Grosphus Simon, 1880
|
publication ID |
https://doi.org/10.18590/euscorpius.2019.vol2019.iss281.1 |
|
publication LSID |
lsid:zoobank.org:pub:FEBA0106-02A3-4465-8D39-9FF32634EEF |
|
DOI |
https://doi.org/10.5281/zenodo.7143616 |
|
persistent identifier |
https://treatment.plazi.org/id/03BB8789-FFB9-FFAA-27A9-D257FD18FF05 |
|
treatment provided by |
Felipe |
|
scientific name |
Grosphus Simon, 1880 |
| status |
|
Genus Grosphus Simon, 1880 View in CoL
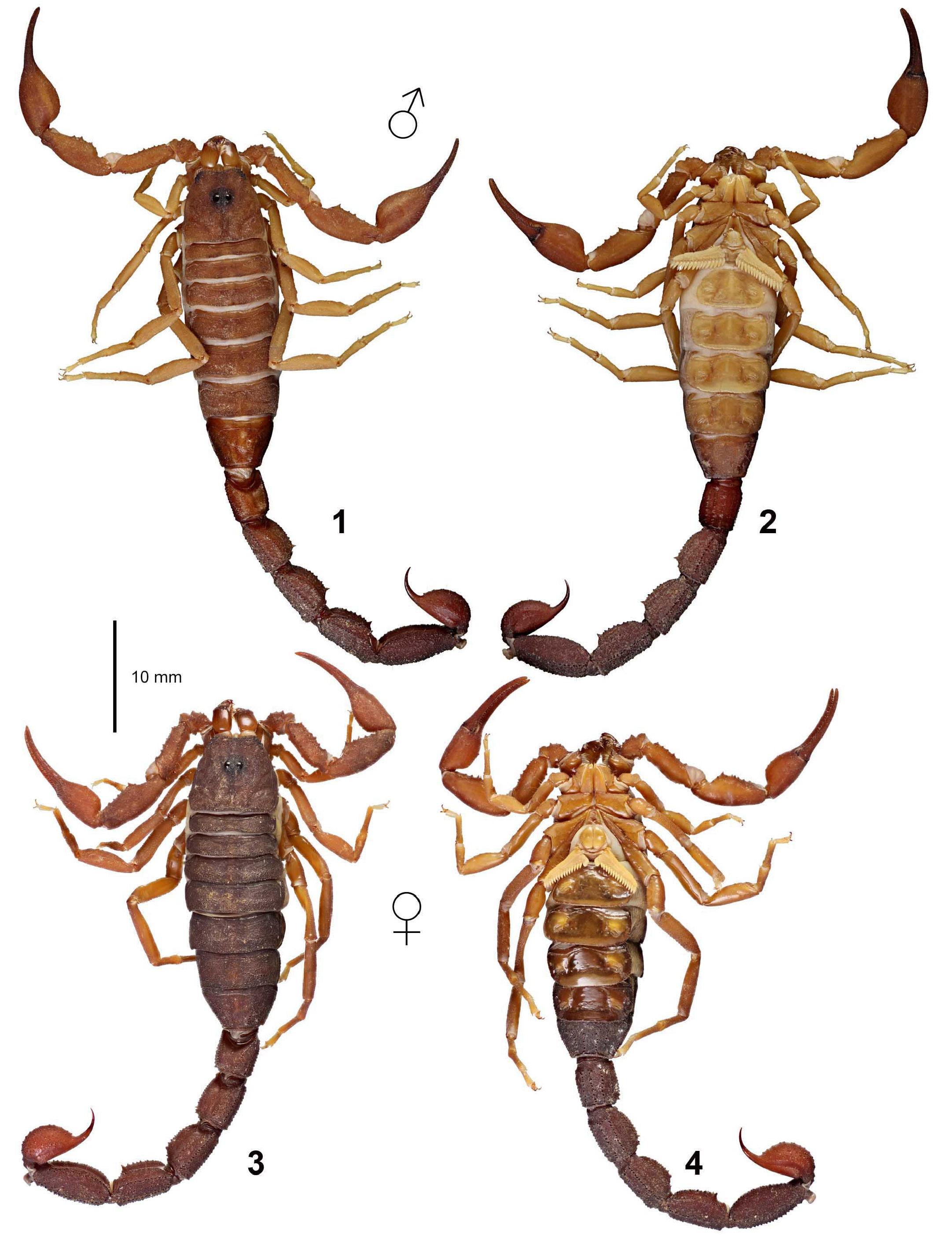
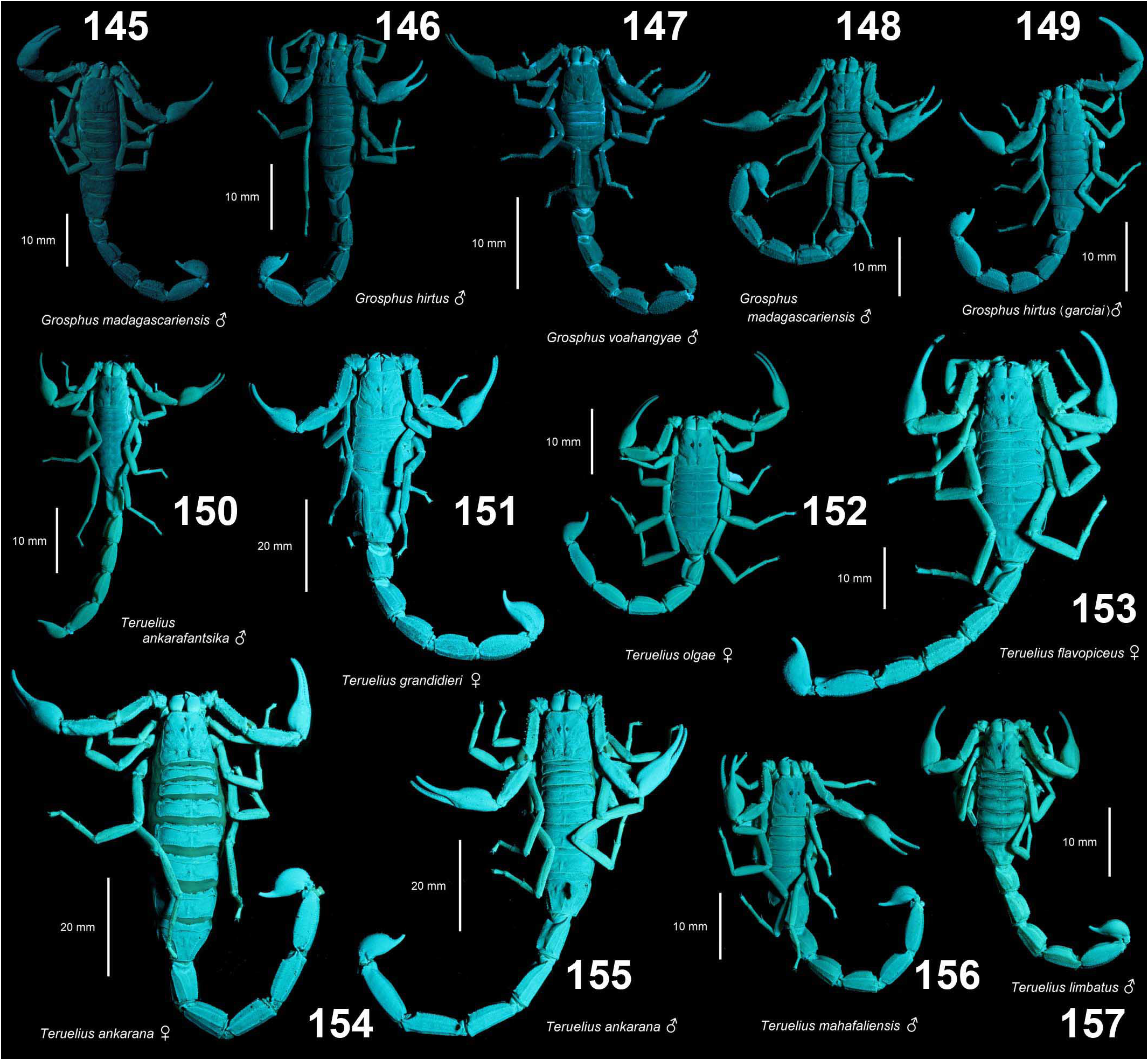
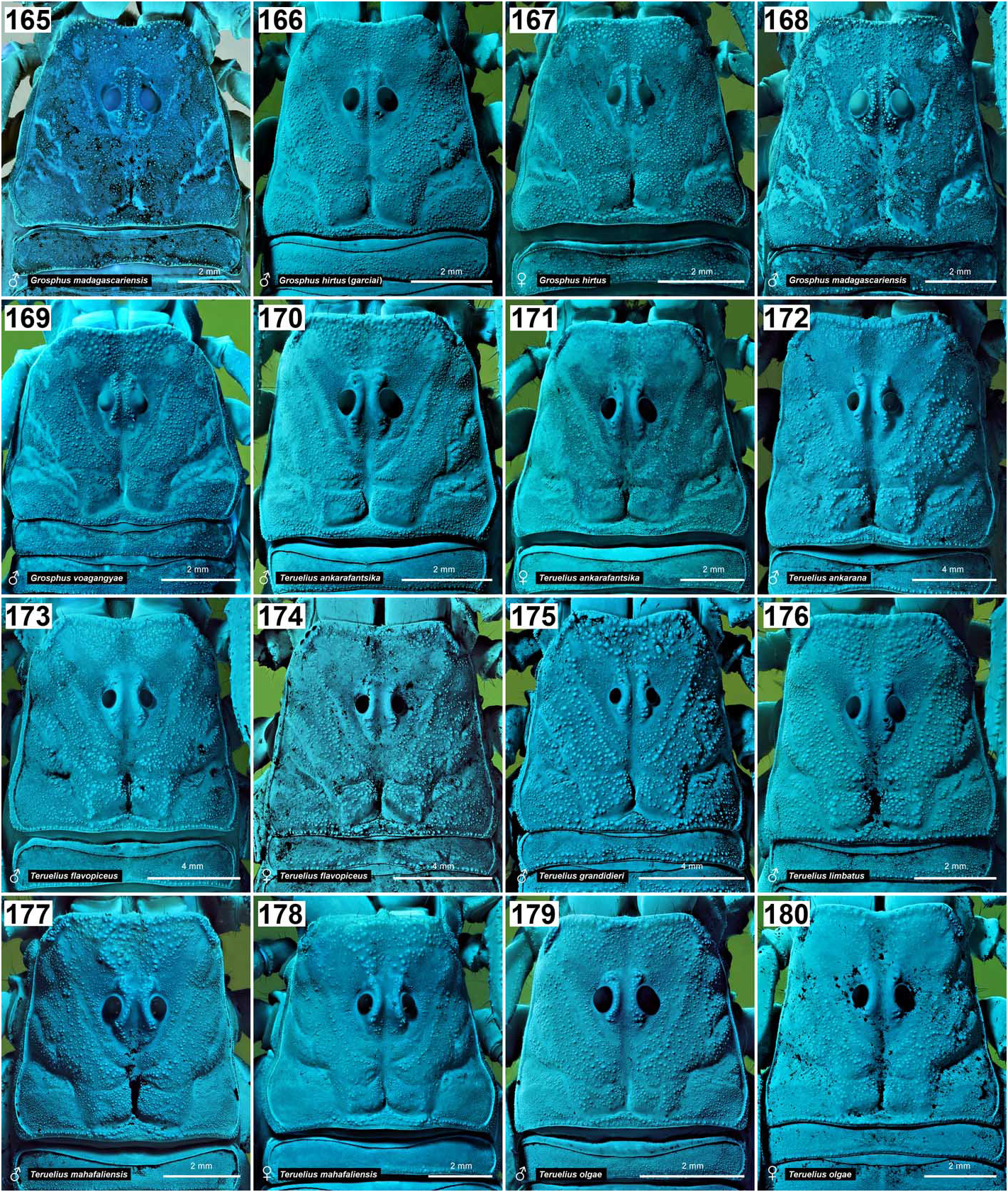
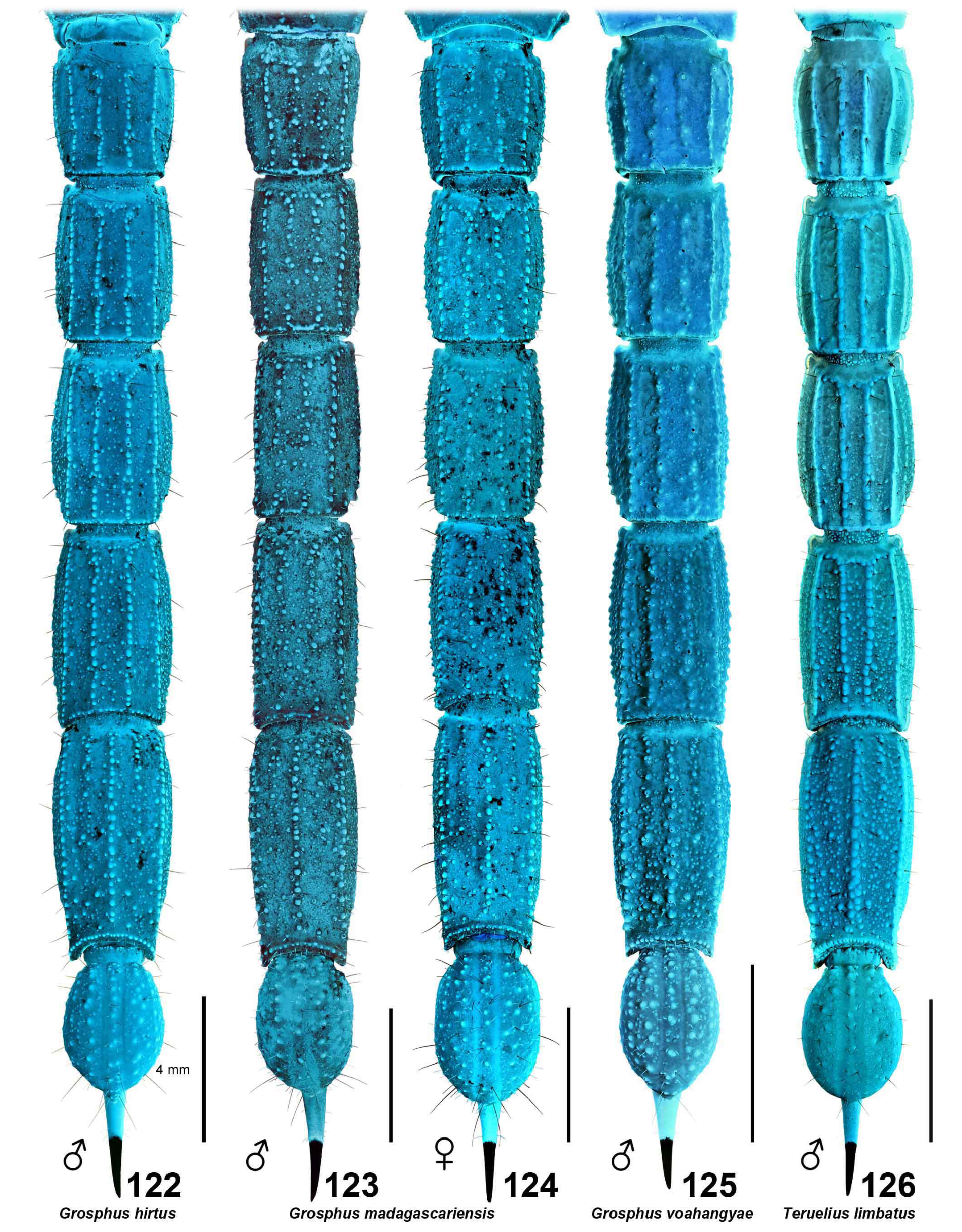
( Figs. 1–4 View Figures 1–4 , 9–12 View Figures 9–20 , 21–22, 25–43 View Figures 21–27 View Figures 28–29 View Figures 30–35 View Figures 36–39 View Figures 40–51 , 52–68 View Figures 52–70 , 86 View Figures 86–89 , 94–98, 106–125 View Figures 94–105 , 133–136 View Figures 133–144 , 145–149 View Figures 145–157 , 158–160 View Figures 158-160 , 165–169 View Figures 165–180 , 181–185 View Figures 181–195 , 196–200 View Figures 196–210 , 211–215 View Figures 211–226 , 227–228 View Figures 227–230 , 231–234 View Figures 231–238 , 239–386 View Figures 239–243 View Figures 244–258 View Figures 259–262 View Figures 263–266 View Figures 267–274 View Figures 275–290 View Figures 291–292 View Figures 293–305 View Figures 306–309 View Figures 310–315 View Figures 316–319 View Figures 320–340 View Figures 341–347 View Figures 348–351 View Figures 352–355 View Figures 356–361 View Figures 362–365 View Figures 366–386 , 580–583 View Figures 580–581 View Figures 582–583 , Tabs. 1–4 View Table 1 )
Grosphus Simon, 1880: 377–378 View in CoL ; Karsch, 1886: 77; Pocock, 1889a: 348–349; Kraepelin, 1891: 70 (in part); Pocock, 1893: 312 (in part); Kraepelin, 1895: 84 (in part); Kraepelin, 1899: 32 (in part); Kraepelin, 1900: 11–12 (in part); Birula, 1917a: 164 (in part); Birula, 1917b: 55 (in part); Fage, 1929: 640–642 (in part); Werner, 1934: 270 (in part); Vachon, 1969: 483 (in part); Legendre, 1972: 428 (in part); Stahnke, 1972: 130 (in part); Vachon, 1974: 906 (in part); Vachon, 1975: 1598 (in part); Lamoral & Reynders, 1975: 507 (in part); Francke, 1985: 8, 15 (in part); Sissom, 1990: 101 (in part); Lourenço, 1995a: 101 (in part); Lourenço, 1996a: 44; Lourenço, 1996b: 5, 8 (in part); Kovařík, 1998: 109 (in part); Fet & Lowe, 2000: 130 (in part); Lourenço, 2001b: 640 (in part); Prendini, 2001: 16–17, 32, 33–35; Fet et al., 2003: 2 View Cited Treatment , 5–6; Lourenço, 2003a: 577 (in part); Lourenço, 2003c: 153–154 (in part); Lourenço & Goodman, 2003a: 26–27; Soleglad & Fet, 2003a: 26; Soleglad & Fet, 2003b: 19, 66–68, 78–79, 88, 90, 154 (in part); Lourenço, 2004a: 31– 33 (in part); Lourenço et al., 2004: 232 (in part); Prendini, 2004a: 39, 41–42; Prendini, 2004b: 115; Fet et al., 2005: 3, 7–8, 10, 23, 26, 29; Prendini & Wheeler, 2005: 481 (in part); Dupré, 2007: 5, 13, 17 (in part); Lourenço et al., 2007a: 176 (in part); Lourenço et al., 2007b: 369 (in part); Kamenz & Prendini, 2008: 6, 8 (in part); Volschenk et al., 2008: 63 (in part); Kovařík, 2009: 22, 31 (in part); Lourenço et al., 2009b: 145 (in part); Lourenço & Wilmé, 2015: 209, 211; Lourenço et al., 2017: 62; Lourenço et al., 2018b: 74 (in part).
Buthus ( Grosphus) : Pocock, 1890: 123 (in part).
TYPE SPECIES. Scorpio ( Androctonus) madagascariensis Gervais, 1843 .
