Calotelea elegans (Masi, 1933)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3664.2.7 |
|
publication LSID |
lsid:zoobank.org:pub:C2464E06-B096-4D86-B755-D3741434D60F |
|
DOI |
https://doi.org/10.5281/zenodo.5624025 |
|
persistent identifier |
https://treatment.plazi.org/id/03B97808-FFAA-2361-30EB-A10E92E3FC4A |
|
treatment provided by |
Plazi |
|
scientific name |
Calotelea elegans (Masi, 1933) |
| status |
|
Calotelea elegans (Masi, 1933) View in CoL
(Figures: 5, 7, 8, 9, 10, 12, 13, 19, 20, 25, 26, 29, 34, 35, 38, 41, 44, 49, 52, 54, 55, 58–66, 68)
Baryconus fasciatipennis Sarra, 1930: 225 . Preoccupied by Lamproteleia fasciatipennis Kieffer, 1910 Ceratoteleia elegans Masi, 1933: 21 . Pegoteleia elegans: Bin, 1974: 457 . Pegoteleia fasciatipennis: Bin, 1974: 457 , 464. Calotelea sarrai Masner, 1976: 44 ; Mineo & Caleca, 1994: 132. Calotelea elegans: Masner, 1976: 44 ; Kononova & Kozlov, 2008: 286, 291.
Body size: 1.6–2.3 mm.
Female: Colour: highly variable ( Figures 58–66 View FIGURES 58 – 66 ), in some specimens body yellow, except for 2 pairs of small brown spots on the sides of T3 and T4 and the last five brown antennomeres. At the other extreme are specimens with the head and mesosoma dark brown and metasoma light brown, with dark spots on the sides of all tergites. In all specimens fore wing with two dark-brown transverse bands. In one specimen, antenna, except scape and radicle, entirely dark-brown. Legs yellow, except distal parts of femora and tibiae, which are brown.
Head shape, in dorsal view transverse ( Figure 19 View FIGURES 15 – 20 ), WH 1.4–1.7 times LH, 1.1–1.3 times width of mesoscutum. Hyperoccipital carina absent. Occipital carina present, sharp and conspicuous. Compound eye large, silvery, glabrous ( Figures 19–20 View FIGURES 15 – 20 ). LE 1.6–2.0 times Lt, HE 1.2–1.3 times LE, 1.6–1.9 times length of cheek. Inner orbits diverging. OOL 0.2–06 times length of TDPO, POL 1.4–2.0 times LOL. Distance between compound eyes (measured at level of anterior ocellus) 1.3–1.6 times POL. Orbital carina absent; frontal depression absent, submedian carina absent; antennal scrobe absent; frons without central keel ( Figure 29 View FIGURES 27 – 35 ). Interantennal prominence triangular, not prominent, torulus opening on anterofrontal surface of prominence. Malar sulcus present, genal carina absent ( Figure 29 View FIGURES 27 – 35 ). Facial striae present, inconspicuous, short, radiating from the base of the mandible. Clypeus short, almost rectangular with rounded corners and 4–6 long hairs ( Figure 29 View FIGURES 27 – 35 ).
Sculpture of head: coriaceous to alutaceous.
Antenna 12-segmented ( Figure 34 View FIGURES 27 – 35 ). Radicle long, well developed ( Figure 29 View FIGURES 27 – 35 ). LA1 4.0–4.3 times wA1, 2.4– 2.6 times length of radicle and 2.6–2.7 times LA2. LA2 2.3–2.5 times wA2 and 1.0–1.3 times LA3. LA3, 2.0–3.3 times wA3 and 1.0–1.3 times LA4. LA4 2.0–2.5 times wA4 and 1.5–2.2 times LA5. A4 have the same width as A5. LA5 1.0–2.0 times wA5 and 1.0–1.5 times LA6. LA6 equal with LA7 and 0.8–1.0 times wA6. LA12 1.2–1.6 times wA12 and 1.2–1.3 times LA11. Mesosoma short and convex, length of mesoscutum 0.8–1.2 times width. Dorsal margin of mesosoma, convex in lateral view ( Figures 20 View FIGURES 15 – 20 , 41 View FIGURES 36 – 55 ).
Dorsal epomial carina absent, pronotal shoulders not developed. Longitudinal epomial carina absent. Cervical pronotal area small, oblique in lateral view, largely hidden in dorsal view ( Figure 38 View FIGURES 36 – 55 ). Lateral pronotal area broad, flat; propleural epicoxal sulcus distinct, crenulate. Pronotal suprahumeral sulcus not conspicuous and not crenulate. Dorsal pronotal area absent. Netrion conspicuous, open, carinate, smooth and lustrous, its anterior margin flanked by a row of foveolae. Central part of netrion, barely convex, almost flat ( Figure 41 View FIGURES 36 – 55 ).
Mesoscutum convex, 2.3–4.0 times Lscut. Skaphion present, with conspicuous carina ( Figure 38 View FIGURES 36 – 55 ). Anteroadmedian lines absent. Notauli absent. Humeral and suprahumeral sulci deep and narrow, indistinct. Parapsidal line present, short ( Figure 38 View FIGURES 36 – 55 ). Longitudinal median mesoscutal line absent. Parascutal carina present. Sculpture of mesoscutum imbricate coriaceous. Transscutal articulation deep and conspicuous. Scutoscutellar sulcus, foveolate laterally. Scutellum transverse, Wscut 1.8–2.5 times Lscut. Mesoscutellum convex, with coriaceous sculpture, axillae crenulate, unarmed, posterior rim crenulate. Metascutellum short and broad, barely visible in dorsal view, not produced ( Figure 38 View FIGURES 36 – 55 ). Mesopleuron glabrous ( Figure 41 View FIGURES 36 – 55 ). Prespecular sulcus with conspicuous oval foveae, continuous with long mesopleural carina. Transpleural sulcus not visible. Speculum smooth and lustrous without complete transverse ridge. Femoral depression smooth and lustrous, long, relatively narrow where it meets the mesepimeral sulcus ( Figure 41 View FIGURES 36 – 55 ). Dorsal femoral depression, near speculum with a large, circular pleural pit. Posterodorsal corner of mesopleuron obtuse. Mesepimeral sulcus crenulate, with small, dense foveae. Posterior mesepimeral area broad and lustrous. Sternaulus indistinct. Propodeum barely visible in dorsal view because horn of T1 totally covers the metasomal depression ( Figures 19, 20 View FIGURES 15 – 20 , 38, 41 View FIGURES 36 – 55 ). Lateral propodeal area triangular, externally with short, silvery hairs. Lateral propodeal carina distinct, not crenulate.
