Teresaspis lineata ( Canu & Bassler, 1928 ), 2018
|
publication ID |
https://doi.org/10.11646/zootaxa.4524.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:22A47EBE-338A-4AAF-A63F-45EDE9727F18 |
|
DOI |
https://doi.org/10.5281/zenodo.5979045 |
|
persistent identifier |
https://treatment.plazi.org/id/03BA0525-3A7D-FFAB-5CAA-7A7D719BD15B |
|
treatment provided by |
Plazi |
|
scientific name |
Teresaspis lineata ( Canu & Bassler, 1928 ) |
| status |
comb. nov. |
Teresaspis lineata ( Canu & Bassler, 1928) View in CoL n. comb.
( Figs 46–84 View FIGURES 46–48 View FIGURES 49–54 View FIGURES 55–64 View FIGURES 65–69 View FIGURES 70–76 View FIGURES 77, 78 View FIGURES 79–84 ; Tables 1, 2)
Cribrilina lineata Canu & Bassler, 1928: p. 38 View in CoL , pl. 3, figs 5, 6.
Cribrilina lineata: Harmelin, 1978: p. 177 View in CoL .
Cribrilina lineata: Winston & Maturo, 2009: p. 1155 View in CoL . Material examined. Holotype: USNM 7828 About USNM . A colony including ovicellate zooids and kenozooids, encrusting corals, Caribbean Sea, 30.04.1884, Albatross station D 2152, 2.5 miles northwest of Habana Light , 704 m depth. Further material: Great Bahama Bank Slope, R / V Maria S. Merian Cruise MSM20 View Materials /4. Station GeoB16367-2 (two live and seven dead colonies on coral fragments, except one on barnacle)—SMF-45.515; Station GeoB16368-1 (four dead colonies on corals, except one on brachiopod shell)—SMF-45.516; Station GeoB16373-2 (one living and one dead colony on corals)—SMF-45.517; Station GeoB16375-1 (two dead colonies on coral fragments)— SMF-45.518; Station GeoB16376-1 (six dead colonies on