Leposoma annectans Ruibal, 1952
|
publication ID |
https://doi.org/10.1590/S0031-10492002000500001 |
|
persistent identifier |
https://treatment.plazi.org/id/03B587BD-3A58-FFDE-FE11-FD31FD3A1F3F |
|
treatment provided by |
Carolina |
|
scientific name |
Leposoma annectans Ruibal, 1952 |
| status |
|
Leposoma annectans Ruibal, 1952
Description of the new specimens (Fig. 1). Rostral broad, wider than high, contacting first supralabial, nasal and frontonasal. Frontonasal longitudinally divided, as wide as long, just reaching anterior supraocular and in broad contact with rostral, nasal, loreal and prefrontal, always indenting posteriorly the latter. Frontonasal entirely divided longitudinally and medially in four specimens; in ten other specimens, an irregular azygous subtriangular scale, wider posteriorly, and symmetric with respect to the median suture, occurring between the longitudinally divided frontonasal and the prefrontals. Prefrontals as long as wide, in broad contact, indented anteriorly by the azygous scale (when present) and posteriorly by the frontal. Frontal hexagonal, with strongly concave lateral margins; approximately three times as long as broad, slightly wider and indenting a pair of frontoparietals posteriorly. Frontoparietals pentagonal, larger than prefrontals, in slight contact. Interparietal wider and as long as frontal, with slightly concave and diverging lateral margins, longer than wide; wider and rounded posteriorly. In one specimen ( MZUSP 87.804) the posterior part of interparietal is symmetrically fused with the enlarged adjacent temporals forming two relatively enlarged scales that contact slightly medially, shortening the interparietal and preventing it from contacting the dorsals. In one specimen ( MZUSP 87.794) there is no medial contact among these scales; in another this modification affects only the left side leaving a long and narrow posterior interparietal process contacting dorsals. Parietals irregularly hexagonal, almost as long as wide; narrower and shorter than interparietal and never reaching its posterior margin. Parietals followed posteriorly by several enlarged occipito-temporal scales. Supraoculars four; the first smallest and longer than wide; the third the largest, squarish, much larger than second and fourth that are subrectangular and wider than long; all contacting superciliaries. Nasal above first supralabial, large, longer than wide, entire, with the nostril in the center; ventral margin slightly rounded, dorsal margin straight. Loreal posterior to nasal, narrow and diagonally oriented; anterior to frenocular; the last followed by a series of 4 or 5 infraorbital elongate granules. Five supralabials, first longest, fifth highest, third and fourth eye; fifth supralabial followed by a scale approximately as large as the last supralabial and separated from the tympanum by 3 much smaller scales. Four or five superciliaries, first largest, larger than or as large as first supraocular, expanded on lateral surface of head. Eyelid with semitransparent disc formed by 3-4 scales. Temporal region covered with strongly keeled juxtaposed scales that are smaller between the area delimited by the tympanum and much larger in the occipito-temporal region around parietals. Lateral surface of neck with conical and juxtaposed, sometimes keeled granules, arranged in irregularly transverse rows. Ear opening bordered by a series of very small, smooth, granules; tympanum distinct, subovoid. All scales of the top of head with sharp and irregularly longitudinal striations.
Mental broad, wider than long. Postmental single. First pair of genials smallest in broad contact at middline, second largest, in contact anteriorly, both in contact with infralabials. Third pair of genials the smallest, approximately as long as wide, separated medially by a series of elongate scutes and from the infralabials by an enlarged and elongate scute. Mental, postmental and genials strongly marked with sharp longitudinal striations. Infralabials five; second and third largest. Scales immediately posterior and lateral to third pair of genials elongate, juxtaposed, strongly keeled or striated. From there on they gradually grade into lanceolate, imbricate and keeled gulars. Gulars in 9-11 transverse rows, lanceolate, imbricate, strongly keeled. Collar fold indistinct.
Dorsal scales large, strongly keeled, mucronate, imbricate, becoming laterally juxtaposed at midbody, in 26-30 regular transverse rows between interparietal and posterior level of hind limbs. Lateral scales resemble dorsals; grade into ventrals except for an area with small, flat, almost smooth and juxtaposed scutes near groin and a region with small and conical granules around arm level. Scales around midbody 21-27. Ventrals leaf-shaped, keeled, mucronate, imbricate, in 16-20 regular transverse and diagonal rows from interbrachials (included) to preanals. Posterior margin of the vent with five scales; central and paramedial scales the largest. Total pores (preanal plus femoral) 10-12 in males (4 preanal). Pores absent in females.
Scales of tail imbricate, keeled, in complete rings, more regular ventrally; dorsal and lateral tail scales more strongly keeled and wider than ventral scales proximally, becoming gradually identical towards the extremity.
Limb scales keeled and imbricate, except on ventral surface of brachium and on posterior surface of thigh which are mostly subimbricate or juxtaposed, sometimes granular. Palmar and plantar surfaces with small, conical granules. Subdigital lamellae mostly double, 9-12 on Finger IV and 12-16 on Toe IV. Fingers and toes clawed, with the following relative sizes: 1 <2 = 5<3 <4 and 1 <2 <5 <3 <4, respectively.