Metapleuron weakly convex, smooth and lustrous, divided by metapleural sulcus into a large dorsal and small ventral areas. Metapleural sulcus conspicuous, large, metapleural pit indistinct ( Figures 20 View FIGURES 15 – 20 , 41 View FIGURES 36 – 55 ).
Macropterous, fore wing covered with short, dense microtrichia, with two dark-brown transverse bands; first band at the level of basal vein and second at stigmal vein; fore wing apex infuscate ( Figures 19–20 View FIGURES 15 – 20 , 58–66 View FIGURES 58 – 66 ); fore wings narrow, Lfw 4.0–4.3 times wFw, 1.1–1.2 times Lhw, 3.6–4.3 times width of mesosoma; submarginal, marginal, postmarginal and stigmal veins tubular; basal vein nebulous. Lpmv 2.2– 3.1 times Lmv. Lmv 0.9–1.4 times Lsv.
Lhw 7.1–8.3 times whw, hind wings with 3 hamuli and complete submarginal vein. Marginal fringe well developed, whw 2.1–3.0 times length of marginal fringe.
Metasoma elongate, fusiform, with 7 tergites ( Figures 58–66 View FIGURES 58 – 66 ), the last tergite extruded or retracted with ovipositor. Laterotergites well developed, narrow. Length of metasoma 3.0–4.3 times width. T1 longitudinally costate except for horn; horn rugose with smooth sculpture ( Figures 38, 44, 54–55 View FIGURES 36 – 55 ); LT1 1.2–1.7 times wT1min, ratio between wT1max and wT1min 1.1 – 1.5. LT2, 1.2–1.6 times LT1, wT2max 1.0–1.5 LT2, ratio between wT2max and wT2min 1.8–2.3. Sculpture of T2 with longitudinal striae, in some specimens strongly, almost costate, in others smooth, striate only at base of T2 ( Figures 54–55 View FIGURES 36 – 55 ). T3 the longest tergite, LT3 1.2–1.7 times LT2 and 1.1–1.6 times LT4. wT3max 0.9–1.2 times LT3, ratio between wT3max and wT3min 1.1–1.2. T3 imbricatecoriaceous to reticulate medially. LT4 1.5–2.1 times LT5 and LT5 1.1–1.7 times LT6. Ratio between wT4max and wT4min 1.2–1.4 and ratio between wT5max and wT5min 1.4–1.9. LT6 0.5–0.9 times wT6max. Sculpture of T4– T6 similare for T3. T1 laterally with many silvery hairs. T2 and T3 with hairs laterally, but also each with a row of hairs posteriorly; T4–T6 with sparse hairs all over.
Male: differs from female in body colour, and antenna structure. Body colour dark-brown ( Figures 25–26 View FIGURES 21 – 26 ). LA1, 3.5 times wA1, 3.0 times LA2 and 1.5 times LA3. LA3 equal to LA5 and shorter than LA4 (LA3, 0.8 times LA4). LA5, 1.2 times LA6. Lscut, 2.6–2.8 times length of metascutellum. Metascutellum not produced as a lamina ( Figures 49, 52 View FIGURES 36 – 55 ). Metasoma elongate, LT3, 1.1–1.2 times LT2, 2.8 times LT1 and 1.5–1.6 times LT4. LT4, 1.4 times LT5 and 2.3 times LT6.
Material examined. GREECE: 1 Ƥ, Lake Kerkini, Stratiom site, 5–11.v.2008, N41º17’44.9” E23º17’36.6”, leg. G. Ramel, Malaise trap (OPPC); 2 Ƥ, Lake Kerkini, Ecotourism site, 19–25.ix.2006, N41º18’15.6” E23º13’1.2”, leg. G. Ramel, Malaise trap (OPPC); 3 Ƥ, Lake Kerkini, Ecotourism site, 29.viii–4.ix.2006, N41º18’15.6” E23º13’1.2”, leg. G. Ramel, Malaise trap (OPPC); 1 Ƥ, Lake Kerkini, Petritsi site, 8–14.ix.2008, N41º17’43.7” E23º17’12.6”, leg. G. Ramel, Malaise trap (OPPC); 1 Ƥ, Lake Kerkini, Ecotourism site, 2.v– 8.v.2006, N41º18’15.6” E23º13’1.2”, leg. G. Ramel, Malaise trap (OPPC); 1 Ƥ, Lake Kerkini, Ecotourism site, 9– 15.v.2006, N41º18’15.6” E23º13’1.2”, leg. G. Ramel, Malaise trap (OPPC); 1 Ƥ, Elea near Kyparissia, 29.viii.1979, leg. Bouček (NMPC); 7 Ƥ & 1 3, Rhodes, Ixia, 1529. viii.1984, leg. M.C. Day, Malaise trap (CNCI); 1 Ƥ, Corfu, Velonades, 30.viii.1987, leg. J.S. Noyes (CNCI); 1 Ƥ, Kos Kos town, 28–29.viii.1994, leg. J.S. Noyes (CNCI); 1 Ƥ, Rhodes, Ixia, 9–22.viii.1985, leg. M.C. Day, yellow pan trap (CNCI); 1 3, Lithotopos Village, N41°07’52,2” E23°12’53,3”, 28.viii.–4.ix.2004, leg. G. Ramel, Malaise trap, (OPPC); 1 3, Lithotopos Village, N41°07’52,2” E23°12’53,3”, 19.ix.–25.ix.2004, leg. G. Ramel, Malaise trap, (OPPC); 1 3, Lithotopos Village, N41°07’52,2” E23°12’53,3”, 26.ix.–2.x.2004, leg. G. Ramel, Malaise trap, (OPPC); 1 3, Krousia Mts site N41°11’32,4”, E23°03’59,5”, 22–28.viii.2007, leg. G. Ramel, Malaise trap (OPPC); 1 3, Corfu, Velonades, 30.viii.1987, leg. J.S. Noyes (CNCI); 1 3, Corfu, 10.x.2001, N39,28.68 E19,53.11, leg. M. v. Tschirnhaus, eclector (OPPC).
FRANCE: 1 Ƥ, Var, 8 km s. St Tropez, 19.viii.1988, leg. Bouček (NMPC); 1 Ƥ, Var, 8 km s. St Tropez, 2.ix.1986, leg. Bouček (NMPC); 1 Ƥ, Montpellier, 2–9.x.1979, leg. J.T. Huber, pan trap (BNMH); 1 Ƥ, Var, 8 km s. St Tropez, 5.ix.1986, leg. Bouček (BNMH); 1 Ƥ, Bouches-du-Rhône, Fonscolombe, 23.viii.1986, leg. M.W.R. de V. Graham (BNMH); 1 Ƥ, Hérault Montpellier, 39. ix.1980, leg. J.F. Vayssières, yellow pan trap (CNCI); 1 Ƥ, Grabels, Dépt. Hérault, 15.ix.1979, leg. J.T. Huber (CNCI); 1 Ƥ, Montpellier, 2–9.x.1979, leg. J.T. Huber (CNCI); 2 Ƥ, Montpellier, 22–25.ix.1979, leg. J.T. Huber (CNCI); 1 Ƥ, Hérault, Balliarguet, CSIRO Laboratory, N43º41’12” E3º62’24” E, 10–18.iv.1993, leg. P. Mason, Malaise trap (CNCI); 1 Ƥ, Hérault, Balliarguet, CSIRO Laboratory, N43º41’12” E3º62’24” E, 18.iv–3.v.1993, leg. P. Mason, Malaise trap (CNCI); 1 Ƥ, Montpellier, 15– 19.ix.1978, leg. J.T. Huber, pan trap (CNCI); 1 3, Montpellier, 29.x.1979, leg. J.T. Huber, pan trap (BMNH); 1 3, Montpellier, 8–14.ix.1979, leg. J.T. Huber, pan trap (CNCI); 1 3, Montpellier, 15.v.1980, leg. J.T. Huber, pan trap (CNCI); 1 3, Montpellier, 14–18.ix.1979, leg. J.T. Huber, pan trap (CNCI); 1 3, Montpellier, 22–25.ix.1979, leg. J.T. Huber, pan trap (CNCI).
MONTENEGRO: 1 Ƥ & 2 3, Cela,?. viii.1986, leg. N.D. Springate (CNCI).
CROATIA: 1 3, Orsac, 27.viii.2007, leg M. Mitroiu, swept (OPPC).
SPAIN: 1 Ƥ, Mallorca, 5km ESE Polenca, 21.viii.1996, leg. J.S. Noyes (CNCI); 4 3, Mallorca, Polenca, 28.viii.1996, leg. J.S. Noyes (CNCI).
ITALY: 1 Ƥ, Savona, Ortovero, near Albenga, 5.x.1969, leg. Bouček (NMPC); 1 Ƥ, Savona, Ceriale, near Albenga, 3.ix.1972, leg. Bouček (BNMH); 1 Ƥ, Calabria, Capo Vaticano, 18.viii.1988, leg. J.S. Noyes (CNCI); 3 Ƥ, Sardinia, Tempio Pausania, 9–16.x.1978, leg. F. Bin, Malaise trap (CNCI); 1 Ƥ, Sardinia, Tempio Pausania, 28.v–5.vi.1978, leg. F. Bin, Malaise trap (CNCI); 1 Ƥ, Sardinia, Tempio Pausania, 28.viii–4.ix.1978, leg. F. Bin, Malaise trap (CNCI); 1 Ƥ, Calabria, 15.viii.1988, leg. J.S. Noyes (CNCI); 2 Ƥ, Sardinia, Tempio Pausania, 11– 18.ix.1978 (CNCI); 1 3, Savona, Ceriale, near Albenga, 3.ix.1972, leg. Bouček (BNMH); 3 Ƥ and 3 3, Matera, 1930 (paralectotype of Baryconus fasciatipennis Sarra, 1930 ) (IEFS); 1 Ƥ Isl. Capraia?. vi.1931, F. Capra-C. Mancini (type of Ceratoteleia elegans Masi, 1933 ) (MCSN); 3 Ƥ, Guspini, 3.x.1933, reared from Asphodelus (CNCI); 1 3, Sicilia, Madonia,?. vi.2009, leg. P. Jansta, swept, (CNCI); 1 3, Sardinia, Tempio Pausania, 29.v– 5vi.1978, leg. F. Bin, Malaise trap (CNCI); 1 3, Sardinia, Tempio Pausania 25.ix–2x.1978, leg. F. Bin, Malaise trap, (CNCI); 2 3, Sicily, Balestrate, 2.x.1981, leg. G. Mineo, pan trap, (CNCI); 1 3, Perugia, 28.vi.1976, leg. F. Bin (CNCI); 1 3, Sardinia, Tempio Pausania, 2029. v.1978, leg. F. Bin, Malaise trap, (CNCI); 1 3, Sardinia, Tempio Pausania, 916. x.1978, leg. F. Bin, Malaise trap (CNCI); 1 3, Sardinia, Tempio Pausania, 1118. ix.1978, leg. F. Bin, Malaise trap (CNCI); 2 3, Sardinia, Guspini, 1933, reared from Asphodelus (CNCI).
ROMANIA: 1 3, Constanţa, Agigea marine dune reservation, 1.x.2004, N44º05’11.1” E28º38’31”, leg. I. Popescu, swept, (OPPC); 1 Ƥ, Constanţa, Agigea marine dune reservation, 14.ix.2006, N44º05’11.1” E28º38’31”, leg. O. Popovici & C. Ailenei, yellow pan trap (OPPC); 2 3, Constanţa, Agigea marine dune reservation, 15– 16.ix.2006, N44º05’11.1” E28º38’31”, leg. O. Popovici & L. Fusu, yellow pan trap (OPPC).