corals, except one on erect bryozoan and one on foraminiferan test)—SMF-45.519; Station GeoB16377-1 (two live and two dead colonies on corals)—SMF- 45.520; Station GeoB16378-1 (one dead colony on a coral fragment)—SMF-45.521; Station GeoB16381-4 (one dead colony on a coral)—SMF-45.522; Station GeoB16382-1 (12 living and 22 dead colonies on corals, except one on a stylasterid hydrozoan and one on Aphrocallistes sponge)—SMF-45.523. Five specimens, one from Station GeoB16375-1, one from Station GeoB16377-1, two from Station GeoB16382-1, and one from Station GeoB16388-3: PMC-Rosso Bahama Collection Bah.H. B 43a.
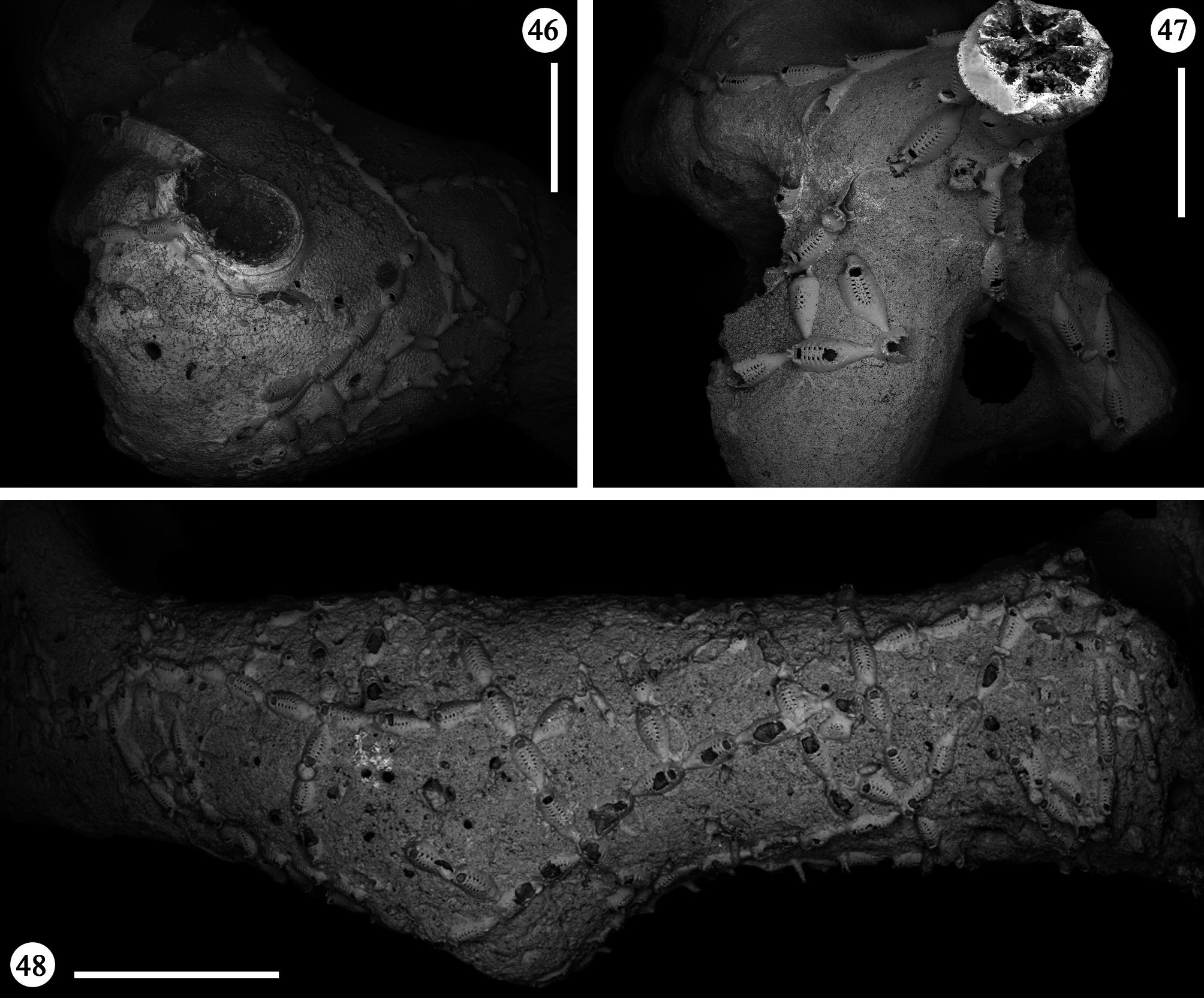
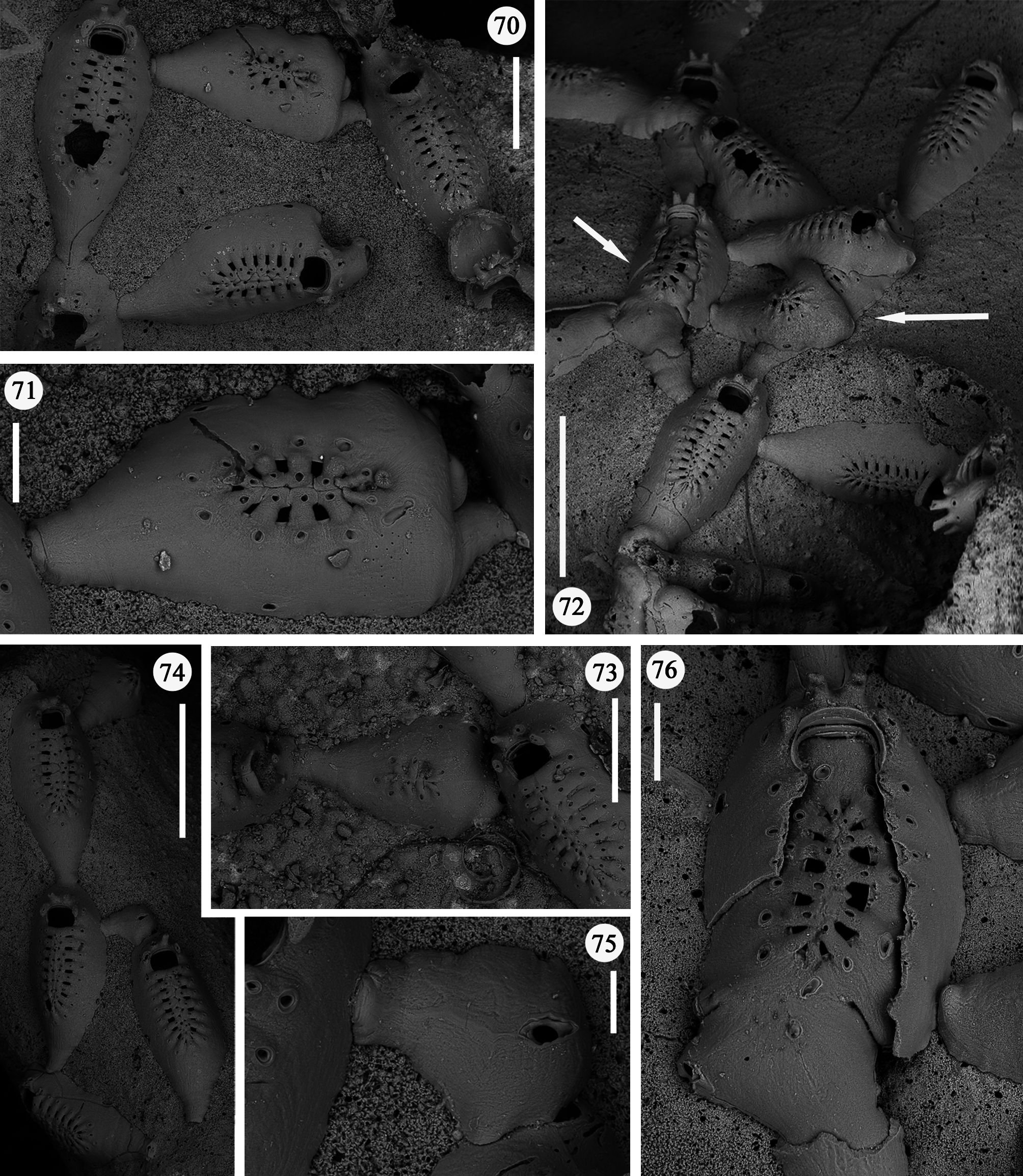
Description. Colony encrusting, uniserial, branching, often extensively spreading on the whole surface of large coral fragments, forming irregular networks ( Figs 46–48 View FIGURES 46–48 ), occasionally producing junctions of branches that could superimpose each other. Budding usually distal, giving rise to linear rows of variable length formed by chains of one to few and up to a dozen autozooids ( Figs 46, 47 View FIGURES 46–48 ). Rows usually straight or only gently curving. New branches borne from distolateral buds usually unilateral but sometimes formed symmetrically on both sides, at high angles to the parental zooid, in an almost cross-like pattern ( Fig. 48 View FIGURES 46–48 ). Additional, usually unpaired, branches occasionally formed proximolaterally. In a single occasion twin zooids were budded distally from the parental one ( Fig. 69 View FIGURES 65–69 ), nearly parallel and abutting each other.
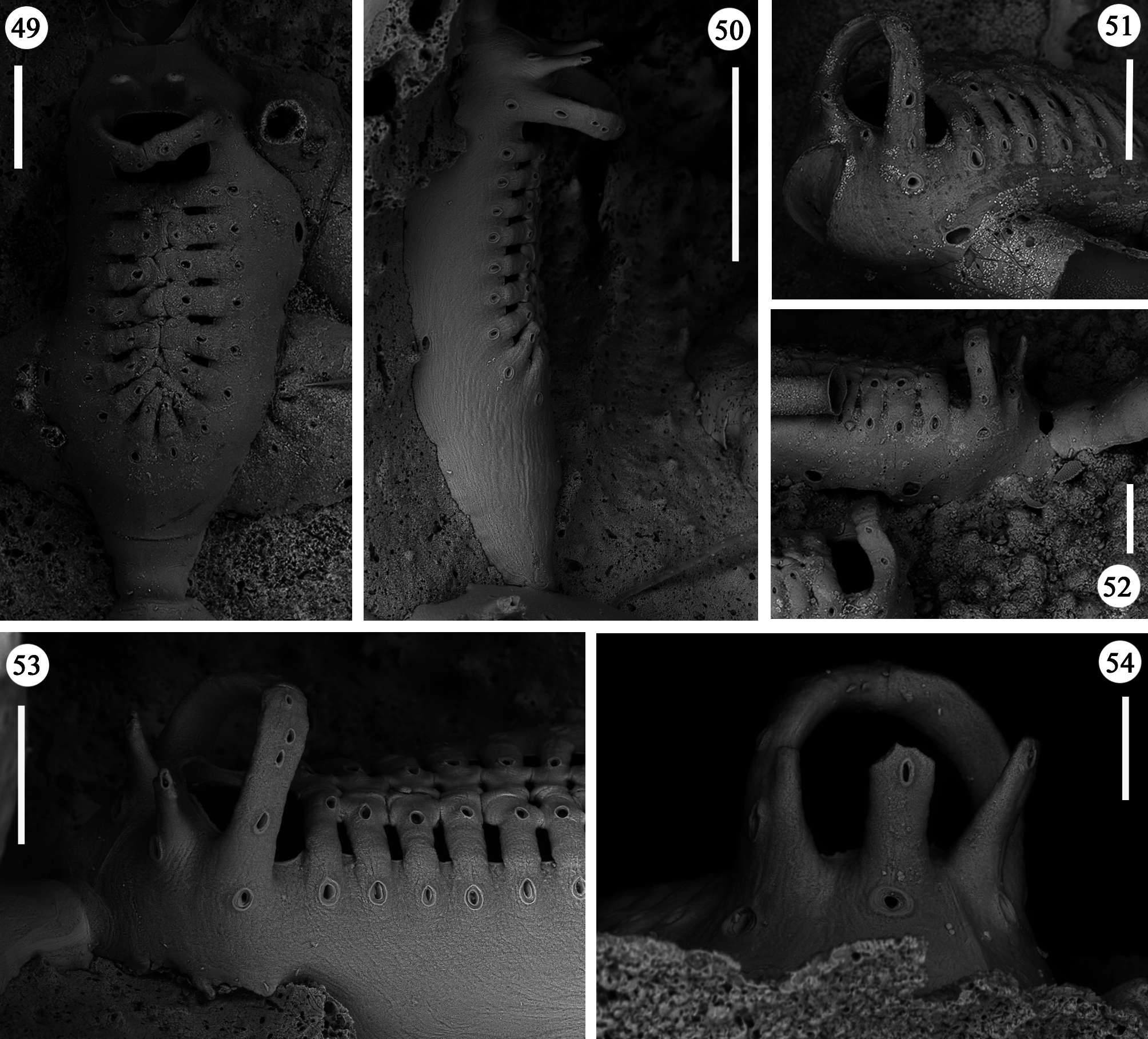
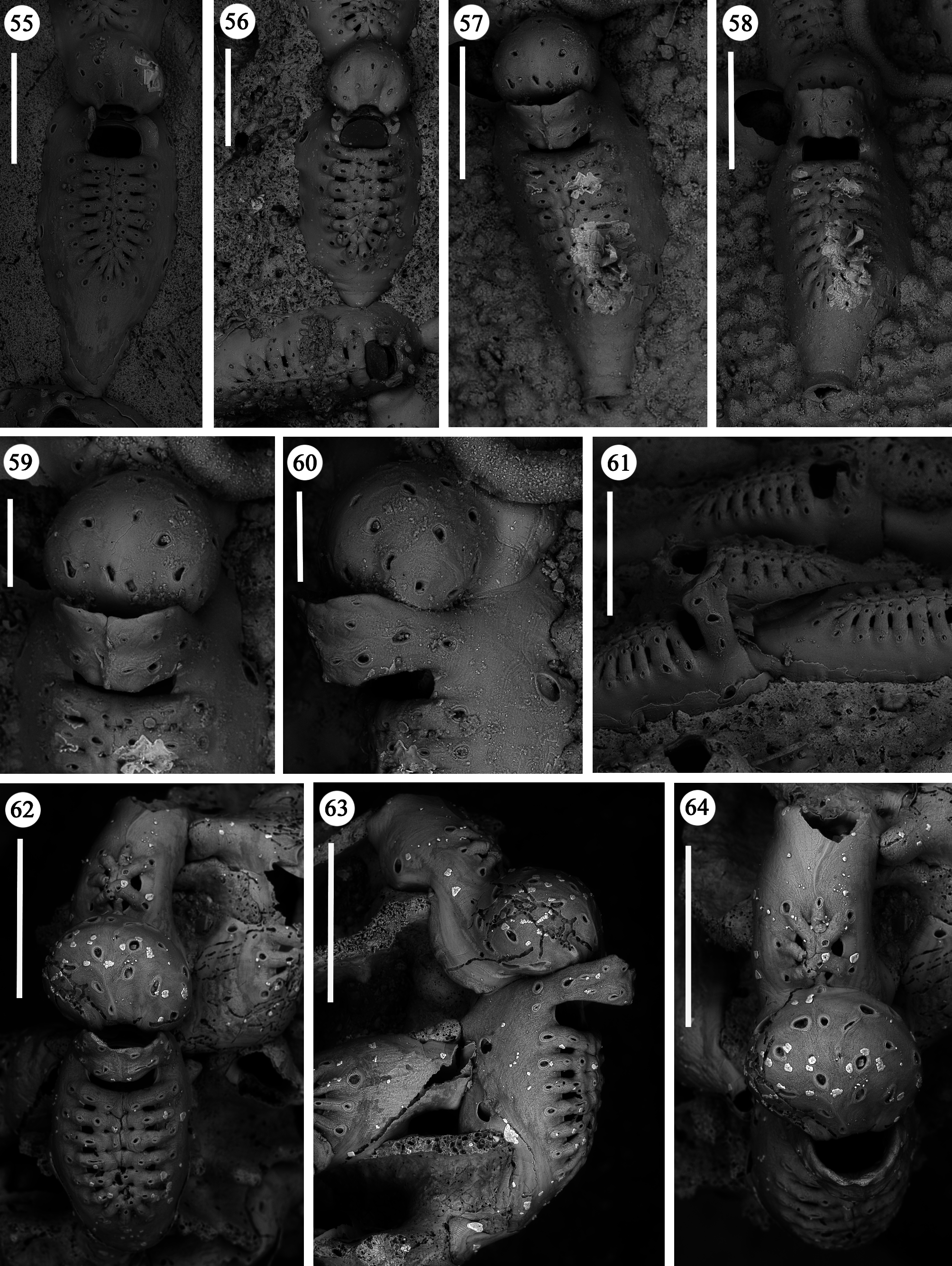
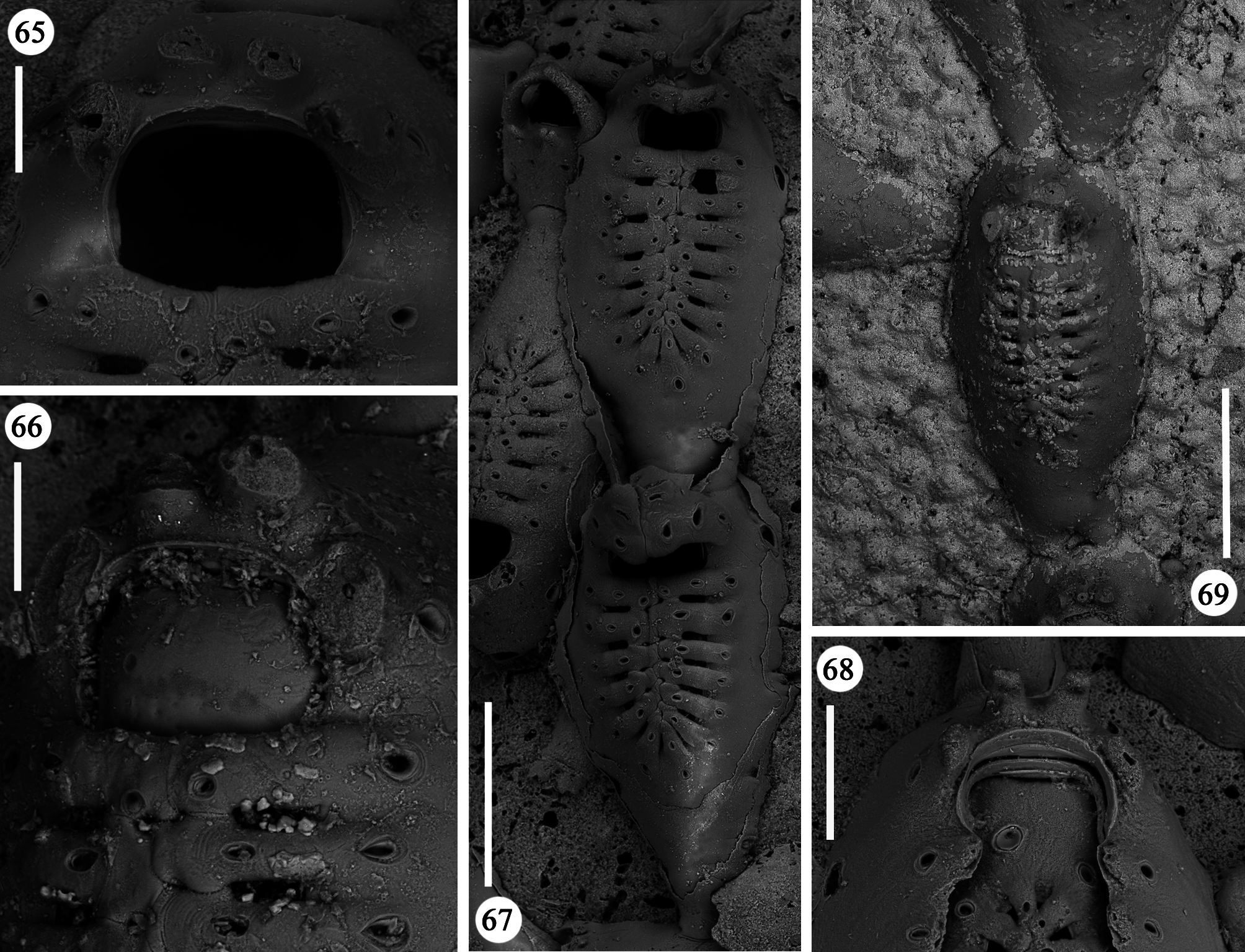
Zooids large (well-distinguished with the naked eye), club-shaped, with a short, proximal caudal portion and a longer, almost parallel-sided, dilated distal portion ( Figs 49, 50 View FIGURES 49–54 , 55–58 View FIGURES 55–64 , 67 View FIGURES 65–69 , 72, 74 View FIGURES 70–76 , 79–84 View FIGURES 79–84 ). Gymnocyst extensive, forming caudal portions; the lateral zooidal walls covering more than one-third of the frontal surface, slightly narrowing only around the orifice, but sometimes wide even distally. Interzooidal communication through a single, distal, basal pore-chamber with a tiny central window and two distolateral ones on each side at the level of the orifice, and 1–2 additional proximolateral dietellae, all visible by corresponding small round pore windows in the gymnocyst. Frontal shield elongated ovoidal, gently arching in continuity with the gymnocyst, formed by 14–19 (usually 16–17) large (60–90 µm wide) costae, parallel sided except for the more proximal ones that are wedgeshaped. Adjacent costae widely separated from each other, with a single large (30–50 µm) connecting bridge on both sides, close to zooidal midline, where further, usually very irregular connections occur between roughly fanshaped ends of costae meeting in an alternating pattern from the opposite sides and producing a somewhat zigzag suture, usually leaving two further small intercostal spaces. The resulting shield shows a regular peripheral row of intercostal spaces, about 70–100 µm long by 15–20 µm wide, and a median set of very small triangular to rounded lacunae, often absent between some costae, especially the proximal ones ( Fig. 67 View FIGURES 65–69 ). Each costa gently curving, flat and smooth, without any differentiation between