Dorsal surface of body and tail light brown with irregular dark brown or black spots one scale wide. An inconspicuous dorsolateral light stripe one-half to one scale wide running from midbody level toward the ear level, their anteriormost parts converging to occipital region. Between stripes a double series of irregularly disposed black spots extends from the occipital region to the base of tail. Flanks and lateral surface of tail slightly darker than back with a few small light spots more conspicuous on neck. Dorsal and lateral surface of head light brown with scattered dark brown reticulation. Ventral parts of head, body and tail yellowish cream, immaculate. Limbs dark brown dorsally, mottled with yellow; ventrally yellowish cream, immaculate, like for the ventral parts of body and tail.
COMPARISONS WITH LEPOSOMA SCINCOIDES (Figs 1 and 2)
Leposoma annectans and Leposoma scincoides are the only Atlantic
Forest Leposoma presenting a longitudinally divided frontonasal and highly
striated scales on head. The two species can be separated by the following list
of characters. Except when noted, differences refers to all specimens of L.
scincoides examined.
1) Leposoma annectans presents a posteriorly enlarged interparietal with lateral margins slightly concave and divergent; in L. scincoides the interparietal is not enlarged posteriorly and its lateral margins are almost parallel.
2) Leposoma annectans has parietals as long as wide and shorter than the interparietal; in L. scincoides the parietals are longer than wide and as the long as the interparietal.
3) Leposoma annectans has a small scale separating the third pair of chinshields from the infralabials; in scincoides no such scale exists and the third pair of chinshields contacts the infralabials.
4) Leposoma annectans has a very large and almost quadrangular third supraocular; in scincoides the third supraocular is clearly wider than long having approximately the same size as the second supraocular.
5) Leposoma annectans presents conspicuous sharp striations on the scales of the ventral part of head; in scincoides these scales are almost smooth.
6) Leposoma annectans is more dark colored and has more irregular dorsal black spots than Leposoma scincoides .
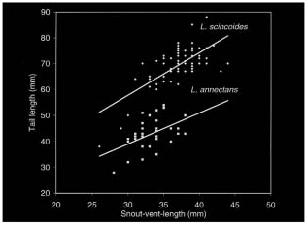
7) Leposoma annectans has a shorter body length and relative tail length when compared with scincoides ( Fig. 4 View Figure 4 ).
8) In Leposoma annectans , femoral and preanal pores are restricted to males, and are absent in females; in females of scincoides pores are present or absent.
9) Leposoma annectans additionally differs from L. scincoides in having a lower number of dorsal and ventral scales, lower counts of scales around midbody, a lower number of fourth finger and fourth toe infradigital lamellae and a lower number of total femoral pores. Table 1 summarizes these data for the sample obtained syntopically at Una, State of Bahia. Differences between the two species in number of dorsal and ventral scales, scales around body, total number of pores and number of fourth finger and fourth toe lamellae are all significant (t-test and Kruskall-Wallis test; P<0.01). Except for femoral pores, there are no sexual differences in the caracters studied for Leposoma annectans (t-test; P<0.01). The same is true for Leposoma scincoides except that females of this species have slightly higher counts of ventrals and scales around body than males (t-test and Kruskal-Wallis test; P>0.05).
COMPARISONS WITH OTHER LEPOSOMA
The twelve species presently included in the genus Leposoma can be separated in two clearly distinctive groups: parietale and scincoides (Rodrigues, 1997) . Species of the scincoides group ( scincoides , nanodactylus and baturitensis ) are restricted to eastern Brazil and have elongate dorsal scales and lanceolate ventrals that are arranged in diagonal rather than in longitudinal rows. In the more diverse parietale group (9 species) ranging from Amazonia to Costa Rica, dorsal scales are wider and shorter, and ventral scales are arranged in regular longitudinal rows. Leposoma annectans is a member of the scincoides group as it has lanceolate ventrals arranged in diagonal rather than in regular longitudinal rows. In the scincoides group it differs from Leposoma nanodactylus and L. baturitensis by characters 1-5 referred above, by having a longitudinally divided frontonasal (single in nanodactylus and baturitensis ), and by presenting the dorsal and ventral scales of the head heavily striated (weakly striated or smooth in nanodactylus and baturitensis ). From Leposoma baturitensis it additionally differs by the number of IV toe lamellae (12-16; 16-18 in baturitensis ), total pores (10-13; 13-15 in baturitensis ) and color pattern.
Three specimens of Leposoma nanodactylus , the first record since the holotype, were obtained along with specimens of the present series of Leposoma scincoides and Leposoma annectans . They are adult males ( 27-34 mm SVL) and agree perfectly with the original description. Even characters supposed to be variable like the presence of five supraoculars, the ventrolateral black pigmentation and the arrangement of preanal scales are identical to the conditions reported for the type. The new meristic data, combined with those reported for the holotype, provides additional characters to diagnose Leposoma nanodactylus from Leposoma annectans (characters from the latter in parenthesis): dorsal scales: 31-33 (26-30); ventral scales: 18-20 (16-20); scales around body: 27-31 (21-27); IV finger lamellae: 7-8 (9-12); IV toe lamellae: 10-12 (12-16); total pores: 10 (10-13).