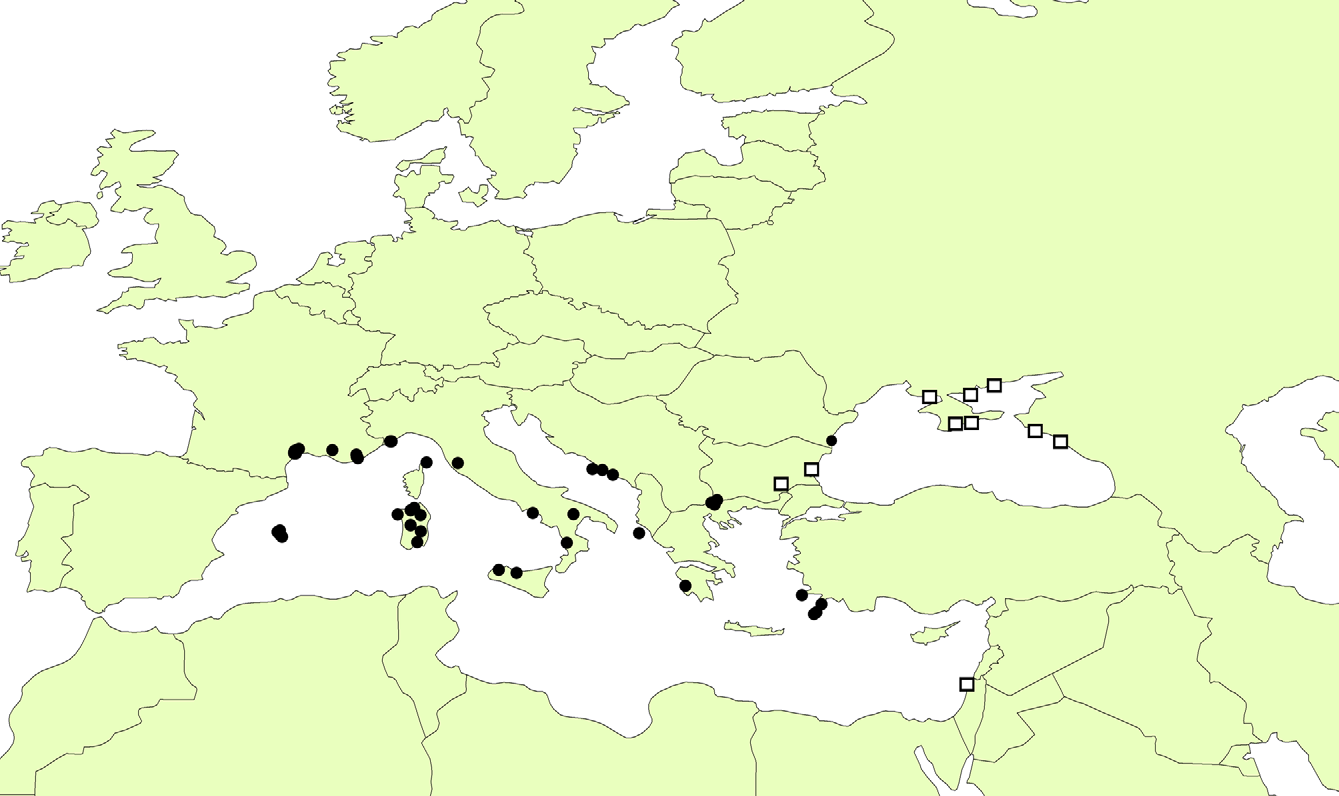
Distribution: the available data indicate that Calotelea elegans is a Palearctic species with a Ponto- Mediterranean distribution, the distribution of this species are mapped in Figure 68 View FIGURE 68 .
Discussion
The present study has shown that Calotelea is poorly represented in Europe by just three species. All three are found in the Mediterranean area, although Calotelea elegans extends into the region bordering the Black Sea (i.e. Ponto-Mediterranean), however these ranges may well be extended by further collecting. The main problems encountered in the taxonomy of this genus in Europe result from previous misunderstandings of some morphological terms and over the concept of Calotelea sensu Ashmead. Amongst European species, two show sexual dichromatism and the other is the same colour in both sexes. In species with sexual dichromatism, the females are lighter than males and show a disruptive colour pattern with transverse dark wing bands. This kind of colour is unusual among European Platygastroidea, but is often found in tropical platygastroids. For Calotelea elegans , the fore wing pattern is reinforced by the metasomal pattern, and in some specimens also the legs. In the darker males, the fore wing is only weakly banded at the level of the basal vein. In Calotelea carbonaria , although it is a melanic species, the fore wings have dark-brown transverse bands, the disruptive effect being less. However in Calotelea laminata , the fore wings are without transverse bands, the body pattern still produces a disruptive effect. In its body colour, Calotelea elegans is close to Calotelea laminata , but closer to Calotelea carbonaria in the colour of fore wings. Even though the body colour and the structure of the metascutellum show Calotelea carbonaria is clearly different from Calotelea elegans , the statistical analysis (traditional and geometric morphometric analysis) placed these species very close to one another and less similar to Calotelea laminata . Indeed the males of these two species are morphologically very close, the main character of separation being the sculpture of T2 and the structure of the aedeago-volsellar complex, which is different in these two species. The main differences between females of Calotelea laminata and Calotelea elegans , with respect to Calotelea carbonaria is in the metascutellum and T2 sculpture. The same differences are found for males, but the differences between the aedeago-volsellar complexes are striking. Characters of the aedeago-volsellar complex have been used previously in the taxonomy of platygastroids, Fabritius (1974), Johnson (1984), Johnson & Bin (1982, 1988), Nixon (1935, 1940, 1958), Mineo (1979) showed the utility of this character in the systematics and taxonomy of platygastroids. However, Kozlov & Kononova (1983) point to the lack of utility of external male genitalia in the taxonomy of Palaearctic Trissolcus . Mikó (personal communication), has also found it very useful for the taxonomy of Palaearctic Trimorus . For females, both traditional morphometric and geometric morphometric analysis have shown differences between Calotelea laminata and of the other two Calotelea . Kononova (2001) considers that the hosts have a strong influence on the morphology of parasitoids, and Kononova and Fursov (2007) showed the influence of two different hosts ( Planaeschna milnei and Aeshna nigroflava (Insecta: Odonata )) on the phenotype of Calotelea shimurai . Taking into account the differences in shape of the metasoma among the sympatric European species of Calotelea , we can speculate that although they are found in the same kinds of habitat and often at the same time, they use different hosts. The flight period of Calotelea carbonaria and Calotelea laminata is from August to October, whereas for Calotelea elegans there are two flight periods: April- June and August–October. Possibly specimens from April–June have emerged from hibernation, however it seems more likely that there are two generations because the first flight period covers almost three months and because males are found then. Morphologically there are no notable differences between specimens from these two generations. All specimens of the first generation have T2 distinctly longitudinally striated. Specimens showing this character are also present in the second generation, but a lot of second generation specimens also have the sculpture of T2 evident only at the base of T2 which is otherwise smooth. Specimens with an intermediate sculpture on T2 are found in the second generation.
Distribution: Calotelea elegans seems to have the widest distribution of the European species of Calotelea . Although this species was noted before under various names (see synonymy) from Italy (Sarra, 1930; Masi, 1933; Bin et al.; Kononova & Kozlov, 2008), Romania (Fabritius, 1973; Fabritius & Popovici, 2007; Popovici, 2007) and Bulgaria, Ukraine and Russia (Kononova & Kozlov, 2008) it is recorded here for the first time from Greece, Spain, Croatia, Montenegro and France (Maneval, 1940 recorded the genus Pegoteleia from France without a species).
Although the biology of Calotelea is poorly known, we examined three females from Guspini ( Italy) reared from Asphodelus (Asphodelaceae) . We have also seen Triteleia peyerimhoffi with the same collection data (Popovici et al., 2011).
Masner, (1980a), proposed the ocularis group for species of Calotelea with the metanotal lamina (or plate) produced. Previously this group included only Nearctic and Neotropical species. Calotelea laminata is the first recorded and only European species of Calotelea belonging to the ocularis species group. Calotelea elegans belongs to another group characterized by the absence of a metanotal lamina—the elegans group. The affinity of Calotelea carbonaria for one of these two groups is debatable (although the female has a metanotal lamina, the male does not; also all statistical analyses undertaken here place this species close to Calotelea elegans ). So, to clarify the species group placement of Calotelea carbonaria , it will be necessary to analyze the Old World species of Calotelea in a future study.
Acknowledgements
The senior author thanks to Gordon Ramel, for his huge effort in collecting and sorting samples from Greece; to Dr John Noyes, Dr Ferdinando Bin, Dr John Huber and Dr Michael von Tschirnhaus for the gift of valuable specimens. Thanks are due to Dr Andrey Khalaim and Dr Yulia Astafurova for providing photos of type material of Calotelea affinis Kozlov and Kononova ; to Dr Jan Macek for his help with the material housed in NMPC; to Dr Giuliano Doria and Dr Maria Tavano for the type material from MCSN; to Dr Marco Gebiola and Dr Bruno Espinoza for their help with the type material from IEFS; also his colleagues and friends Dr L. Fusu and Dr M. Mitroiu for collaborative collecting and travel; Dr Luciana Musetti for her invaluable help with information about specimens from ZMAS and UASK; Dr I. Moglan, Dr M. v.Tschirnhaus and Catrinel Damian for their support; Dr I. Gostin for allowing the senior author access to her laboratory; Dr Popescu Irinel for the gift of specimen; Baltag Emanuel for his suggestions regarding the maps; Dr. N. Johnson and Dr L. Musetti for access to The genera of Platygastroidea ( Hymenoptera ) of the World and Hymenoptera on-line database, valuable resources for any Platygastroidea student. Popovici Ovidiu is supported by POSDRU/89/1.5/S/63663 and Synthesys grant (GB-TAF- 1303); Popovici Mariana is supported by POSDRU/89/1.5/S/49944.
References
Agassiz, L. (1846) Nomenclatoris zoologici index universalis, continens nomina systematica classium, ordinum, familiarum et generum animalium omnium, tam viventium quam fossilium, secundum ordinem alphabeticum unicum disposita, adjectis homonymiis plantarum, nec non variis adnotationibus et emendationibus. Jent & Gassmann, Soloduri, 393 pp.
Ashmead, W.H. (1893) A monograph of the North American Proctotrypidae. Bulletin of the United States National Museum, 45, 1–472.
http://dx.doi.org/10.5479/si.03629236.45.1
Ashmead, W.H. (1894) Report on the parasitic Cynipidae, part of the Braconidae, the Ichneumonidae, the Proctotrypidae, and part of the Chalcidinae. Part III. Zoological Journal of the Linnean Society of London, 25, 188 –254.
Ashmead, W.H. (1900) Report upon the aculeate Hymenoptera of the islands of St Vincent and Grenada, with additions to the parasitic Hymenoptera and a list of the described Hymenoptera of the West Indies. Transactions of the Royal Entomological Society of London, 1900, 207 –367.
http://dx.doi.org/10.1111/j.1365-2311.1900.tb02379.x
Ashmead, W.H. (1903) Classification of the pointed-tailed wasps, or the superfamily Proctotrypoidea. - III. Journal of the New York Entomological Society, 11, 86–99.
Austin, A.D. & Field, S.A. (1997) The ovipositor system of scelionid and platygastrid wasps (Hymenoptera: Platygastroidea): comparative morphology and phylogenetic implications. Invertebrate Taxonomy, 11, 1–87. http://dx.doi.org/10.1071/IT95048
Austin, A.D., Johnson, N.F. & Dowton, M. (2005) Systematics, evolution and biology of scelionid and platygastrid wasps. Annual Review of Entomology, 50, 553 –582.
http://dx.doi.org/10.1146/annurev.ento.50.071803.130500
Bernardo, U., Monti, M.M., Nappo, A.G., Gebiola, M., Russo, A., Pedata, P.A. & Viggiani, G. (2008) Species status of two populations of Pnigalio soemius (Hymenoptera: Eulophidae) reared from two different hosts: An integrative approach. Biological Control, 46, 293 –303.
http://dx.doi.org/10.1016/j.biocontrol.2008.05.009
Bin, F., Caleca, V., Casale, A., Mineo, G. & Pagliano, G. (1995) Checklist delle specie della fauna italiana. Hymenoptera Proctotrupoidea, Ceraphronoidea. Calderini, Bologna, 98, 1–19.
Boldaruyev, V.O. (1969) Egg parasites of the subfamily Telenominae (Hymenoptera, Scelionidae), reared from the eggs of harmful insects. Trudy Buryatskogo Instituta Estestvennykh Nauk Buryatskii Filial Siirskogo Otdeleniya Akademii Nauk SSSR, 7, 156 –171.
Bookstein, F.L. (1991) Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge, 435 pp.
Bookstein, F.L. (1997) Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Medical Image Analysis, 1 (3), 1–225.
http://dx.doi.org/10.1016/S1361-8415(97)85012-8
Brues, C.T. (1908) Hymenoptera. Fam. Scelionidae. Genera Insectorum, 80, 1–59.
Brullé, A. (1846) Histoire naturelle des insectes. Hyménoptères. Tome quatrième. Librairie Encyclopédique de Roret, Paris, 680 pp.
Cameron, P. (1912) On the parasitic Hymenoptera reared at Dehra Dun, northern India, from the lac (Tachardia) and sal insects. Indian Forest Records, 4, 91–110.
Clausen, C.P. (1976) Phoresy among entomophagous insects. Annual Review of Entomology, 21, 343 –368.
Dalla Torre, K.W. (1898) Catalogus hymenopterorum hucusque descriptiorum systematicus et synonymicus. Vol. V. Chalcididae et Proctotrupidae. Sumptibus Guilelmi Engelmann, Lipsiae, 598 pp.
De Santis, L. (1967) Catalogo de los Argentinos de la serie parasitica, incluyendo Bethyloidea. Provincia de Buenos Aires Gobernacion. Comision de Investigacion Cientifica, La Plata, 337 pp.
Delucchi, V.L. (1961) Le complexe des Asolcus Nakagawa (Microphanurus Kieffer) (Hymenoptera, Proctotrupoidea) parasites oophages des punaises des céréales au Maroc et au Moyen-Orient. Cahiers de la Recherche Agronomique, 14, 41–67.
Dodd, A.P. (1920) Notes on the exotic Proctotrupoidea in the British and Oxford University Museums, with descriptions of new genera and species. Transactions of the Entomological Society of London, 1919, 321 –382. http://dx.doi.org/10.1111/j.1365-2311.1920.tb00008.x
Fabritius, K. (1973) Contribu ţ ii la studiul Proctotrupoidelor (Hymenoptera) din R.S. România. PhD Thesis, Iaşi, Faculty of Biology, “Al. I. Cuza University ”.
Fabritius, K. (1974) Die Telenominen (Hymenoptera: Scelionidae) Rumäniens, eine faunistische Studie in unmittelbarer Verbindung mit der biologischen Schädlingsbekämpfung. Folia Entomologica Hungarica, 27 Suppl., 339–344.
Fabritius, K. & Popovici, O. (2007) A Catalogue of Scelionidae from Romania (Hymenoptera, Platygastroidea). Entomologica Romanica, 12, 133 –161.
Ferrière, C. (1952) Deux nouveaux parasites des oeufs de Locusta migratoria migratorioides en Afrique. Bulletin de la Société Entomologique de France, 56, 114 –118.
Förster, A. (1856) Hymenopterologische Studien. II. Heft. Chalcidae und Proctotrupii. Ernst ter Meer, Aachen, 152 pp.
Fusu, L. (2010) Species status of two colour morphs of Eupelmus vesicularis (Hymenoptera: Eupelmidae) as revealed by allozyme electrophoresis, morphometric and host preference data. Journal of Natural History, 44, 1113–1129. http://dx.doi.org/10.1080/00222931003632773
Galloway, I.D. & Austin, A.D. (1984) Revision of the Scelioninae (Hymenoptera: Scelionidae) in Australia. Australian Journal of Zoology Supplementary Series, 99, 1–138.
http://dx.doi.org/10.1071/AJZS099
Gibson, G.A. (2009) Revision of New World Spalangiinae (Hymenoptera: Pteromalidae). Zootaxa, 2259, 1–159.
Hammer, Ø., Harper, D.A.T. & Ryan, P.D. (2001) PAST: Palaeontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica, 4 (1), 1–9.
Harris, R.A. (1979) A glossary of surface sculpturing. Occasional Papers in Entomology, 28, 1–32.
Hope, F.W. (1837) Observations on succinic insects. Transactions of the Entomological Society of London, 2, 46–57.
Johnson, N.F. (1984) Systematics of Nearctic Telenomus: classification and revisions of the podisi and phymatae species groups (Hymenoptera: Scelionidae). Bulletin of the Ohio Biological Survey, 6 (3), 1–113.
Johnson, N.F. (1992) Catalog of world Proctotrupoidea excluding Platygastridae. Memoirs of the American Entomological Institute, 51, 1–825.
Johnson, N.F. (2004) The genera of Platygastroidea (Hymenoptera) of the World. Available from: http://osuc.biosci.ohiostate.edu/hymenoptera/eol_scelionidae.home (Accessed 18 June 2012)
Johnson, N.F. (1997) Hymenoptera On-Line. Available from: http://purl.oclc.org/NET/hymenoptera/hol (accessed 18 June 2012)
Johnson, N.F. & Bin, F. (1982) Species of Telenomus (Hymenoptera, Scelionidae), parasitoids of stalked eggs of Neuroptera (Chrysopidae and Berothidae). Redia, 65, 189 –206.
Johnson, N.F. & Bin, F. (1988) Telenomus species (Hymenoptera: Scelionidae) associated with the eggs of Zygaenidae (Lepidoptera). Proceedings of the Entomological Society of Washington, 90, 244 –247.
Kieffer, J.-J. (1908) Révision des Scelionidae (Hyménoptères). Annales de la Société Scientifique de Bruxelles, 32, 111 –250.
Kieffer, J.-J. (1910 a) Diagnoses de nouveaux genres et de nouvelles espèces de Scélionides (Hym.) des Îles Sechêlles. Bulletin de la Société Entomologique de France, 1910, 292 –294.
Kieffer, J.-J. (1910 b) Hymenoptera. Fam. Scelionidae. Addenda et corrigenda. Genera Insectorum, 80, 61–112.
Kieffer, J.-J. (1912) Hymenoptera, Proctotrupoidea. Transactions of the Linnean Society of London, Zoology, 15, 45–80.
Kieffer, J.-J. (1913) Proctotrypidae (3e partie). Species des Hyménoptères d'Europe et d’Algérie, 11, 161 –304.
Kieffer, J.-J. (1926) Scelionidae. Das Tierreich, 48, Walter de Gruyter & Co., Berlin, 885 pp.
Klingenberg, C.P. (2011) MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources, 11, 353 –357.
http://dx.doi.org/10.1111/j.1755-0998.2010.02924.x
Kononova, S.V. (1995) Fam. Scelionidae. In: Lehr, P.A. (Ed.), Key to insects of Russian Far East. Vol. 4. Neuropteroidea, Mecoptera, Hymenoptera. Part 2. Hymenoptera. Dal'nauka, Vladivostok, pp. 57 – 121.
Kononova, S.V. (2001) Morphological adaptations: ecological aspects and importance for evolution of egg parasites of the family Scelionidae (Hymenoptera, Proctotrupoidea). Entomological Review, 81 (6), 701–714.
Kononova, S.V. & Kozlov, M.A. (2008) Scelionids of the Palaearctic (Hymenoptera, Scelionidae). Subfamily Scelioninae. Tovarishchestvo Nauchnykh Izdanii KMK, Saint Petersburg, 489 pp.
Kononova, S.V. & Petrov, S. (2000) A review of the genera Triteleia, Paridris and Calotelea (Hymenoptera, Scelionidae, Scelioninae) of Palaearctic region. Vestnik Zoologii, 34 (6), 27–35.
Kononova, S.V. & Fursov, V.N. (2007) A review of the genera Calotelea, Calliscelio and Oxyscelio (Scelioninae, Scelionidae, Proctotrupoidea) from the Palaearctic fauna. Zoologicheskii Zhurnal, 86, 52–65.
http://dx.doi.org/10.1134/S0013873807010101
Kozlov, M.A. (1967) Palaearctic species of egg parasites of the genus Telenomus Haliday (Hymenoptera, Scelionidae, Telenominae). Entomologicheskoye Obozreniye, 46, 361 –378.
Kozlov, M.A. (1971) Proctotrupoids (Hymenoptera, Proctotrupoidea) of the USSR. Trudy Vsesoyuznogo Entomologicheskogo Obshchestva, 54, 3–67.
Kozlov, M.A. (1978) Superfamily Proctotrupoidea. In: Medvedev, G.S. Determination of insects of the European portion of the USSR. Nauka, Leningrad, 3 (2), 758 pp.
Kozlov, M.A. & Kononova, S.V. (1983) Telenominae of the Fauna of USSR. Zoological Institute of Scientific Academy of USSR, 136, Nauka, Leningrad, 336 pp.
Kozlov, M.A. & Kononova, S.V. (1985) A review of the genera Triteleia and Calliscelio (Proctotrupoidea, Scelionidae). Vestnik Zoologii, 1985 (4), 15–24.
Kozlov, M.A. & Kononova, S.V. (1989) New species of the genus Calotelea Westwood (Hymenoptera, Scelionidae) of the USSR and Japan. Trudy Zoologicheskogo Instituta Akademii Nauk SSSR, 188, 101 –108.
Kozlov, M.A. & Kononova, S.V. (1990) Scelioninae of the Fauna of the USSR (Hymenoptera, Scelionidae, Scelioninae). Nauka, Leningrad, 344 pp.
Maneval, H. (1940) Fam. XVII. Proctotrypides. In: Perrier, R. (Ed.), La Faune de la France en tableaux synoptiques illustrés. Tome VII. Hyménoptères par Lucien Berland avec la collaboration de MM. Raymond Benoit, Francis Bernard, Henri Maneval. Paris, pp. 93–118.
Mani, M.S. (1941) Serphoidea. Catalogue of Indian Insects, 26, 1–60.
Mani, M.S. & Sharma, S.K. (1982) Proctotrupoidea (Hymenoptera) from India: A review. Oriental Insects, 16, 135 –258.
Masi, L. (1933) Raccolte entomologiche nell'isola di Capraia fatte da C. Mancini e F. Capra (1927–1931). IV. Hymenoptera Terebrantia et Phytophaga. Memorie della Societa Entomologica Italiana, 12, 16–48.
Masner, L. (1958) A new egg-parasite of gipsy moth Lymantria dispar (L.). Entomophaga, 3, 39–44. http://dx.doi.org/10.1007/BF02372198
Masner, L. (1959) Some problems of the taxonomy of the subfamily Telenominae (Hym., Scelionidae). Transaction of the 1st International Conference Insect Pathology and Biological Control, Prague, 1958, 375 –382.
Masner, L. (1964) A comparison of some Nearctic and Palearctic genera of Proctotrupoidea (Hymenoptera) with revisional notes. Č asopis Č eskoslovenské spole č nosti entomologické, 61, 123 –155.
Masner, L. (1965) The types of Proctotrupoidea (Hymenoptera) in the British Museum (Natural History) and in the Hope Department of Entomology, Oxford. Bulletin of the British Museum (Natural History) Entomology Supplement, 1, 1–154.
Masner, L. (1976) Revisionary notes and keys to world genera of Scelionidae (Hymenoptera: Proctotrupoidea). Memoirs of the Entomological Society of Canada, 97, 1–87.
http://dx.doi.org/10.4039/entm10897fv
Masner, L. (1979) Pleural morphology in scelionid wasps (Hymenoptera, Scelionidae) - an aid to higher classification. The Canadian Entomologist, 111, 1079–1087.
http://dx.doi.org/10.4039/Ent1111079-9
Masner, L. (1980 a) The identity of Calotelea ocularis Ashmead, 1894 (Hymenoptera, Proctotrupoidea, Scelionidae). The Canadian Entomologist, 112, 393 –396.
http://dx.doi.org/10.4039/Ent112393-4
Masner, L. (1980 b) A revision of the Nearctic species of Calotelea Westwood (Hymenoptera, Proctotrupoidea, Scelionidae). The Canadian Entomologist, 112, 397 –408.
http://dx.doi.org/10.4039/Ent112397-4
Masner, L. (1980 c) Key to genera of Scelionidae of the Holarctic region, with descriptions of new genera and species (Hymenoptera: Proctotrupoidea). Memoirs of the Entomological Society of Canada, 113, 1–54. http://dx.doi.org/10.4039/entm112113fv
Mikó, I., Vilhelmsen, L., Johnson, N.F., Masner, L. & Pénzes, Z. (2007) Skeletomusculature of Scelionidae (Hymenoptera: Platygastroidea): head and mesosoma. Zootaxa, 1571, 1–78.
Mineo, G. (1979) Studies of the Scelionidae (Hym. Proctotrupoidea). IX. Material for a revision of the genus Gryon Hal., with description of 4 new species (G. austrafricanum, G. eremiogryon, G. laraichii, G. n i c o l a i) and notes on other scelionids. Bollettino del Laboratorio di Entomologia Agraria Filippo Silvestri, 36, 234 –265.
Muesebeck, C.F.W. (1979) Superfamily Proctotrupoidea. In: Krombein, K.V., Hurd P.D. et al. (Eds.), Catalog of Hymenoptera in America north of Mexico. Smithsonian Institution Press, Washington, DC, pp. 1121–1186.
Muesebeck, C.F.W. & Masner, L. (1967) Superfamily Proctotrupoidea. In: Krombein, K.V. & Burks, B.D. (Eds.), Hymenoptera of America north of Mexico. Synoptic Catalog (Agriculture Monograph No. 2). Second supplement. United States Government Printing Office, Washington, pp. 285–304.
Muesebeck, C.F.W. & Walkley, L.M. (1951) Superfamily Proctotrupoidea. In: Muesebeck, C.F.W., Krombein, K.V. & Townes, H.K. (Eds.), Hymenoptera of America north of Mexico: Synoptic Catalog. U.S. Dept. Agriculture Monograph, 2, pp. 655– 718.
Muesebeck, C.F.W. & Walkley, L.M. (1956) Type species of the genera and subgenera of parasitic wasps comprising the superfamily Proctotrupoidea (order Hymenoptera). Proceedings of the U.S. National Museum, 105, 319 –419.
Murphy, N., Carey, D., Castro, L., Dowton, M. & Austin, A. (2007) Phylogeny of the platygastroid wasps (Hymenoptera) based on sequences from the 18SrRNA, 28S rRNA and cytochrome oxidase I genes: implications for the evolution of the ovipositor system and host relationships. Biological Journal of the Linnean Society, 91, 653 –669. http://dx.doi.org/10.1111/j.1095-8312.2007.00825.x
Narendran, T.C. (1998) A new species and a key to species of Calotelea Westwood (Hymenoptera: Scelionidae) from India. Proceedings of the Zoological Society of Calcutta, 51 (1), 70–74.
Nikol'skaya, M.N. (1948) Species of the genus Telenomus (Hymenoptera, Scelionidae), parasites of the eggs of gadflies. Doklady Akademii Nauk SSSR, 62, 729 –732.
Nixon, G.E.J. (1935) A revision of the African Telenominae (Proctotrupoidea, Scelionidae). Transactions of the Royal Entomological Society of London, 83, 73–103.
http://dx.doi.org/10.1111/j.1365-2311.1935.tb00416.x
Nixon, G.E.J. (1939) Parasites of hemipterous grain-pests in Europe (Hymenoptera: Proctotrupoidea). Arbeiten über Morphologische und Taxonomische Entomologie aus Berlin-Dahlem, 6, 129 –136.
Nixon, G.E.J. (1940) New species of Proctotrupoidea. Annals and Magazine of Natural History, 11 (6), 497–512.
Nixon, G.E.J. (1958) A synopsis of the African species of Scelio Latreille (Hymenoptera: Proctotrupoidea, Scelionidae). Transactions of the Royal Entomological Society of London, 110, 303 –318.
http://dx.doi.org/10.1111/j.1365-2311.1958.tb00785.x
Ogloblin, A.A. (1927) Two new scelionid parasites of Locusta migratoria L., from Russia. Bulletin of Entomological Research, 17, 393 –404.
http://dx.doi.org/10.1017/S0007485300019490
Polaszek, A., Manzari, S. & Quicke, D.L.J. (2004) Morphological and molecular taxonomic analysis of the Encarsia meritoria species-complex (Hymenoptera, Aphelinidae), parasitoids of whiteflies (Hemiptera, Aleyrodidae) of economic importance. Zoologica Scripta, 33, 403 –421.
http://dx.doi.org/10.1111/j.0300-3256.2004.00161.x
Popovici, O.A. (2007) Biodiversitatea familiilor scelionide ş i platigastride (Hymenoptera, Scelionidae, Platygastridae) din estul României. PhD Thesis, Faculty of Biology, “Al. I. Cuza” University Iaşi.
Popovici, O.A. & Buhl, P.N. (2010) The West Palaearctic species of Fidiobia Ashmead, 1894 (Hymenoptera: Platygastroidea). Journal of Natural History, 44, 1131–1164.
http://dx.doi.org/10.1080/00222931003632740
Popovici, O.A., Bin, F., Masner, L., Popovici, M. & Notton, D.G. (2011) Triteleia peyerimhoffi comb. n., a remarkably variable circum-Mediterranean scelionid (Hymenoptera, Platygastroidea). ZooKeys, 140, 71–99. http://dx.doi.org/10.3897/zookeys.140.1925
Rajmohana, K. (2006) Studies on Proctotrupoidea and Platygastroidea (Hymenoptera: Insecta) of Kerala. Memoirs of the Zoological Survey of India, 21 (1), 1–153.
Rohlf, F.J. (1999) Shape statistics: Procrustes superimpositions and tangent spaces. Journal of Classification, 16, 197 –223. http://dx.doi.org/10.1007/s003579900054
Rohlf, F.J. (2003) TpsRelw, Version 1.35. Department of Ecology and Evolution, State University of New York, Stony Brook.
Rohlf, F.J. (2004) tpsDig 2. Department of Ecology and Evolution, State University of New York, Stony Brook.
Sadeghi, S., Adriaens, D. & Dumont, H.J. (2009) Geometric morphometric analysis of wing shape variation in ten European populations of Calopteryx splendens (Harris, 1782) (Zygoptera: Odonata). Odonatologica, 38 (4), 343–360.
Safavi, M. (1968) Étude biologique et écologique des Hyménoptères parasites des oeufs des punaises des céréales. Entomophaga, 13 (5), 381–495.
Slice, D.E. (2001) Landmark coordinates aligned by Procrustes analysis do not lie in Kendall’s shape space. Systematic Biology, 50, 141 –149.
http://dx.doi.org/10.1080/10635150119110
Sarra, R. (1930) Due nuovi imenotteri italiani. Bollettino del Laboratorio di Zoologia Generale e Agraria della R. Scuola Superiore d'Agricultura, 24, 223 –227.
Szelényi, G. (1941) Neue Gattungen und Arten der palaearktischen Scelioniden (Hym., Proctotrupoidea). Zoologischer Anzeiger, 134, 158 –168.
Zelditch, M.L., Swiderski, D.L., Sheets, A.D. & Fink, W.L. (2004) Geometric Morphometrics for Biologists: A Primer. Elsevier, Berlin, 444 pp.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.