basal and subsequent portion, bearing one large pelmatidium on its base (the proximal ones in a very proximal position), and a second slightly smaller one close to the fusion area ( Figs 49, 53 View FIGURES 49–54 , 66, 67 View FIGURES 65–69 ). Occasionally, additional pelmatidia on some costae, more often on the first suboral pair ( Fig. 67 View FIGURES 65–69 ). Pelmatidia ovoidal to irregularly shaped, infundibular, showing an internal ring of calcification. Suture lines between pelmatidia visible at high magnification on each costa. Orifice of non-ovicellate autozooids subrectangular, with blunt corners, a straight to gently concave proximal border, a slightly arched distal border and barely developed lateral constrictions ( Fig. 65 View FIGURES 65–69 ). Four robust, erect oral processes in non-ovicellate zooids ( Figs 49, 50, 52–54 View FIGURES 49–54 ), reduced to two in the ovicellate ones ( Figs 55, 56 View FIGURES 55–64 ); each process bearing a pelmatidium near the base and a second one near the tip, more or less flattened and non-articulated, revealing to be upward-directed costae consisting of c. 20 µm thick walls surrounding internal cavities of less than 10 µm in diameter ( Figs 65, 66 View FIGURES 65–69 ). The two distal processes are straight and slightly tapering upwards, sometimes compressed. Lateral processes levelled with distal corners of the orifice, long and with three pelmatidia, overarching above the orifice to eventually fuse along midline to form a handle-like structure some distance above it, slightly tilted proximally ( Figs 49–54 View FIGURES 49–54 ). This arched oral structure becomes much wider (up to about 200 µm) in ovicellate zooids ( Figs 57–60, 62, 63 View FIGURES 55–64 ), and forms a proximally inclined hanging roof above the orifice and the proximal part of the ooecium, leaving uncovered a larger subquadrangular proximal passage and a narrower semi-circular distal one above the ovicell.
Ovicells hyperstomial, acleithral. Ooecium formed by the distal autozooid ( Figs 55–60 View FIGURES 55–64 , 70 View FIGURES 70–76 , 81, 82 View FIGURES 79–84 ) [ type 1 sensu Ostrovsky (1998, 2008, 2013) and type A sensu Bishop & Househam (1987)], but occasionally (one single observation) by a distal kenozooid ( Figs 62–64 View FIGURES 55–64 ) [ type 1 sensu Ostrovsky (1998, 2008, 2013), and type B sensu Bishop & Househam (1987)]. Ooecium often (but not always) recumbent on the costal shield of the distal zooid, slightly wider than long, the surface smooth, bearing about 20 pseudopores (nearly 12 visible in frontal view, plus about eight near the base and only visible in lateral view); pseudopores large (up to 50 µm in their maximum dimension), irregularly shaped. Central part of ectooecium slightly deformed in some ovicells, with central swollen part bordered by two lateral furrows ( Figs 55, 56 View FIGURES 55–64 , 81, 82 View FIGURES 79–84 ).
Kenozooids of two different types: 1) irregularly triangular kenozooids, as large as autozooids, with a very extensive gymnocyst, usually with five budding loci as autozooids (only one of which seems to be active) and a centrally placed, round to oval shield, formed by a dozen costae or less, similar in the general appearance to autozooidal frontal shield but comparably smaller and exhibiting shorter costae and wider intercostal spaces ( Figs 70–73, 76 View FIGURES 70–76 ); 2) occasional, smaller (160 x 200 µm) kenozooids, more irregularly-shaped, with a frontal surface entirely consisting of gymnocyst and a centrally placed opesia at the end of a longitudinal suture ( Figs 74, 75 View FIGURES 70–76 ). Both types of kenozooids often placed between zooids belonging to different branches. Avicularia seemingly absent.
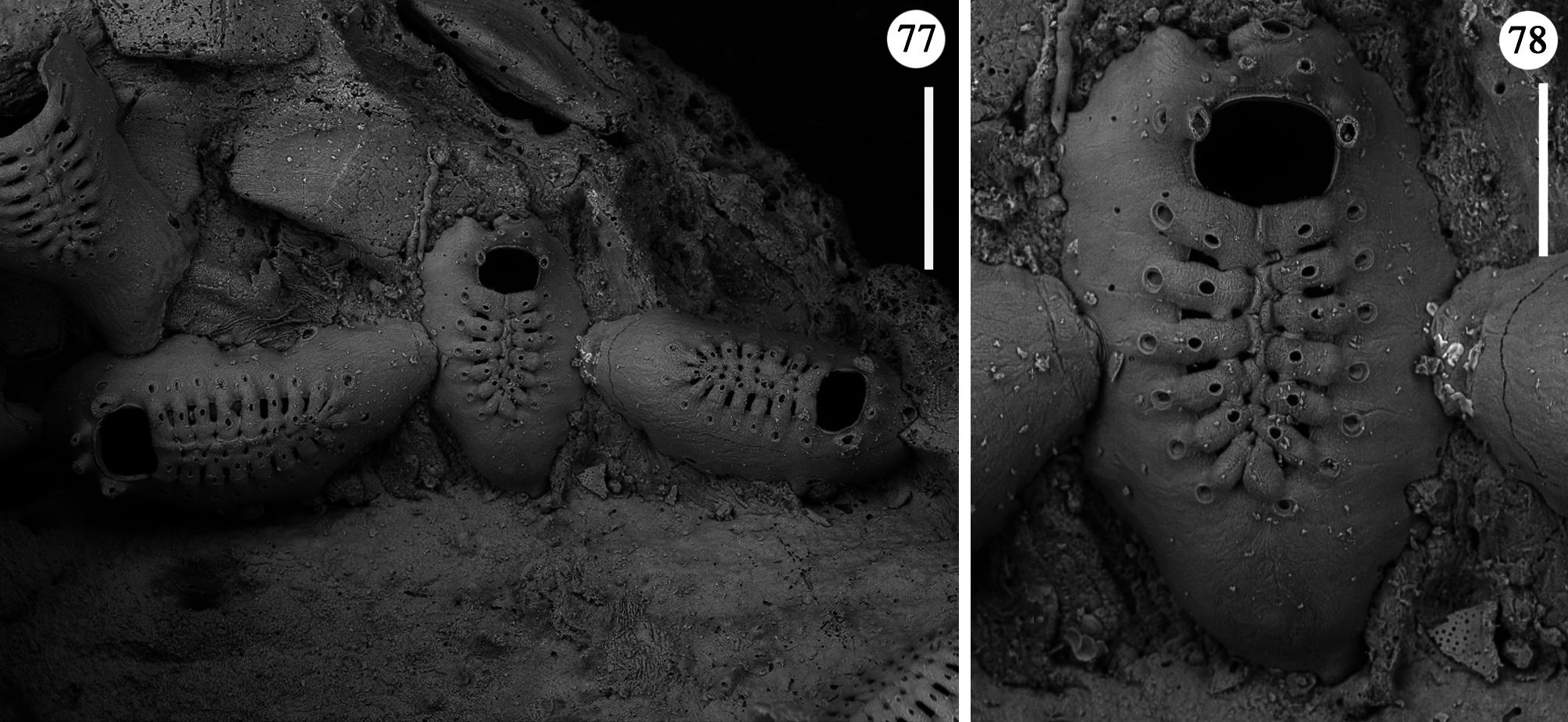
Ancestrula and periancestrular area hardly detectable in the colony network, surely observed only twice ( Figs 77, 78 View FIGURES 77, 78 ). Ancestrula smaller than autozooids, but comparable in morphology except for the short and proximally roundish gymnocyst, and its smaller frontal shield consisting of 13 costae. In the two observed ancestrulae, two zooids originate about at mid-length on each lateral side. Both show relatively short and non-tapering caudal parts, and have frontal shields of 14 costae, and a smaller size than subsequent autozooids.
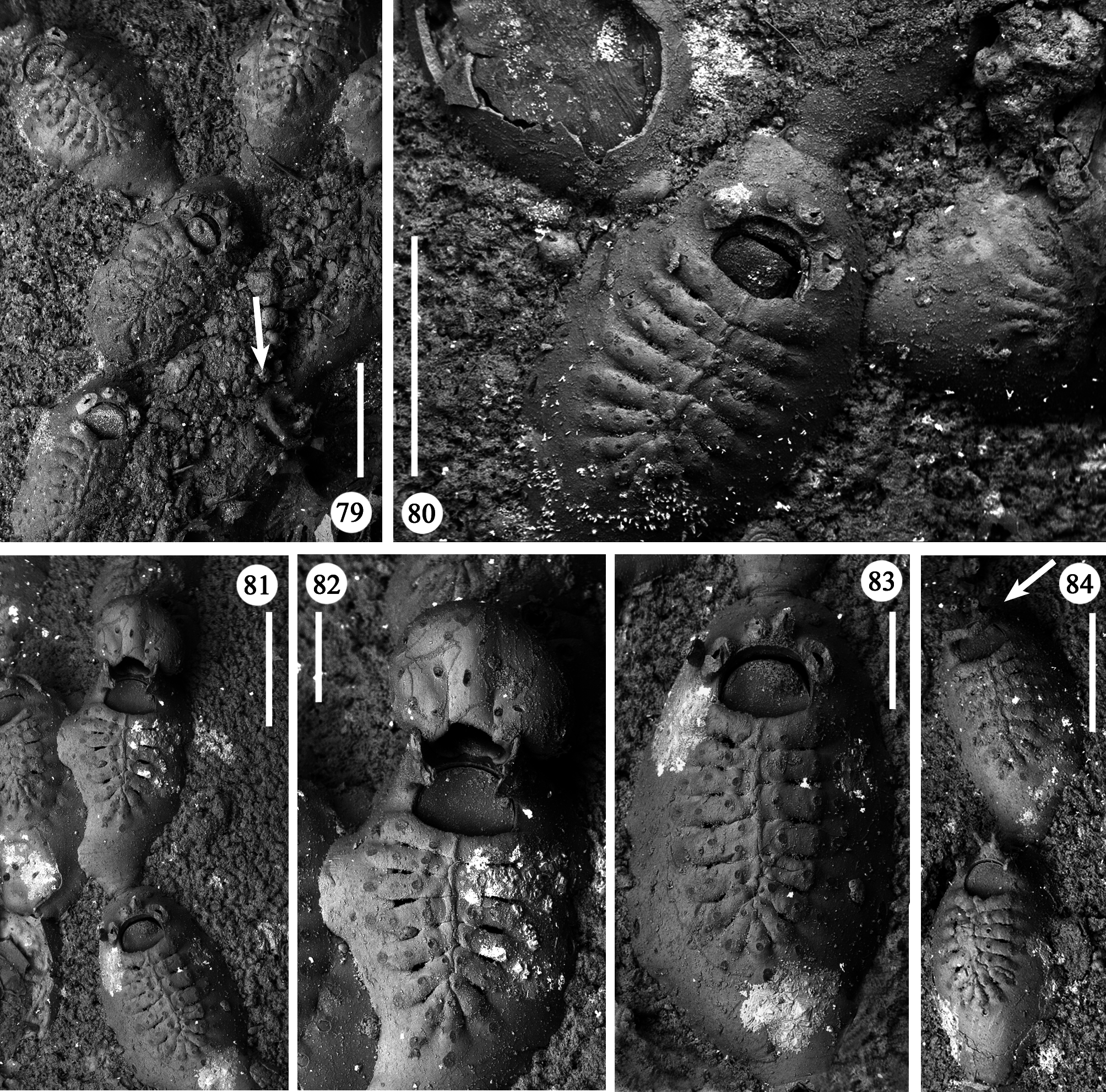
Remarks. Characters observed in the GBBS material fit well with the examination of the holotype ( Figs 79– 84 View FIGURES 79–84 ) and the description by Canu & Bassler (1928). Describing the ectooecial surface, these authors mention “a longitudinal keel and two lateral circular scars”, a character exaggerated in their figure ( Canu & Bassler 1928, pl. 3, fig. 5). In fact, it is not so pronounced (compare Figs 55, 56 View FIGURES 55–64 , 81, 82 View FIGURES 79–84 ). The "keel" is likely the result of a growth defect during the development of the ovicell. Plate 3, fig. 5 in Canu & Bassler (1928) also shows the arch formed by the lateral oral processes associated with the ovicell, which is now broken in the holotype ( Fig. 82 View FIGURES 79–84 ). Canu & Bassler (1928) described this characteristical arched structure as exclusively associated with ovicellate zooids but photos of the holotype point to its occurrence also in non-ovicellate zooids ( Figs 79, 84 View FIGURES 79–84 , arrowed), as frequently observed in our material.
A certain variability occurs in the number of autozooidal costae between the holotype [14–16 were counted in seven zooids (mean 15)], and the colonies from different stations (Station GeoB 16382-1: 15.7; Station GeoB 1638276-1: 16.7; Station GeoB 1638288-3: 17; Station GeoB 1638277-1: 17.5). However, no correlation was noted between the number of costae and the dimensions of the zooids. Occasionally, autozooids can be irregularly shaped, sometimes with lateral concave indentations, largely caused by the roughness of the encrusted substratum. Examples of regeneration include broken zooids with reparative budding (often intramural) of new autozooids ( Figs 61 View FIGURES 55–64 , 67 View FIGURES 65–69 ) or kenozooids ( Figs 68 View FIGURES 65–69 , 72, 76 View FIGURES 70–76 ), and are also visible as double-edged orifices ( Figs 68 View FIGURES 65–69 , 76 View FIGURES 70–76 ). Reparations also occur in the holotype. Ovicells are not common in the numerous colonies and/or colony fragments studied.
The function of the costal structure overarching the orifice was discussed by Canu & Bassler (1928: 38) who wrote that “protective influence of the apertural arch is rather difficult to understand: we have observed it only on the ovicelled zooecia; it must retard very much the extrusion of the tentacles.” However, in non-ovicellate zooids the arch is narrow and very high above the orifice, thus obviously not restricting the polypide eversion via the distal ‘passage’ made by the calcified arch and bounded distally by the distal oral processes. In most Cheilostomata the protruding lophophores are slightly inclined distally, and one could hardly imagine that in Teresaspis lineata n. comb. they would be tilted in opposite directions through the regulating action of this arched structure. This behaviour could be different from what is expected to happen in species, such as Gephyrotes nitidopunctatus ( Smitt, 1868) and Spiniflabellum spinosum ( Canu & Bassler, 1928) , which have similar peristomial structures (see above)
but exhibit even smaller proximal passages, possibly not suitable for tentacle eversion. The wide costal arch developing in ovicellate zooids covers most of the zooidal orifice, although still leaving a proximal sub-rectangular (about 100 µm long by 250 µm wide) passage and a distal semi-circular one above the ooecium. It is unknown if this structure hampers the polypide eversion, but probably not, since oviposition should occur by protruded lophophores in acleithral ovicells ( Ostrovsky 2013). The large size of the costal arch also covering the entrance of the brood chamber could be explained by a protection of developing embryos in the ovicell that would be otherwise separated from the external medium sector by the membranous ooecial vesicle only.
