.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:73F90E50-168F-4441-8B68-8DDFFF8E17D4 |
|
DOI |
https://doi.org/10.5281/zenodo.7786180 |
|
persistent identifier |
https://treatment.plazi.org/id/03BB87B1-FF80-0F37-43D5-FB19FD2DFE5E |
|
treatment provided by |
Plazi |
|
scientific name |
Siren sphagnicola |
| status |
sp. nov. |
Siren sphagnicola sp. nov.
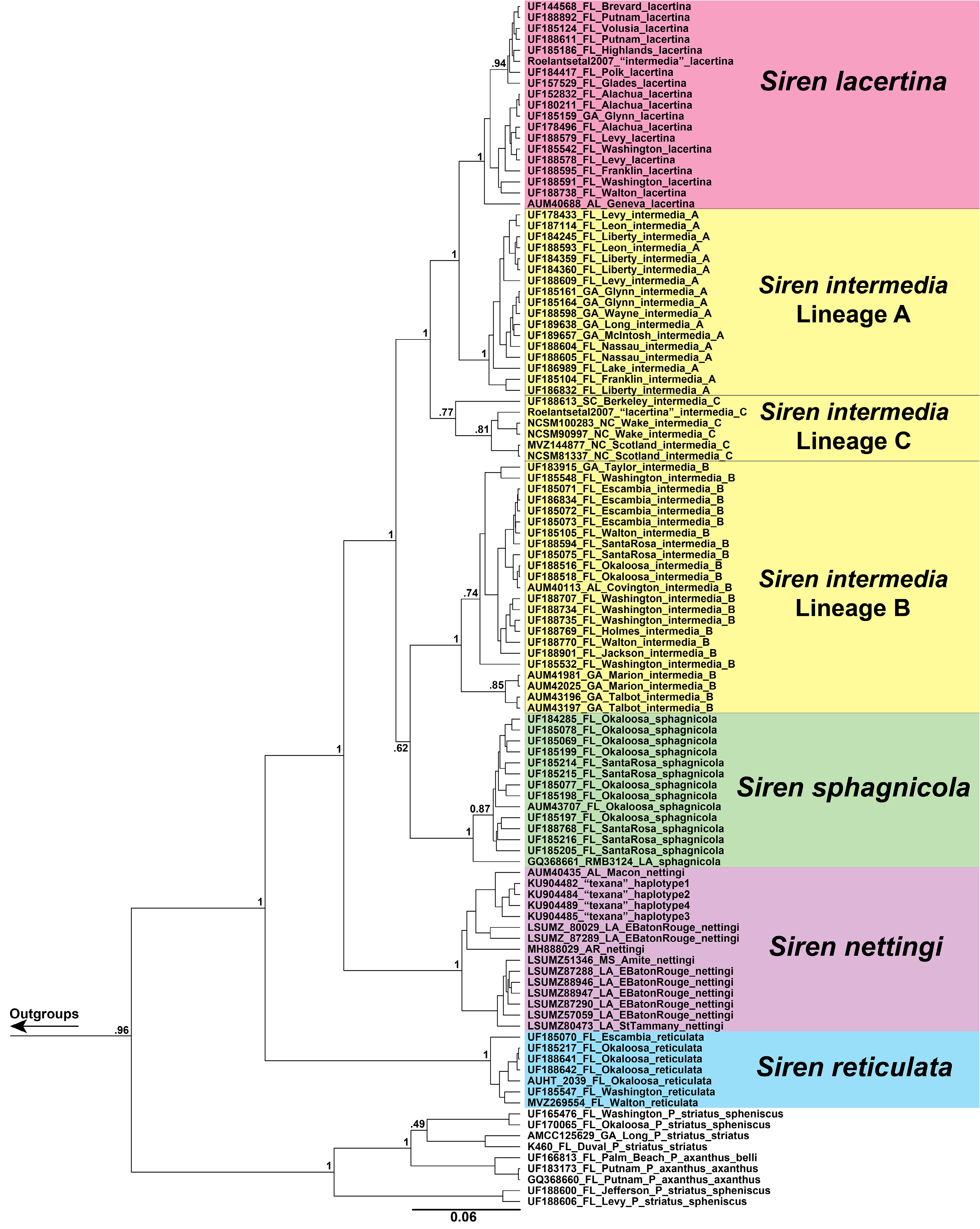
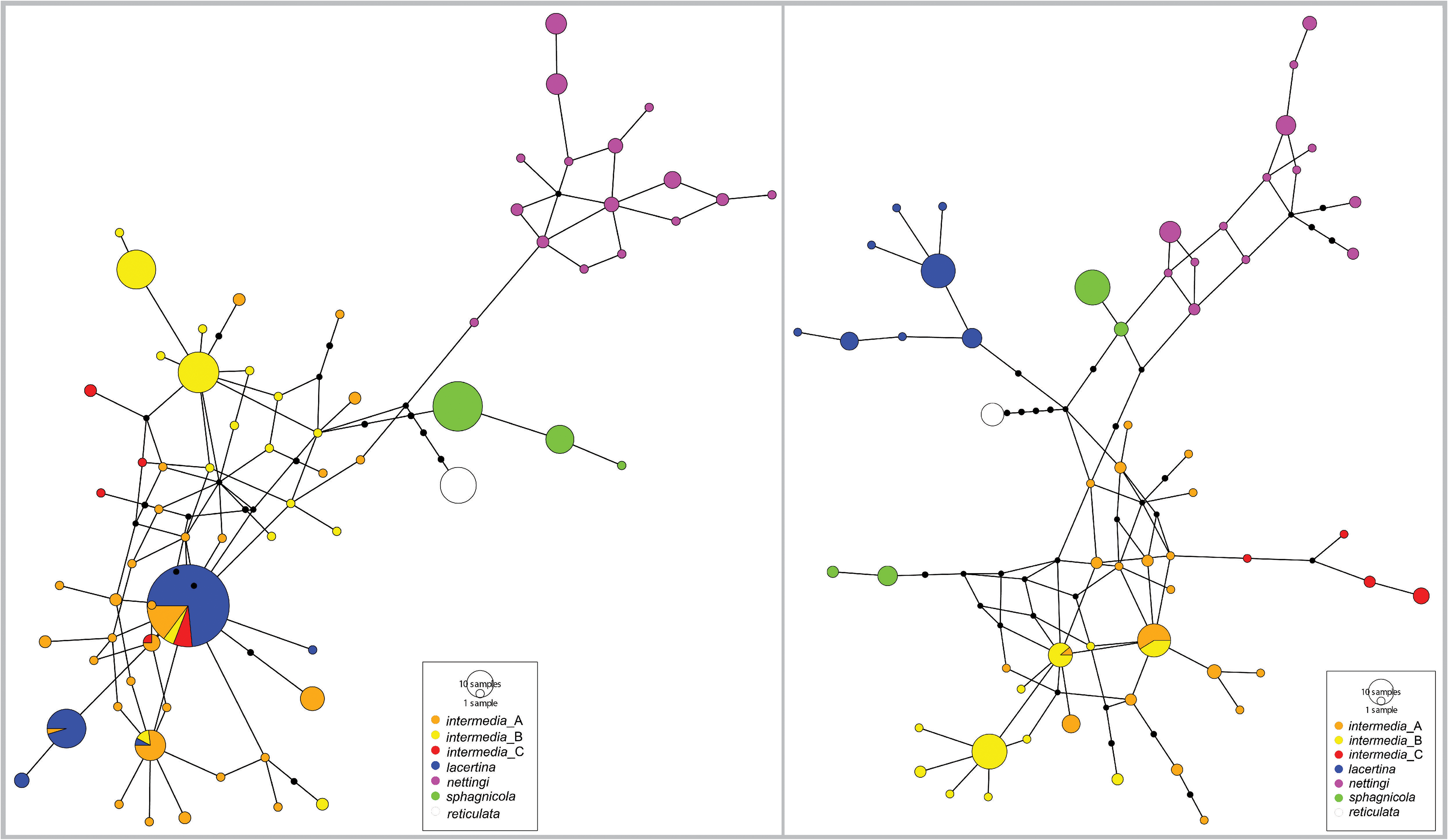
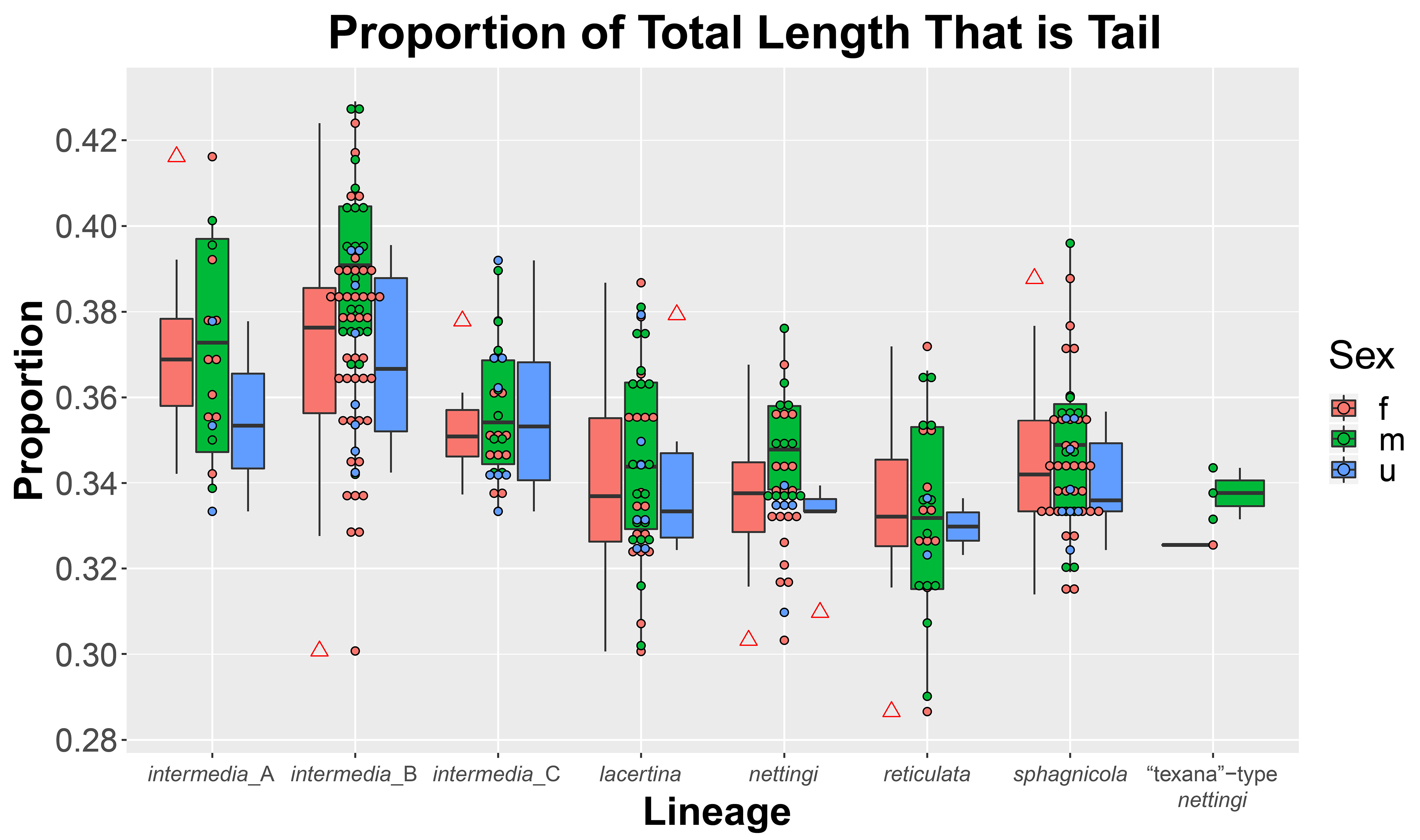
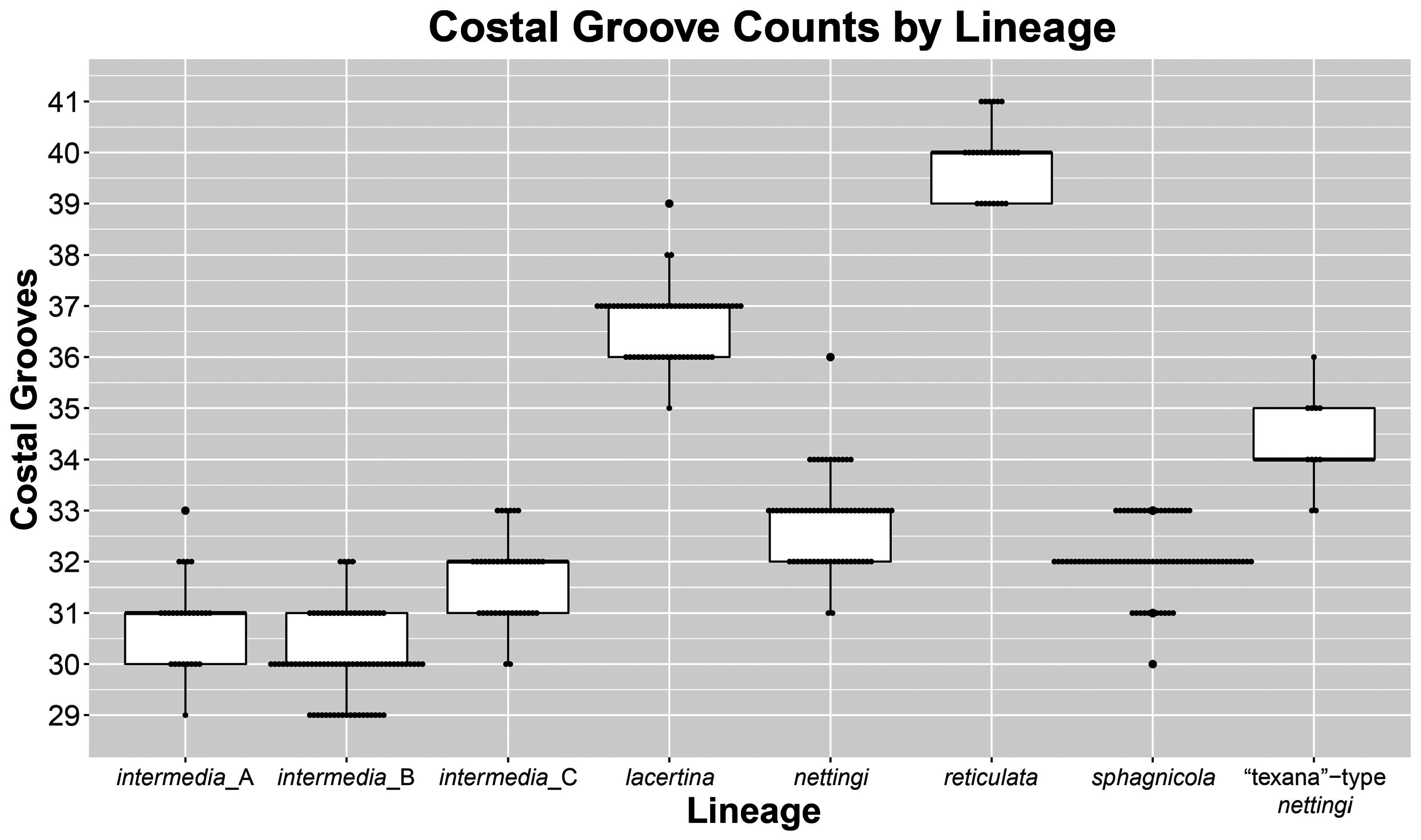
( Figs. 1‒6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 11–14 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 )
Common name. Seepage Siren
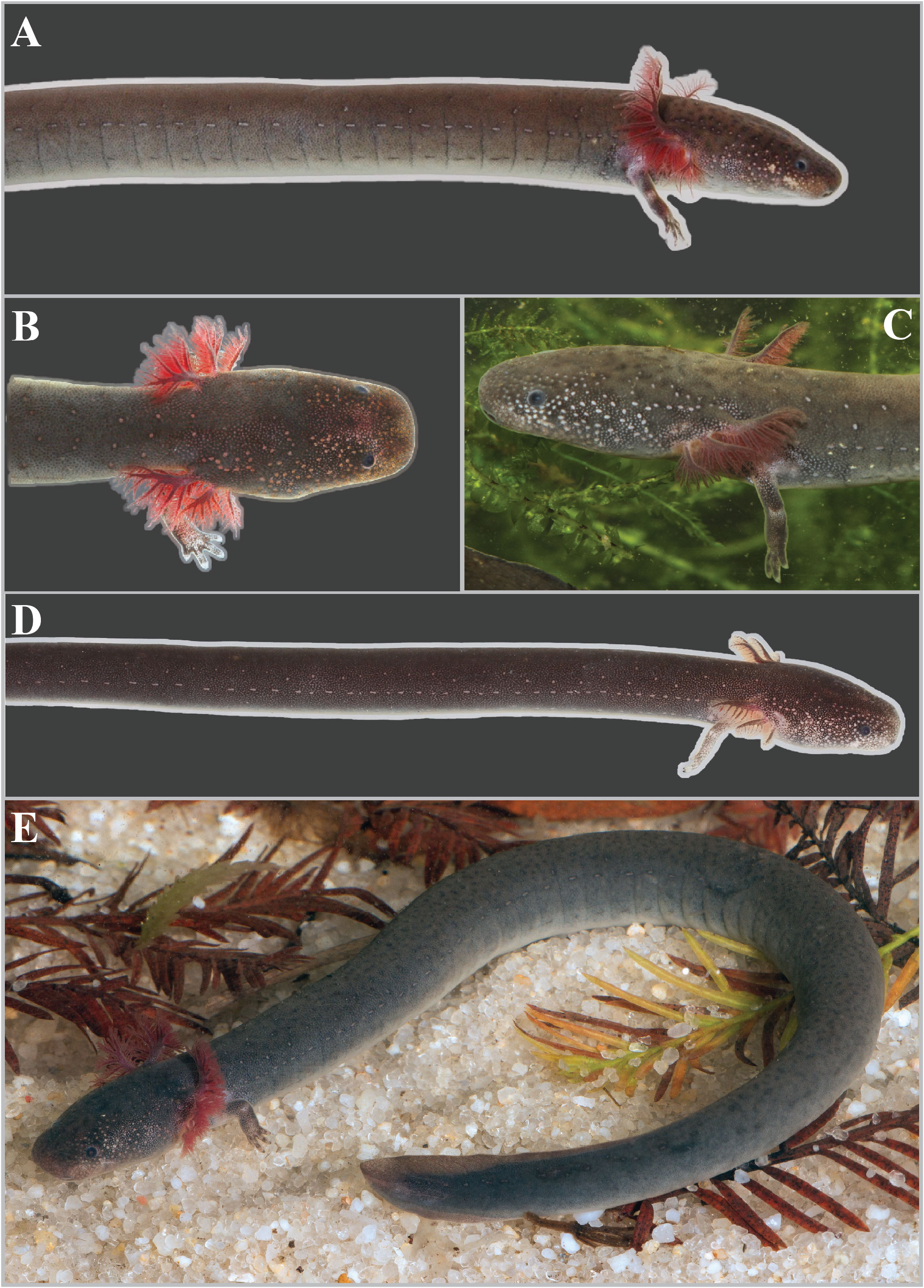
Holotype. UF Herp 185209 ( Fig. 11A View FIGURE 11 ), adult female from Junior Walton Pond in Okaloosa Co., Florida, USA ( 30.69270°N, 86.47250°W, datum WGS84, elev. 30 m) ( Fig. 12 A & 12B View FIGURE 12 ). Collected on 18 January 2019 by Matthew Fedler, Paul Moler, and Pierson Hill. GoogleMaps
Paratypes. UF Herp 161516, 162498, 162568, 163271, 164240, 164241, 164242, 164243, 184285, 185195, 185200, 185201, 185208, 185209, 185214, 185215, 188766, 188767, 190036, 190037, 185205, 185216, 185197, 185198, 185210. Locality information for the various paratype localities is available via FLMNH’s UF Herpetology database (http://specifyportal.flmnh.ufl.edu/herps/).
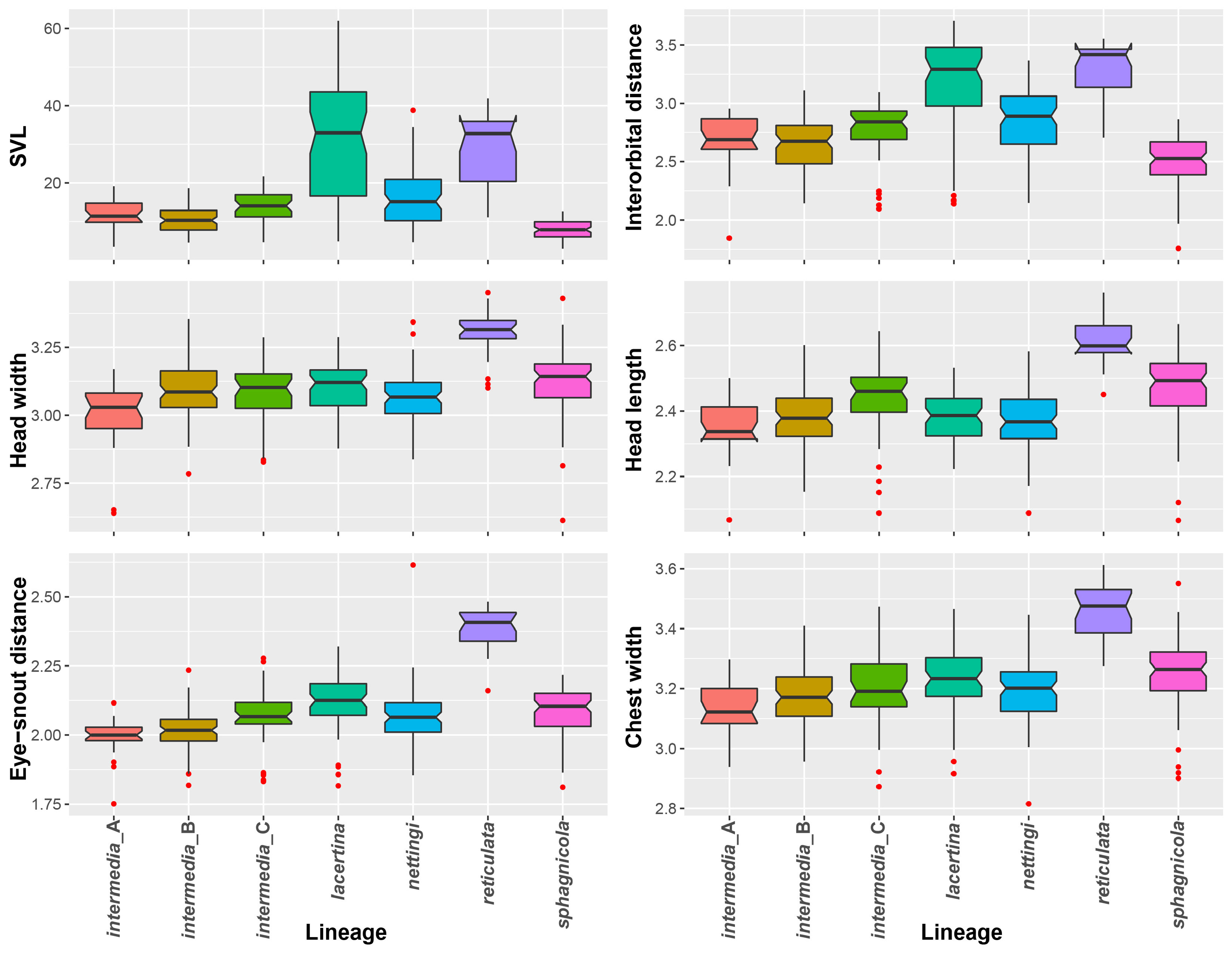
Description of holotype. The holotype has 32 costal grooves and faint black dorsal spots extending from the head to the forelimbs. It lacks approximately 3 mm of its tail tip. In life, it was mouse gray on its venter and sides with a grayish brown dorsum. Sensory pits on the head are well defined and beige in color. Measurements are 97 mm SVL, 49 mm TaL, 3.9 mm interorbital distance, 7.1 mm head width, 11.1 mm head length, 3.6 mm eye-snout distance, and 4.4 mm chest width.
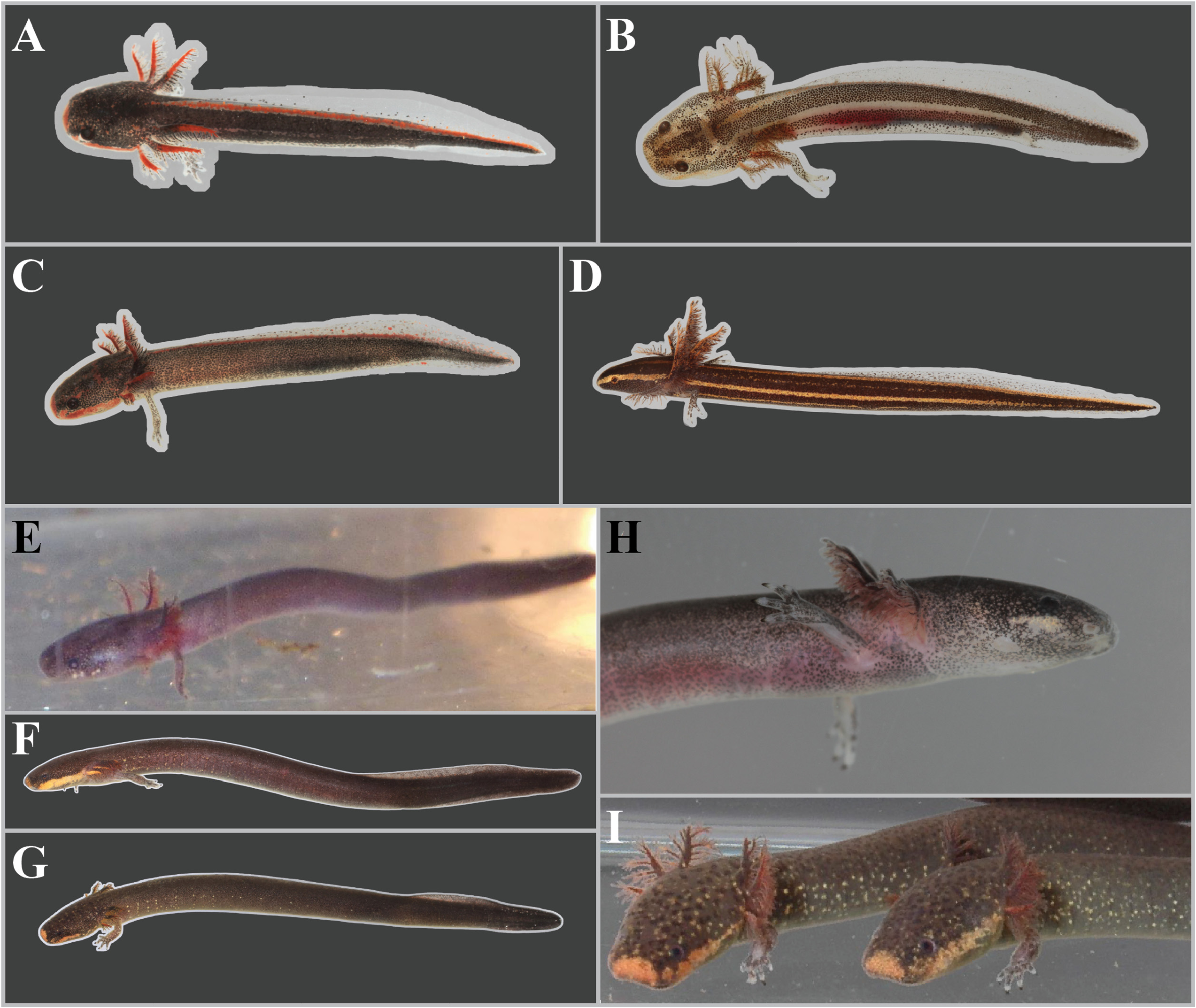
Diagnosis. Siren sphagnicola has typical Siren characteristics: external gills with three fimbriate gill stalks, three associated gill slits, four toes on the forelimbs, lack of pelvic girdle and hindlimbs, and a thin, pigment-bearing mucus layer that overlies the keratinized skin. A combination of traits distinguishes it from other members of the genus. It has 30‒33 costal grooves ( Fig. 3 View FIGURE 3 ) and a mouse gray base color with occasionally a light, grayish brown sheen on the dorsum ( Fig. 11 View FIGURE 11 ). Small juveniles in the post-macrocephalic larval stage, which is>2 months of age according to diagrams of growth/transition rates of S. nettingi provided by Noble & Marshall (1932), have the same gray coloration as adults ( Fig. 11 View FIGURE 11 ) and lack the orange, red, or yellow highlights present on other Siren juveniles of similar age ( Fig. 13 View FIGURE 13 ). A few adult specimens examined have small, well-defined black spotting on the head and occasionally on the dorsum ( Fig. 11 View FIGURE 11 ). Sensory pits on the head are more visible than those on heads of other Siren species and are typically ivory to beige colored, which may denote an absence of gray pigment rather than the presence of chromatophores ( Fig. 11 View FIGURE 11 ). This species lacks the yellow labial stripe present in young S. lacertina ( Figs. 13B & 13F View FIGURE 13 ), S. intermedia ( Figs. 13A, 13C, 13G, & 13I View FIGURE 13 ), and S. nettingi examined from the Mobile Bay drainage. Some juvenile S. intermedia in eastern populations also lack the light labial stripe. A few small juvenile S. sphagnicola have yellow spots or a short, broken stripe where a labial stripe is present in other species ( Figs. 13E & 13H View FIGURE 13 ). Siren sphagnicola also lacks the post-cranial yellow or gold flecking found in many S. lacertina ( Fig. 8 View FIGURE 8 ), S. intermedia ( Fig. 10 View FIGURE 10 ), and S. nettingi . Gill stalk coloration is typically rosy pink to red in recently captured specimens but fades to grayish pink in captivity, likely due to changes in acidity or oxygenation of water. Intact tail tips are rounded, whereas partially regenerated tails (frequently observed) often taper to an abrupt point after the tail fin blade ( Fig. 11 View FIGURE 11 ). Regenerated portions of the tail seem to lack the density of gray pigment found in non-regenerated portions; thus, the regenerated portion is easily distinguished by its pinkish gray hue. Regenerated portions of the tail of other Siren species examined match the normal body coloration or have a brownish hue.
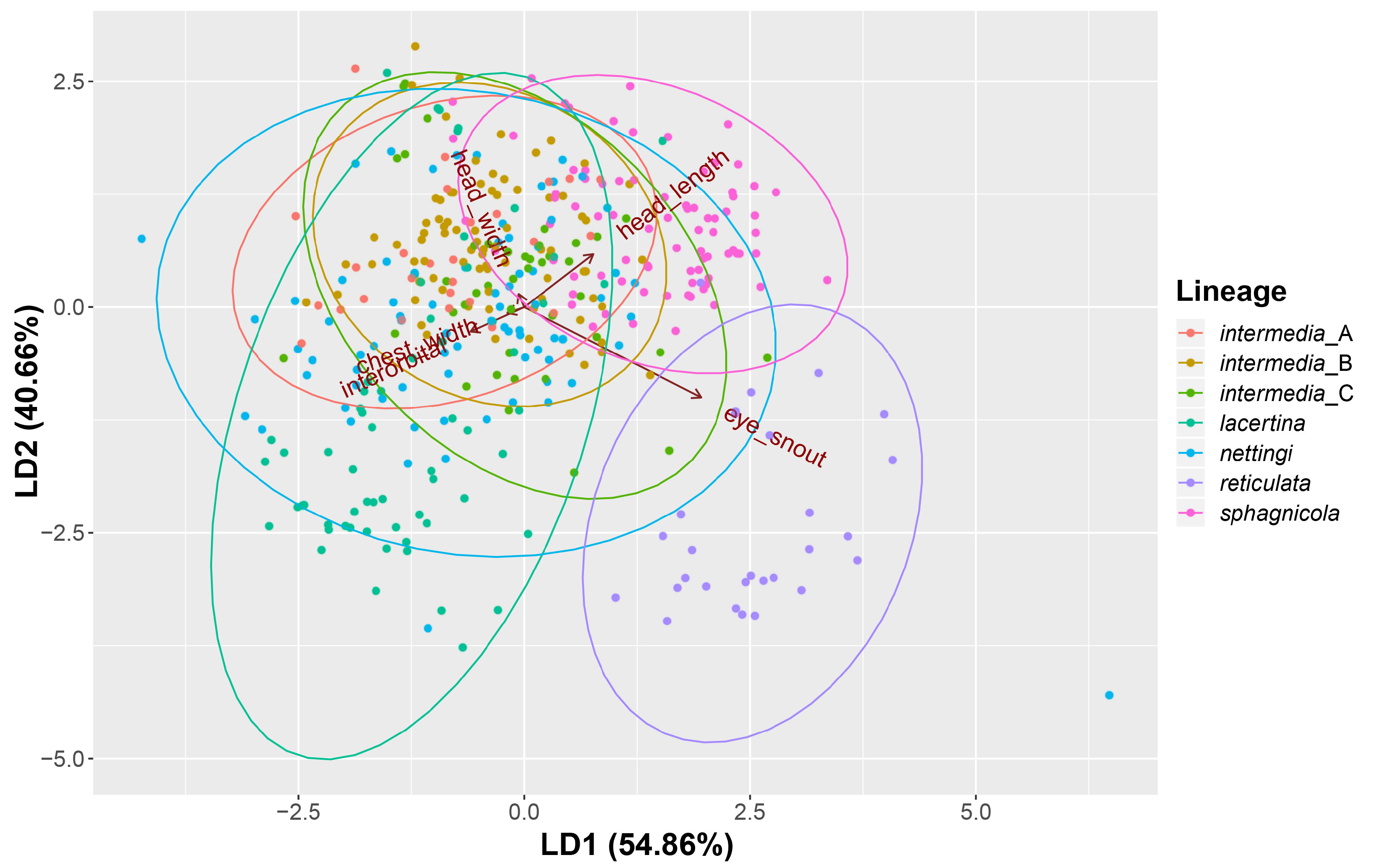
Size. Siren sphagnicola is the smallest known species in the genus Siren . Additionally, all specimens examined are shorter than the maximum length given for both species of Pseudobranchus ( Moler 2019b, c), making S. sphagnicola the smallest member of Sirenidae based on our current understanding. The largest specimen examined (AUM 27973) had an SVL of 126 mm, but it lacked a complete tail. The largest specimen with a complete tail (AUM 8960) had an SVL of 120 mm and a TaL of 76 mm ( 196 mm TL). We attributed these AUM specimens to S. sphagnicola based upon costal groove count, lack of labial striping and gold flecking found on sympatric S. nettingi and S. intermedia , and presence of beige-colored facial pores. Reproductive females have been found as small as 71 mm SVL ( 111 mm TL). When comparing measurement distributions of Siren lineages, S. sphagnicola was not distinct from any other single lineage in, at most, two of seven measurements (Table 4).
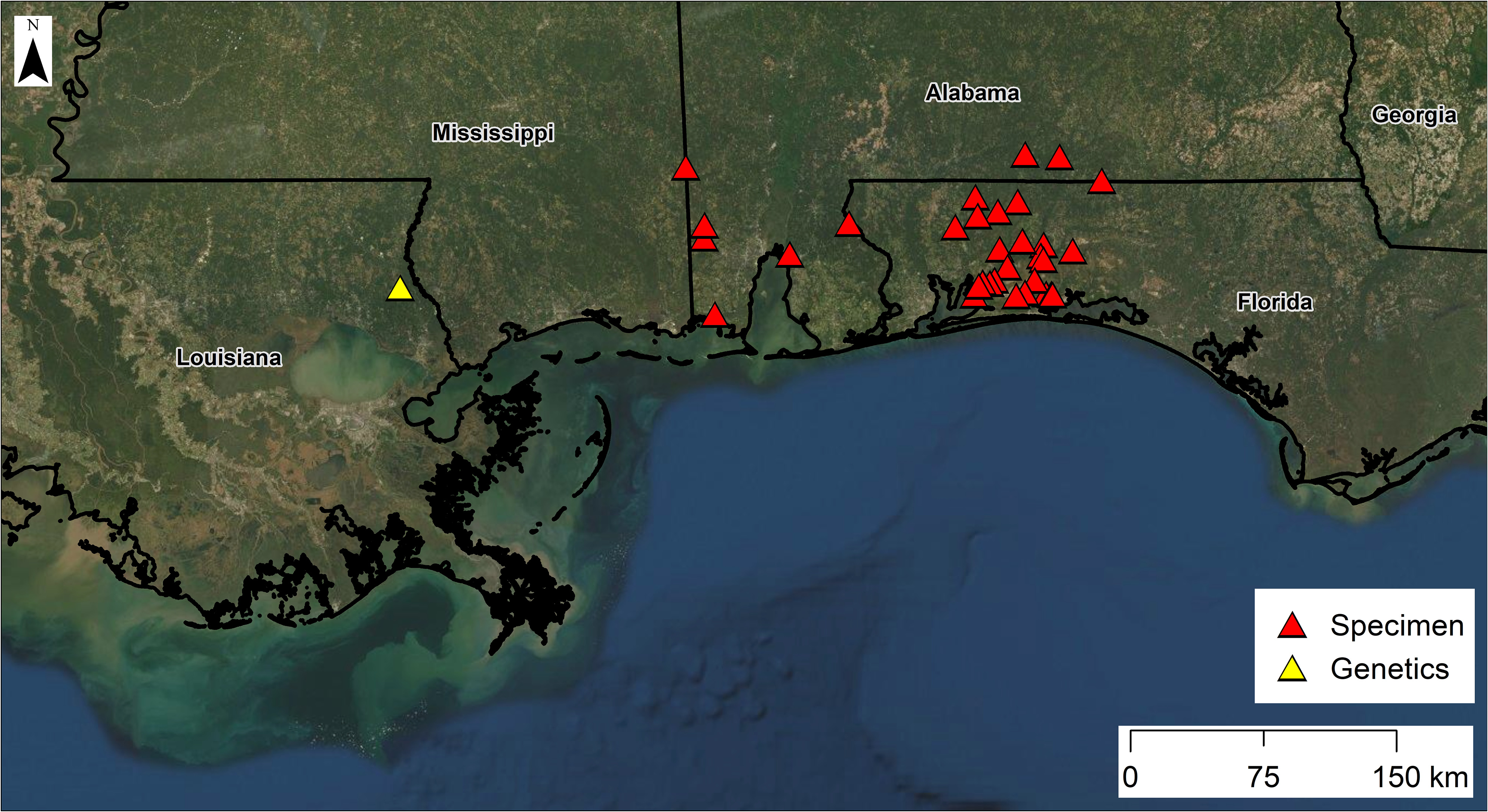
Natural history and distribution. Based on our surveys in Florida, populations appear to be robust and widely distributed in suitable microhabitats in the Blackwater and Yellow river drainages and the western two-thirds of Eglin Air Force Base, including several streams that flow into the western side of Choctawhatchee Bay ( Fig. 14 View FIGURE 14 ). This suspected microhabitat specialist has been found in headwater seepage areas of steephead streams, mucky seeps farther downstream, muddy and/or densely vegetated seepage bogs, shallow-water depressions lined with dense sphagnum moss or filled with leaves along seepage-fed streamside terraces, and other types of shallow streams with mucky, detrital, or sandy bottoms ( Enge 2005) ( Fig. 12 View FIGURE 12 ). In contrast, S. intermedia collected at localities near (< 200 m) S. sphagnicola were found in leaf packs not associated with seeps and adjacent to deeper water. Incised (gully-eroded) first- and second-order streams ( Strahler 1964) lack the microhabitats used by S. sphagnicola (and many other salamander species), because accumulations of leaf litter and other detritus are constantly flushed from streams and scoured from surface pools by heavy rainfall events. Common, syntopic amphibian species are the Southern Cricket Frog ( Acris gryllus [Le Conte]), Bronze Frog ( Lithobates clamitans Latreille ), Southern Two-lined Salamander ( Eurycea cirrigera [Green]), and Southern Red Salamander ( Pseudotriton ruber vioscai Bishop ). Onetoed Amphiuma ( Amphiuma pholeter Neill ) and Two-toed Amphiuma ( A. means Garden ) may be present but are less abundant than the aforementioned species ( Enge 2005).
Deep, steephead ravine systems ( Means 1981, 2000) and more shallow-gradient, seepage bogs in upland habitats near the Gulf of Mexico may have served as “evolutionary engines” during periods of elevated sea levels, producing the Florida Bog Frog ( Lithobates okaloosae Moler, 1985 ), Bog Dwarf Salamander ( Eurycea sphagnicola Wray, Means, & Steppan, 2017 ), and S. sphagnicola . A sea level rise of only 2‒5 m would have led to saltwater inundation of the mouths of these deep steephead valleys, thus isolating ancestral populations of freshwater species ( Means 2000). We suspect the range of S. sphagnicola is similar to that of E. sphagnicola , which also inhabits the sphagnum-lined margins of streams and associated seepage habitats ( Wray et al. 2017).
Siren sphagnicola has a smaller geographic distribution than other Siren species. Most specimens have been found in the Blackwater,Yellow, and Escambia/Conecuh river drainages of Florida and Alabama ( Fig. 14 View FIGURE 14 ). Elsewhere, its range is poorly known, but we believe it is restricted to the environs of sandy, seepage-fed creeks in the lower Gulf Coastal Plain as far west as the Florida Parishes of Louisiana ( Fig. 14 View FIGURE 14 ). Locality information from outside Florida is entirely based on preserved AUM specimens and sequence data from a GenBank specimen that match both mtDNA and scnDNA markers. Few Siren museum vouchers with genetic material exist from Mississippi (42 total specimens via Vertnet search and only one with available tissue, which we sequenced) and the Florida Parishes of Louisiana (63 total via Vertnet search; one of two tissues requested yielded DNA), and we did not examine most museum specimens from this area that lacked tissue samples.
Etymology and common name. The specific epithet is derived from Sphagnum, the generic name for sphagnum moss, and the Latin suffix -cola, meaning inhabitant or dweller. The species epithet is used as noun in apposition. This siren is frequently found in and under mats of Sphagnum in and along streams and the margins of other bodies of water. Because of its affinity for seepage-fed streams and wetlands, we suggest Seepage Siren as the common name.
Specimens examined. See Supplemental Table 1 View TABLE 1 .
| UF |
Florida Museum of Natural History- Zoology, Paleontology and Paleobotany |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.