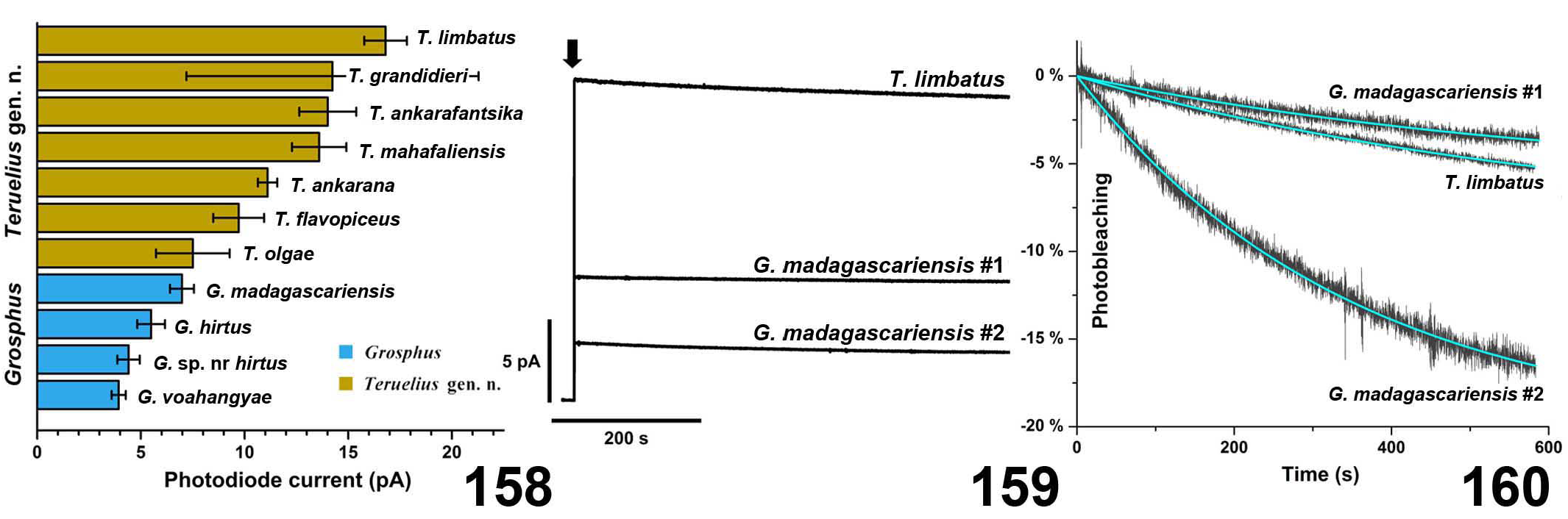
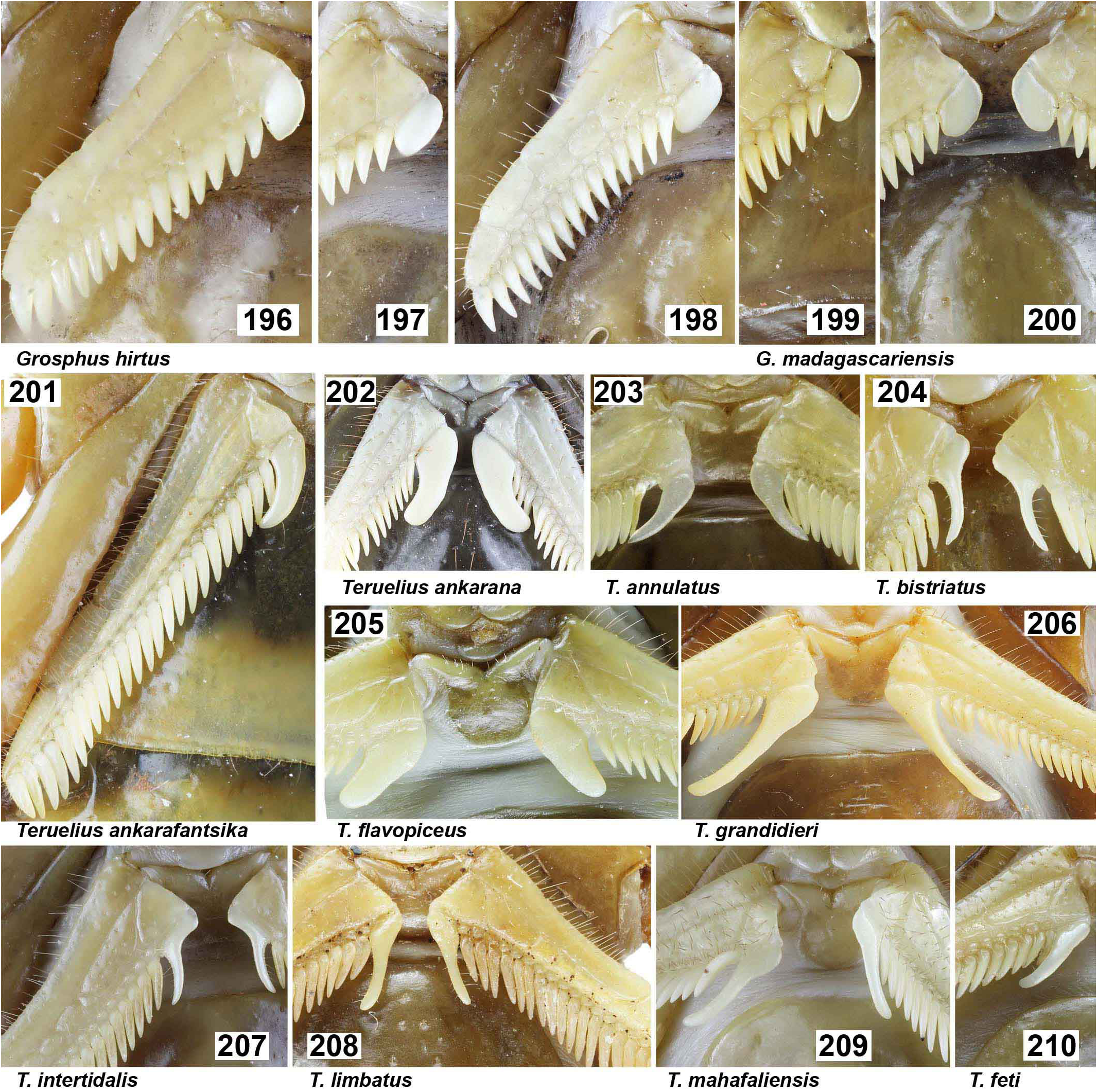
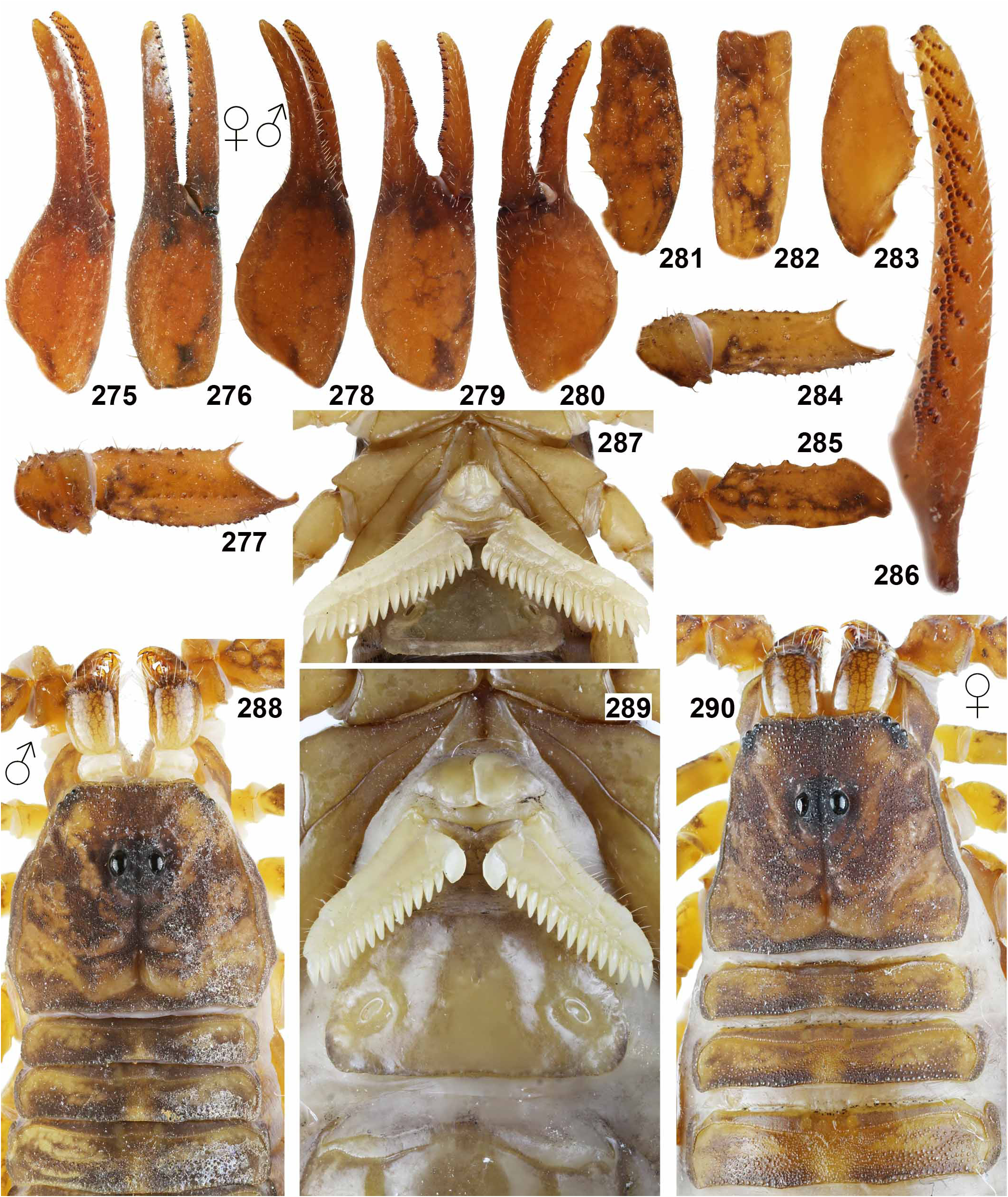
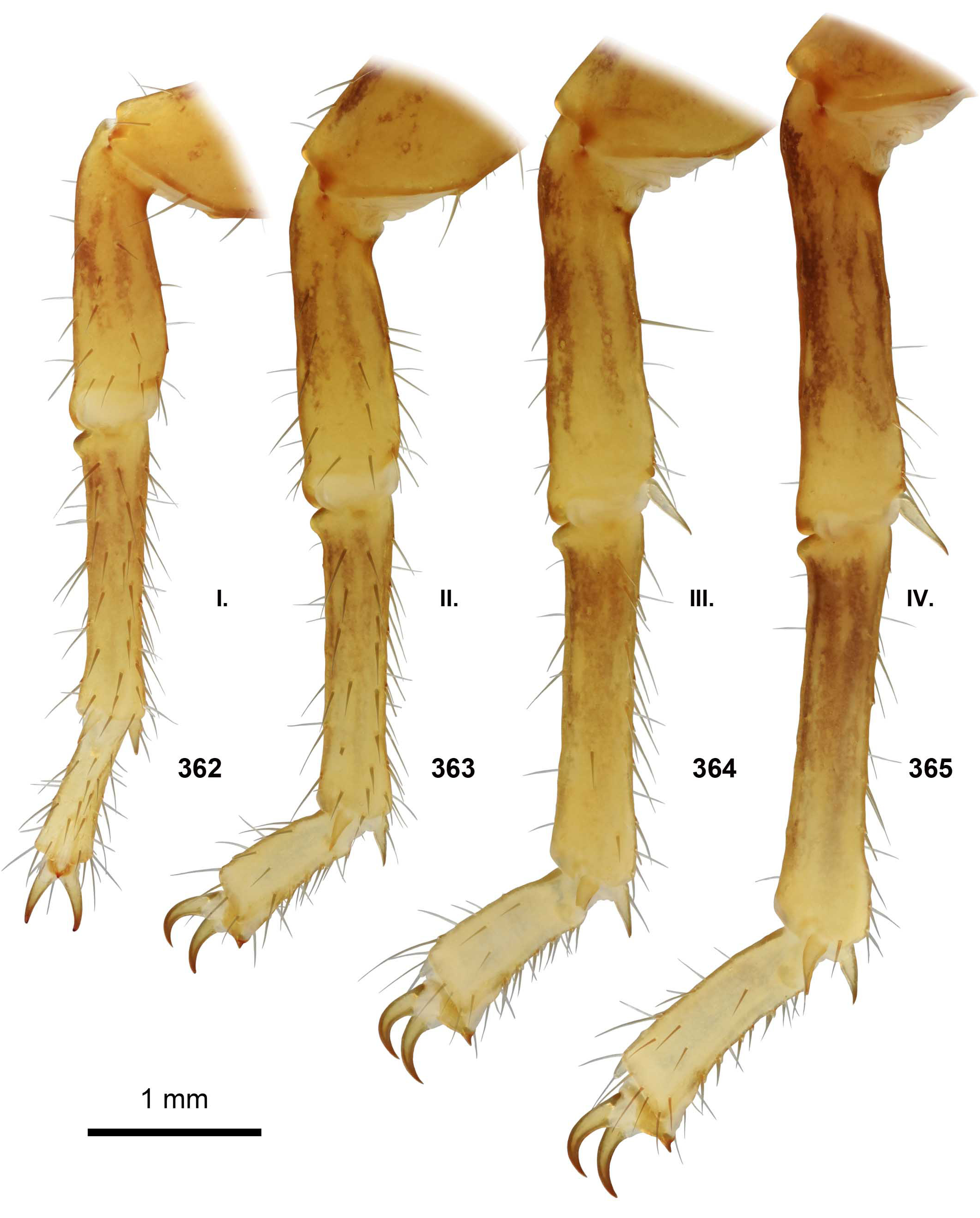
DIAGNOSIS. A member of the ‘ Grosphus ’ group differentiated as follows: medium-sized scorpions, adults ca. 25–75 mm in length; pedipalp finger granule rows 11–14 ( Figs. 252 View Figures 244–258 , 286 View Figures 275–290 , 302 View Figures 293–305 , 330 View Figures 320–340 , 376 View Figures 366–386 ), movable finger typically with 4 external subdistal granules; femur trichobothrium d 2 located on internal surface, or straddling dorsointernal carina ( Figs. 9–12 View Figures 9–20 ); chela manus with petite trichobothrium Eb 3 usually well separated from Eb 2, by more than half the distance between Eb 1 and Eb 2 ( Figs. 21–22 View Figures 21–27 ); manus trichobothrium V 2 roughly collinear with V 1 along chela axis or slightly displaced internally; lower pectinal tooth counts: ♂ 15–23, ♀ 12–19 ( Figs. 28–31 View Figures 28–29 View Figures 30–35 ); basal pectinal tooth of females wide, oval to subrectangular, not distinctly longer than other teeth ( Figs. 40–43 View Figures 40–51 , 196–200 View Figures 196–210 , 289 View Figures 275–290 ); hemispermatophore capsule long or short, posterior lobe with long, lanceolate extension ( Figs. 52–68 View Figures 52–70 ); sternites with broad ovoid, elliptical or hemi-elliptical spiracles ( Figs. 94–98 View Figures 94–105 ); metasoma I with ventromedian carinae moderately to strongly crenulate or granulate ( Figs. 122–125 View Figures 122–126 ); telson with oval or bulbous vesicle, with or without subaculear tubercle in adults ( Figs. 181–185 View Figures 181–195 ); legs with ventral surface of telotarsus sparsely setose, with two rows of <20 short, setiform macrosetae ( Figs. 133–137 View Figures 133–144 , 211–215 View Figures 211–226 , 259–262 View Figures 259–262 , 316–319 View Figures 316–319 , 362–365 View Figures 362–365 ); telotarsus with dorsal terminal process of normal size; cuticle with weak UV fluorescence ( Figs. 145–149 View Figures 145–157 ).
SUBORDINATE TAXA.
Grosphus ambre Lourenço, Wilmé & Waeber, 2018
Grosphus darainensis Lourenço, Goodman & Ramilijaona, 2004
Grosphus goudoti Lourenço & Goodman, 2006
Grosphus hirtus Kraepelin, 1900
Grosphus madagascariensis ( Gervais, 1843)
Grosphus mayottensis Lourenço & Goodman, 2009 Grosphus polskyi Lourenço, Qi & Goodman, 2007
Grosphus rakotoariveloi Lourenço, Wilmé, Soarimalala & Waeber, 2017
Grosphus tavaratra Lourenço, Soarimalala & Goodman, 2009 Grosphus voahangyae Lourenço & Wilmé, 2015
See Tables 1–3 View Table 1 for diagnostic characters used to place the above taxa under Grosphus .
REMARKS. We consider Grosphus paraphyletic and define two species groups distinguished by major differences in hemispermatophore capsule form: (i)‘ madagascariensis ’ group: capsule elongate, monocarinate, with basal lobe located far proximal to base of flagellum ( G. madagascariensis ); (ii) ‘ hirtus ’ group: capsule short, carination variable, with basal lobe located distally near base of flagellum ( G. goudoti , G. hirtus and G. voahangyae ). Phylogenetic polarity of capsule form is unclear. Possible group affiliations of other species are suggested by some similarities in external characters, e.g.: (i) ‘ madagascariensis ’ group: elliptic spiracles, more elongate metasomal segments, maculation patterns weak or absent, subaculear tubercle small or absent; may include G. ambre , G. mayottensis and G. rakotoariveloi ; (ii) ‘ hirtus ’ group: ovoid spiracles, more stout metasomal segments, stronger maculation patterns, subaculear tubercle more developed; may include G. polskyi , G. tavaratra . However, external characters can be misleading and definitive group assignment requires study of hemispermatophore capsules. For example, G. goudoti resembles species of the ‘ madagascariensis ’ group in external characters (metasoma slender, weak maculation, elliptic spiracles, lack of subaculear tubercle) but possesses a ‘ hirtus ’ group type of capsule.
NEW SYNONYMIES.
Grosphus halleuxi Lourenço, Wilmé, Soarimalala & Waeber, 2017 = Grosphus madagascariensis ( Gervais, 1843) , syn. n.
Grosphus mandena Lourenço, 2005 = Grosphus madagascariensis ( Gervais, 1843) , syn. n.
Grosphus simoni Lourenço, Goodman & Ramilijaona, 2004 = Grosphus madagascariensis ( Gervais, 1843) , syn. n.
The single holotype male of G. madagascariensis used for description by Gervais (1843, 1844) is in poor condition after 176 years. It is disarticulated into several fragments: metasoma III–V + telson, metasoma I–II, hollowed carapace and tergites with most of coxosternal area and sternites III–VI missing, and 4 partial leg fragments (cf. https://science.mnhn. fr/taxon/species/grosphus/madagascariensis). The type locality is given only as ‘Madagascar’. Gervais (1844: pl. XI, figs. 1–3) published a color painting of the dorsal habitus, and drawings of two consecutive metasomal segments in lateral view (segments not specified, but possibly III–IV), showing enlarged spiniform granules on posterior dorsal carinae, and the carapace with median and lateral eyes. With only limited information available about the holotype, which has lost many body parts bearing key taxonomic characters, it is difficult to precisely pin down the identity of G. madagascariensis in relation to a group of several other currently-named similar taxa (i.e., G. darainensis , G. halleuxi , G. mandena , G. rakotoariveloi and G. simoni ). Previous diagnoses of Kraepelin (1900), Fage (1929) and Lourenço (1996b) listed some characters that differentiate G. madagascariensis from G. hirtus or Teruelius gen. n. Meristic characters were: PTC ♂ 18–20, ♀ 16–18, and pedipalp movable finger granule rows 12. Lourenço & Goodman (2006) suggested that Goudot, collector of the holotype, travelled in the north eastern region. They selected a male and female from Forêt de Plateau de Makira, in humid northeastern forest near Antongil Bay, as reference material for a redescription. The redescription is generic for the group, with few diagnostic characters: PTC ♂ 20, ♀ 15–16, pedipalp movable finger granule rows 13. Lateral eyes were incorrectly cited only as only 3 pairs, contradicting Gervais (1844).
Grosphus simoni was described by Lourenço, et al. (2004) from two specimens: the holotype male from Forêt de Plateau de Makira, Forêt de Sahantaha, which is humid tropical forest in the northeast; and a paratype male from Station Forestière d’Ampijoroa, Ankarafantsika National Park, which is dry deciduous forest in the northwest. The differential diagnosis was brief: paler coloration, metasoma with strong granules and carinae, including several larger posterior spiniform granules on dorsal carinae on segments II–IV. Recently, Lourenço, et al. (2017) moved the paratype to a different species, G. rakotoariveloi , invalidating the original diagnosis of G. simoni based on both specimens. They revised the diagnosis of G. simoni as: moderately darker coloration, several larger posterior spiniform granules on dorsal carinae of metasoma II–IV, PTC ♂ 15–17, ♀ 14–15, pedipalp finger granule rows ♂ 11–12, ♀ 12– 13, male chela with weak to moderate scalloping. Photographs were included for G. simoni specimens of both sexes from Forêt de Sahantaha (figs. 2–5), although the male was misidentified as a female, and the female misidentified as a male. The holotype male of G. simoni is well documented in high resolution images published on the FMNH website: https://collections-zoology. fieldmuseum.org/catalogue/963985. We studied FMNH materials ( 5♂, 1♀) from Andasibe determined as G. simoni .We further compared other materials, including a male determined by M. Vachon as G. madagascariensis (MHNG) . We found no convincing diagnostic characters to support a distinction between G. simoni and G. madagascariensis . Diagnostic characters for G. simoni involve relatively minor differences in darker vs. lighter shades of color, differences in size of spiniform granules on dorsal metasomal segments, meristic differences of one or two pectine teeth with contiguous or overlapping ranges of PTC, and/ or pedipalp finger granule row counts. These characters are subject to inter-population and geographic variation in many scorpion taxa. Allowing for typical genetic variation, the metasoma and telson of holotypes of G. simoni and G. madagascariensis do not differ significantly in carination, spination or morphometrics. In the absence of quantitative analysis showing discontinuous variation either in characters or morphometrics to support splitting into discrete species, we regard them as synonyms. Localities of G. simoni overlap or are sympatric with the distribution for G. madagascariensis in northeastern humid forests.
The species G. rakotoariveloi has meristics (PTC ♂ 18– 19, pedipalp granule rows ♂ 13–14) that also overlap or are contiguous with those of G. madagascariensis . However, the type (and only known) locality is in a different bioclimatic region with dry deciduous forest, disjunct from eastern humid forests. It has much lighter coloration and relatively wide pedipalp chelae. We provisionally list this species, until additional data are available.
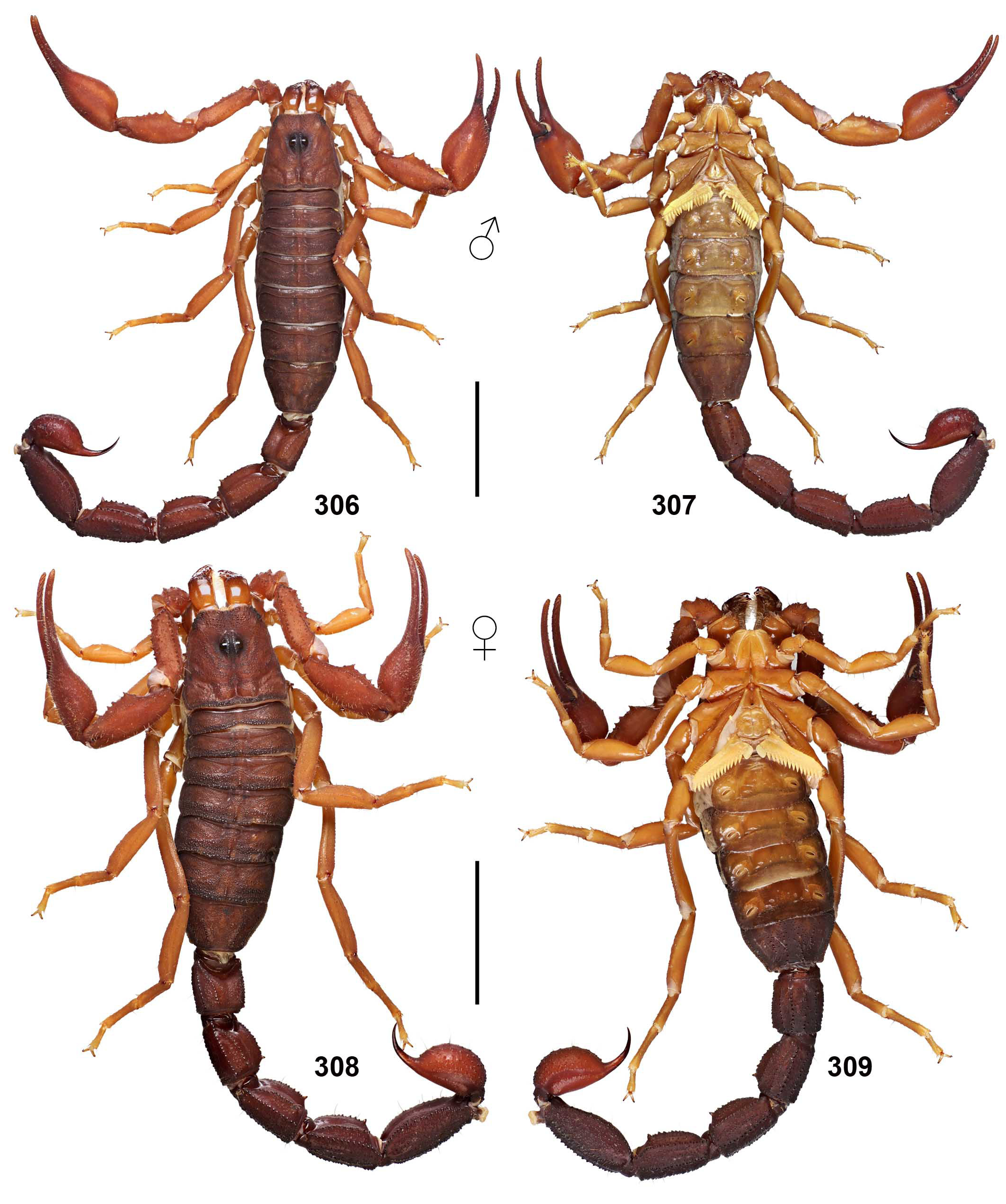
Grosphus halleuxi was described by Lourenço, et al. (2017) from a series of males from Torotorofotsy Forest, ca. 20 km NW of Moramanga, in a central humid forest area that is locally less humid than other eastern forests. The diagnostic characters for differentiating it from G. simoni were: darker coloration, smaller size of 55 mm, PTC ♂ 16–19, pedipalp granule rows ♂ 11–12, and weaker scalloping of pedipalp fingers. The meristic counts do not yield a differential diagnosis as they overlap those of G. simoni (= G. madagascariensis ). We analyzed a series of near topotypic specimens ( 5♂, 6♀) from Moramanga and ca. 30 km E of Moramanga, whose males closely match photos of the G. halleuxi male holotype ( Figs. 1–2 View Figures 1–4 , cf. Lourenço, et al., 2017: figs. 16–17). We obtained meristics: PTC ♂ 15–18, ♀ 13–15, pedipalp finger granule rows ♂ ♀ 12, which are not distinguishable from meristic ranges of G. madagascariensis . Other characters of darker or lighter shades, and degree of pedipalp finger scalloping can also be variable between populations. For example, in Figs. 1 & 3 View Figures 1–4 , a female from Moramanga area is darker than a male from the same area, showing that intensity of coloration varies within the same population, weakening this diagnostic character. G. halleuxi was diagnosed as “much darker”, but ‘ G. simoni ’ appears as dark, if not darker (Lourenço, et al., 2017: figs. 2–5 vs. 16–17). It was argued that G. halleuxi is a narrow-ranged species adapted to a less humid local microclimate. The existence of local microendemic taxa should be supported by strong diagnostic characters. Until such characters are defined, we regard this species as a local population of G. madagascariensis .
Grosphus mandena was described by Lourenço (2005) from near Fort Dauphin, in the Mandena region of southeastern coastal rainforest. Differential diagnostic characters were: lighter coloration, weaker metasomal carination, one larger spiniform granule on metasoma II–IV, and a more granulated telson. Meristics were: PTC ♂ 19–20, ♀ 15–17, pedipalp granule rows ♂ 12–13. As discussed above, these characters fall within ranges of variation for G. madagascariensis , including local populations given other species names. We loaned and studied the male holotype and a female paratype from MHNG and found them to be indistinguishable from G. madagascariensis . We note that the female paratype is considerably darker than the male holotype. This shows that intensity of coloration varies even within the type population, and is not a reliable diagnostic character ( Figs. 348–351 View Figures 348–351 , 580–583 View Figures 580–581 View Figures 582–583 ). Lourenço & Wilmé (2016: fig. 36) showed a nonoverlapping discontinuous transition, at latitude ca. 22°S between the northern range of G. madagascariensis and the southern range of G. mandena . The transition latitude does not correspond to any boundary between centers of endemism as defined by the watershed model of Wilmé et al. (2006). Lourenço et al., (2009b) suggested that the disjunction is recent, due to extirpation of south littoral rainforest by humans. More robust diagnostic characters and analysis of clinal vs. discontinuous variation is necessary to delimit the southern populations as a distinctive species. Until such characters are defined, we regard this species as a southern population of G. madagascariensis . The synonymies of G. halleuxi and G. mandena with G. madagascariensis are further supported by their identical hemispermatophores, all of which have a unique, elongated capsule architecture with a proximal basal lobe (cf. Figs. 52–53, 56–57 View Figures 52–70 vs. Figs. 54–55 View Figures 52–70 ).
Grosphus hirtus garciai Lourenço, 2001 = Grosphus hirtus Kraepelin, 1900 , syn. n.
Grosphus garciai was described by Lourenço (2001b) from Station Forestière d’Ampijoroa, Ankarafantsika National Park, based on an adult male holotype and a juvenile, collected by García Herrero. In the diagnosis, it was differentiated from G. madagascariensis , a quite different species, by: smaller size, maculated light and dark pigmentation, pedipalp granule rows ♂ 13, weaker spiniform granules on pedipalp and metasoma, and weaker scalloping of pedipalp fingers. Curiously, it was not compared to G. hirtus , which bears a much greater similarity. Subsequently, Lourenço & Goodman (2006) redescribed G. hirtus based on material also from Station Forestière d’Ampijoroa, Ankarafantsika National Park, also collected by García Herrero. G. hirtus was separated from G. garciai by yellowish rather than reddish brown color, and larger size ( 40–50 mm vs. 32 mm). Subsequently, Lourenço & Wilmé, (2015a) downgraded G. garciai to the status of subspecies, G. hirtus garciai , supposedly a microendemic taxon in a “local isolated population”. It is morphologically identical to, and differentiated from the nominotypical G. hirtus only by smaller size. The subspecies is known only from the type locality and the nominotypical G. hirtus also occurs in the same area ( Lourenço & Goodman, 2006). The species G. hirtus is distributed more widely over northwest Madagascar ( Lourenço & Wilmé, 2016: fig. 36). We loaned and studied the holotype of G. garciai , as well as male and female topotypes from FMNH. We confirmed that it is morphologically indistinguishable from G. hirtus ( Figs. 263–305 View Figures 263–266 View Figures 267–274 View Figures 275–290 View Figures 291–292 View Figures 293–305 ). We question whether an animal population of somewhat smaller average body size compared to closely neighboring conspecifics, but otherwise identical to them, merits subspecies status. Local size variations of species may be caused by varying environmental conditions that limit growth rates and development. The size differential is exaggerated by the reported body lengths, a measurement that can vary with mesosomal expansion: G. hirtus 40–50 mm ( Lourenço & Goodman, 2006); G. h. garciai 28–32 mm ( Lourenço & Wilmé, 2015a). These numbers imply that G. hirtus is at least 25% longer. A more reliable size comparison for morphometrically similar scorpions would use carapace length. For example, we measured carapaces of G. hirtus : ♂ 5.5 mm, ♀ 5.0 mm, vs. G. h. garciai : ♂ 5.2 mm, ♀ 4.4 mm, i.e., only 6–12% longer. Lourenço et al. (2007a) redescribed G. hirtus with body lengths of ♂ 34.3 mm, ♀ 31.8 mm, and carapace lengths ♂ 4.1 mm, ♀ 4.3 mm. These are small enough to overlap measurements for G. h. garciai . Considering this overlap, we regard G. h. garciai as a synonym of G. hirtus .
Grosphus polskyi is another species that is quite similar to G. hirtus . It was diagnosed by having paler color, with weaker, more diffuse maculate patterns restricted to carapace and tergites, slightly more elongate metasoma segment I, weak spination on dorsal metasomal carinae, and a slightly larger subaculear tubercle. The only known record is the single male holotype from Mikea Forest near Toliara, on the southwestern coast. Although this is quite far south of the southern-most record of G. hirtus ( Lourenço & Wilmé, 2016) , records of the latter are sparse, so it is unclear if it represents a disjunction. We provisionally list this species, until it can be critically evaluated by more material and analysis of variation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Grosphus Simon, 1880
| Lowe, Graeme & Kovařík, František 2019 |
Buthus ( Grosphus )
| POCOCK 1890: 123 |
Grosphus
| LOURENCO, W. R. & L. WILME & P. O. WAEBER 2018: 74 |
| LOURENCO, W. R. & L. WILME L & V. SOARIMALALA & P. O. WAEBER 2017: 62 |
| LOURENCO, W. R. & L. WILME 2015: 209 |
| KOVARIK, F. 2009: 22 |
| LOURENCO, W. R. & V. SOARIMALALA & S. M. GOODMAN 2009: 145 |
| KAMENZ, C. & L. PRENDINI 2008: 6 |
| VOLSCHENK 2008: 63 |
| DUPRE, G. 2007: 5 |
| LOURENCO, W. R. & J. - X. QI & S. M. GOODMAN 2007: 176 |
| LOURENCO, W. R. & V. SOARIMALALA & S. M. GOODMAN 2007: 369 |
| FET, V. & M. E. SOLEGLAD & G. LOWE 2005: 3 |
| PRENDINI, L. & W. WHEELER 2005: 481 |
| LOURENCO, W. R. 2004: 31 |
| LOURENCO, W. R. & S. M. GOODMAN & O. RAMILIJAONA 2004: 232 |
| PRENDINI 2004: 39 |
| PRENDINI 2004: 115 |
| FET, V. & B. GANTENBEIN & A. V. GROMOV & G. LOWE & W. R. LOURENCO 2003: 2 |
| LOURENCO, W. R. 2003: 577 |
| LOURENCO, W. R. 2003: 153 |
| LOURENCO, W. R. & S. M. GOODMAN 2003: 26 |
| SOLEGLAD, M. E. & V. FET 2003: 26 |
| SOLEGLAD, M. E. & V. FET 2003: 19 |
| LOURENCO, W. R. 2001: 640 |
| PRENDINI 2001: 16 |
| FET, V. & G. LOWE 2000: 130 |
| KOVARIK, F. 1998: 109 |
| LOURENCO, W. R. 1996: 44 |
| LOURENCO, W. R. 1996: 5 |
| LOURENCO, W. R. 1995: 101 |
| SISSOM, W. D. 1990: 101 |
| FRANCKE, O. F. 1985: 8 |
| VACHON 1975: 1598 |
| LAMORAL, B. H. & S. C. REYNDERS 1975: 507 |
| VACHON 1974: 906 |
| LEGENDRE, R. 1972: 428 |
| STAHNKE 1972: 130 |
| VACHON 1969: 483 |
| WERNER 1934: 270 |
| FAGE, L. 1929: 640 |
| BIRULA, A. A. 1917: 164 |
| BIRULA, A. A. 1917: 55 |
| KRAEPELIN, K. 1900: 11 |
| KRAEPELIN, K. 1899: 32 |
| KRAEPELIN, K. 1895: 84 |
| POCOCK 1893: 312 |
| KRAEPELIN, K. 1891: 70 |
| POCOCK 1889: 348 |
| KARSCH, F. 1886: 77 |
| SIMON 1880: 378 |