Two colonies from the GBBS possess ancestrulae and periancestrular area, and many have kenozooids that are seemingly missing in the holotype and were not described by Canu & Bassler (1928). Kenozooids include both a costate shield-bearing type, and a smooth-surfaced type with only a small opesia. The first type is supposedly a result of the abortion of the normal autozooidal development, possibly explained by the lack of sufficient space caused by the formation of the neighbour branch or failure of the normal development. Shield-bearing kenozooids commonly occur in several cribrilinid species and genera, but simple opesia-bearing kenozooids have been reported more rarely in some species, such as “ Cribrilina ” uniserialis Harmelin, 1978 ( Harmelin 1978) , Distansescharella alcicornis ( Jullien, 1882) ( Harmelin 1978; Harmelin et al. 1989), D. cervicornis Souto, Berning & Ostrovsky, 2016 ( Souto et al. 2016), and Inferusia taylori Kuklinsky & Barnes, 2009 ( Kuklinski & Barnes 2009), or figured though not described, as in Cinclidia aculeata Denisenko, 2018 ( Denisenko 2018) . Like in other uniserial and pauciserial cheilostome species for which comparable structures have been described (see Rosso & Taylor, 2002; Rosso et al., 2017, and references therein), in T. lineata n. comb., kenozooids sometimes serve to connect neighbouring zooids from different rows, as well as non contiguous zooids of the same curving row. Kenozooid sizes and general outlines seem related to available space, local surface morphology and developmental failure. Kenozooids are often located at the stand-off of a branch encountering zooids of a neighbouring row. Connections between zooids through lateral tubular extensions formed from basal pore-chambers were occasionally observed. Such connections are known in some other uniserial species with runner-like colonies [e.g. in the Cretaceous Unidistelopora krauseae ( Voigt & Schneemilch, 1986) ( Ostrovsky & Taylor 2004) ].
Periancestrular astogenetic pattern seemingly includes the budding of two midlateral zooids, both forming at high angle (100–110°) to the longitudinal axis of the ancestrula, i.e. directing slightly proximally ( Fig. 77 View FIGURES 77, 78 ). In the second ancestrula studied, although concealed beneath a thin layer of encrusting sponge, a distal bud was also present. Its absence in the first ancestrula ( Figs 77, 78 View FIGURES 77, 78 ) is interpreted as related to unsuitabe colonisable surface and/or the possible potential later activation of the ancestrula’s distal bud. This periancestrular budding pattern, which produces three main branches at high angles from each other, favours the rapid exploration of the available substratum all around ancestrula and is reminiscent of that observed in the Cretaceous cyclostome Voigtipora maconensis Taylor & McKinney, 2006 ( Taylor & McKinney 2006) with runner-like colonies. A trifurcate radial arrangement has been also described for the Jurassic species Pyriporopsis portlandensis Pohowsky, 1973 , although not directly resulting from the ancestrula’s trifurcate budding ( Taylor 1986). Further branch production in the colony seems to be governed by the location and activation of budding loci. Each zooid exhibits five potential budding loci: one distal, two distolateral and two proximolateral. However, out of the 355 analysed zooids, none was observed to produce five daughter zooids. Four active budding loci, always including the distal bud and three lateral ones, irrespective of the activation of both distolateral or proximolateral buds, were only occasionally observed (less than 1%). Usually (more than 63% of the cases), new zooids originated from only one distolateral bud plus the distal one, and more rarely (slightly more than 6%) from both distolateral buds plus the distal one.
Exclusive activation of the distal bud is also frequent, as more than 24% of zooids bud only a distal zooid. Nearly 4% of the zooids showed no budding at all. Most of them were kenozooids and several stopped against a previous branch or developed tubular extensions linking the parental branch to the encroached one ( Figs 70–73 View FIGURES 70–76 ). The resulting colony pattern thus includes long chains of zooids with few bifurcations and more rare cruciform trifurcations ( Figs 46–48 View FIGURES 46–48 ). This pattern could be functional to the exploitation of the surfaces offered by the elongated, highly convex and irregularly curved coral skeletons (which seem to constitute the usual substratum for this species). This arrangement substantially differs from the more regular, essentially cruciform pattern reported for the Campanian electrid Herpetopora laxata (d’Orbigny, 1852) encrusting large smooth surfaces offered by a Belemnitella guard ( Taylor 1988b), and for some present-day calloporids, such as Pyriporoides bathyalis ( Rosso & Taylor, 2002) , another uniserial bathyal species, exhibiting a basically cruciform pattern on surfaces larger than those offered by corals. The very rare activation of proximolateral buds in T. lineata n. comb. could prevent encounters with zooids of the parental branches, being incidentally used for spreading of the colony towards previously unexplored surfaces or to restore damaged colony portions. The uniserial running habit, apparently more common during the Cretaceous, seems to be infrequent in more recent cribrilinids, as it is known only for the fossil “ Puellina ” catena ( Wood, 1844), reported from the Pliocene Crag of England, and for the present-day “ Cribrilina ” uniserialis Harmelin, 1978 , from the Azores (see below).
Distribution. Teresaspis lineata n. comb. is presently known from a restricted sector of the western Atlantic from the GBBS (present paper) to the area immediately north of Cuba ( Canu & Bassler 1928). First reported from 708 m water depth, the present material, collected between 641–660 m, extends the species distribution to a slightly shallower zone. Owing to the considerable size of its zooids and the widespread colony network, which is easily visible with the naked eye, it is unlikely that this species goes unnoticed, but its distribution in deep settings, so far scarcely investigated in the GBBS area, presumably explains the absence of records of this species after its description by Canu & Bassler (1928). According to present knowledge, T. lineata n. comb. seems to be associated with deep-water corals, but although particularly abundant on them (especially Enallopsammia ) it also colonises the skeletons of other organisms including bryozoans, brachiopods, barnacles, foraminifera and the spicules of the sponge Aphrocallistes .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Ascophorina |
|
Family |
|
|
Genus |
Teresaspis lineata ( Canu & Bassler, 1928 )
| Rosso, A., Beuck, L., Vertino, A., Sanfilippo, R. & Freiwald, A. 2018 |
Cribrilina lineata :
| Winston & Maturo 2009: 1155 |
Cribrilina lineata :
| Harmelin 1978: 177 |
Cribrilina lineata
| Canu & Bassler 1928: 38 |
Aphrocallistes
| Gray 1858 |