Leposoma annectans is also unique in that the majority of specimens have an azygous scale between the longitudinally divided frontonasal and the prefrontals. The presence of supernumerary scales between frontonasal and prefrontal, although rare, does occur in other gymnophthalmids. It was described as an occasional occurrence in some species of Neusticurus , and recognized as diagnostic in Amapasaurus , a poorly known monotypic genus related to Leposoma .
DISTRIBUTION AND ECOLOGY
The Atlantic rainforests of eastern Bahia are among the top five threatened hotspots of the world ( Mittermeier and Mittermeier, 1997). Enormous amounts of forests have been cut down in the last decades for timber and farming. In 1980, an area of 11.400 ha of remaining forests near Una was declared preserved by the government as the Reserva Biológica de Una ( 15°10’S, 39°03’W). The relief is hilly with some of the valleys dissected by medium size to small streams. Annual precipitation reaches 2000 mm without conspicuous seasonality. Primary forest is composed of high trees, reaching up to 40 m where palms are especially abundant and a characteristic leaf litter is present. The area is also characterized by a high diversity of bromeliads and ferns. Most of the forest surrounding the Una reserve has been selectively cut to accommodate cacao plantations or other crops or cleared for cattle ranching. A few fragments of primary forest remain. In the cacao groves, large and emergent trees (up to 30 m tall and over approximately 50 cm diameter at breast height) have been preserved to shade the understory cacao plants. In these characteristic sheltered cacao groves (cabruca, locally), leaf litter is abundant and formed predominantly by cacao leaves. Despite their high degree of disturbance compared to primary forests, cabrucas provide shelter and humidity for forest animals, apparently protecting them from extirpation in open areas ( Araujo et al., 1998).
In a herpetofaunal survey of Una, a series of pitfall traps were placed in different habitats. We sampled: primary forest of Una Reserve and nearby, forest fragments, secondary forests and, cacao groves. Table 2 shows that Leposoma scincoides and Leposoma annectans were more frequently found in disturbed habitats, specially in cacao groves where their frequency was highest. The sample size of L. nanodactylus is too small to discern habitat preference but two of the three specimens collected along the edge of a forest fragment. A Chi-square test shows no association between habitat categories and species or sex (P>0.05), nor between habitat categories and sex within each species (P>0.05).
The abundance and preference of Leposoma for disturbed habitats in the Atlantic Forests of State of Bahia is not surprising. In October 1986, at São José do Macuco ( 15°09’ S, 39°18’ W) in the same general area of the present study, a collection of Leposoma was also obtained. Of the thirteen specimens collected, one was a male and became the holotype of Leposoma nanodactylus , two specimens (a male and a juvenile) remain unidentified, and 10 specimens ( 5 males and 5 females) were L. scincoides (Rodrigues, 1997) . At the time the area had been recently prepared for cacao farming and large patches of primary forest interdigited with the groves. Although leposomas were spotted and obtained by hand in both habitats, they were definitively more abundant in the cacao groves than in the primary forest GoogleMaps .
The surprise was the sex-ratio of our collection. Table 3 shows for the three species the number of males and females obtained in the pitfall traps. The astonishingly high number of males and the disproportionatly low number of females is puzzling. This sample strongly differs from the balanced sex-ratio obtained in the 1986 sample. As the sampling covers two different periods of year, the number of trapped females is probably not associated with seasonal changes in reproductive behavior. Numbers of females are surprisingly low both in Leposoma scincoides and Leposoma annectans . The same skewed sexratio might also be ocurring in the rare Leposoma nanodactylus , from which only three males were collected. Curiously, the species was previously known only from a male specimen. Unless females are strikingly different from males in terms of habitat selection which in turn could affect their capture rate in pitfalls, there must be another reason for such a skewed sex-ratio. Also, the hypothesis should explain the same phenomenon in two and possibly three syntopic species.
Sex determination by temperature could explain the skewed sex ratio. In species of Leposoma studied cytogenetically ( scincoides , oswaldoi , and guianense ), there are no heteromorphic sex chromosomes suggesting a chromosome mechanism of sex determination (Pellegrino, et al., 1999). No data on temperature sex determination (TSD) exists for gymnophthalmids, although it has been described in Lacertidae ( Viets et al., 1994) , which are the old world scincomorphs most closely related to gymnophthalmids. In several lizard species only males emerge from eggs exposed to high developmental temperatures and the range of temperatures producing both sexes is very small (pattern Ib, Bull (1983), Lang and Andrews, 1994; Viets et al., 1994)).
Temperatures in cacao groves, forest edge, clearings, secondary forests, and small forest fragments are probably higher than mean temperatures in primary forests. Therefore, we suggest that forest disturbance may threaten Leposoma populations by causing elevation of mean ambient temperature in forest fragments which, in turn, may produce sex ratios highly skewed towards males.
If TSD mechanism exists in Leposoma , the natural distribution of edge and treefall gaps in primary forest play a role in maintaining balanced sexratios. However, the range of mean temperature distribution in small forest fragments and cabruca may be significantly altered.
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |